Characteristics of lepidolite-derived lithium in medical battery applications
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lepidolite Lithium Background
Lepidolite, a lithium-rich mica mineral, has emerged as a significant source of lithium for various applications, including medical battery technologies. The growing demand for lithium in the medical sector, particularly for powering implantable devices and portable medical equipment, has led to increased interest in lepidolite-derived lithium.
Historically, lithium extraction primarily focused on brine and spodumene sources. However, the discovery of lepidolite's potential as a lithium source has expanded the industry's horizons. Lepidolite deposits are found in various regions worldwide, including Portugal, Brazil, and parts of Africa, offering a diversified supply chain for lithium production.
The unique characteristics of lepidolite-derived lithium make it particularly suitable for medical battery applications. Its high purity levels and consistent quality are crucial factors in ensuring the reliability and longevity of medical devices. The extraction process from lepidolite also allows for better control over impurities, which is essential in meeting the stringent requirements of medical-grade lithium.
In recent years, advancements in extraction technologies have significantly improved the efficiency and cost-effectiveness of obtaining lithium from lepidolite. These developments have made lepidolite a more viable option for lithium production, competing with traditional sources in terms of both quality and economic feasibility.
The medical battery industry has shown increasing interest in lepidolite-derived lithium due to its potential to meet the growing demand for high-performance, long-lasting batteries in medical devices. The unique properties of this lithium source align well with the requirements of implantable medical devices, which demand exceptional reliability and safety standards.
As the medical technology sector continues to evolve, with a trend towards miniaturization and increased functionality of devices, the demand for high-quality lithium sources like lepidolite is expected to grow. This trend is driving further research and development in lepidolite extraction and processing techniques, aiming to optimize the characteristics of the derived lithium for specific medical applications.
The background of lepidolite lithium in medical battery applications thus represents a convergence of geological resources, technological advancements, and medical innovation. It highlights the importance of exploring alternative lithium sources to meet the specialized needs of the medical industry, ensuring a sustainable and reliable supply chain for critical healthcare technologies.
Historically, lithium extraction primarily focused on brine and spodumene sources. However, the discovery of lepidolite's potential as a lithium source has expanded the industry's horizons. Lepidolite deposits are found in various regions worldwide, including Portugal, Brazil, and parts of Africa, offering a diversified supply chain for lithium production.
The unique characteristics of lepidolite-derived lithium make it particularly suitable for medical battery applications. Its high purity levels and consistent quality are crucial factors in ensuring the reliability and longevity of medical devices. The extraction process from lepidolite also allows for better control over impurities, which is essential in meeting the stringent requirements of medical-grade lithium.
In recent years, advancements in extraction technologies have significantly improved the efficiency and cost-effectiveness of obtaining lithium from lepidolite. These developments have made lepidolite a more viable option for lithium production, competing with traditional sources in terms of both quality and economic feasibility.
The medical battery industry has shown increasing interest in lepidolite-derived lithium due to its potential to meet the growing demand for high-performance, long-lasting batteries in medical devices. The unique properties of this lithium source align well with the requirements of implantable medical devices, which demand exceptional reliability and safety standards.
As the medical technology sector continues to evolve, with a trend towards miniaturization and increased functionality of devices, the demand for high-quality lithium sources like lepidolite is expected to grow. This trend is driving further research and development in lepidolite extraction and processing techniques, aiming to optimize the characteristics of the derived lithium for specific medical applications.
The background of lepidolite lithium in medical battery applications thus represents a convergence of geological resources, technological advancements, and medical innovation. It highlights the importance of exploring alternative lithium sources to meet the specialized needs of the medical industry, ensuring a sustainable and reliable supply chain for critical healthcare technologies.
Medical Battery Market Analysis
The medical battery market has experienced significant growth in recent years, driven by the increasing prevalence of implantable medical devices and portable healthcare equipment. This market segment is characterized by stringent safety requirements, long-term reliability demands, and the need for high energy density. Lithium-ion batteries, particularly those utilizing lepidolite-derived lithium, have emerged as a promising solution for medical applications due to their superior performance characteristics.
The global medical battery market is projected to expand at a robust rate, with North America and Europe leading in terms of market share. This growth is primarily attributed to the rising adoption of wearable medical devices, implantable cardioverter-defibrillators (ICDs), and other battery-powered medical equipment. The aging population in developed countries and the increasing incidence of chronic diseases are key factors contributing to market expansion.
Lepidolite-derived lithium offers several advantages in medical battery applications. Its high purity and consistent quality make it particularly suitable for use in sensitive medical devices. The extraction process for lepidolite-derived lithium is generally more environmentally friendly compared to traditional brine-based methods, aligning with the growing emphasis on sustainability in the healthcare sector.
The demand for medical batteries with enhanced safety features is a significant market driver. Lepidolite-derived lithium batteries have demonstrated improved thermal stability and reduced risk of thermal runaway, addressing critical safety concerns in medical applications. This characteristic is especially valuable in implantable devices where battery failure could have severe consequences.
Market trends indicate a shift towards miniaturization of medical devices, necessitating batteries with higher energy density. Lepidolite-derived lithium batteries are well-positioned to meet this demand, offering compact size without compromising on power output. This trend is particularly evident in the development of next-generation pacemakers, cochlear implants, and neurostimulators.
The COVID-19 pandemic has accelerated the adoption of remote patient monitoring systems and telemedicine devices, further boosting the demand for reliable, long-lasting medical batteries. Lepidolite-derived lithium batteries, with their extended lifecycle and stable performance, are increasingly being integrated into these emerging healthcare technologies.
Regulatory considerations play a crucial role in shaping the medical battery market. Stringent quality control measures and compliance requirements for medical-grade batteries have created entry barriers, potentially favoring established manufacturers with expertise in lepidolite-derived lithium technology. This regulatory landscape is expected to drive innovation in battery design and manufacturing processes to meet evolving safety standards.
The global medical battery market is projected to expand at a robust rate, with North America and Europe leading in terms of market share. This growth is primarily attributed to the rising adoption of wearable medical devices, implantable cardioverter-defibrillators (ICDs), and other battery-powered medical equipment. The aging population in developed countries and the increasing incidence of chronic diseases are key factors contributing to market expansion.
Lepidolite-derived lithium offers several advantages in medical battery applications. Its high purity and consistent quality make it particularly suitable for use in sensitive medical devices. The extraction process for lepidolite-derived lithium is generally more environmentally friendly compared to traditional brine-based methods, aligning with the growing emphasis on sustainability in the healthcare sector.
The demand for medical batteries with enhanced safety features is a significant market driver. Lepidolite-derived lithium batteries have demonstrated improved thermal stability and reduced risk of thermal runaway, addressing critical safety concerns in medical applications. This characteristic is especially valuable in implantable devices where battery failure could have severe consequences.
Market trends indicate a shift towards miniaturization of medical devices, necessitating batteries with higher energy density. Lepidolite-derived lithium batteries are well-positioned to meet this demand, offering compact size without compromising on power output. This trend is particularly evident in the development of next-generation pacemakers, cochlear implants, and neurostimulators.
The COVID-19 pandemic has accelerated the adoption of remote patient monitoring systems and telemedicine devices, further boosting the demand for reliable, long-lasting medical batteries. Lepidolite-derived lithium batteries, with their extended lifecycle and stable performance, are increasingly being integrated into these emerging healthcare technologies.
Regulatory considerations play a crucial role in shaping the medical battery market. Stringent quality control measures and compliance requirements for medical-grade batteries have created entry barriers, potentially favoring established manufacturers with expertise in lepidolite-derived lithium technology. This regulatory landscape is expected to drive innovation in battery design and manufacturing processes to meet evolving safety standards.
Lepidolite Extraction Challenges
The extraction of lithium from lepidolite presents several significant challenges that hinder its widespread adoption in medical battery applications. One of the primary obstacles is the complex mineralogical structure of lepidolite, which requires intensive processing to liberate the lithium content. Unlike other lithium-bearing minerals, lepidolite's lithium is tightly bound within its crystal lattice, necessitating high-energy extraction methods.
The conventional extraction process for lepidolite involves roasting the mineral at temperatures exceeding 800°C, followed by acid leaching. This energy-intensive procedure not only increases production costs but also raises environmental concerns due to high carbon emissions. Moreover, the use of strong acids in the leaching stage poses potential risks to worker safety and environmental integrity, requiring stringent control measures.
Another significant challenge lies in the relatively low lithium content of lepidolite compared to other lithium sources. Typical lepidolite ores contain only 1-1.5% lithium by weight, necessitating the processing of large volumes of material to obtain commercially viable quantities of lithium. This low yield increases the overall extraction costs and generates substantial amounts of waste material, further complicating the environmental management aspects of lepidolite processing.
The presence of impurities in lepidolite, such as rubidium, cesium, and fluorine, also complicates the extraction process. These elements must be carefully separated from the lithium to meet the high purity standards required for medical battery applications. The additional purification steps add complexity to the extraction process and can significantly impact the final product's cost and quality.
Water consumption is another critical challenge in lepidolite extraction. The process requires substantial amounts of water for mineral processing and acid leaching, which can be problematic in water-scarce regions where many lepidolite deposits are located. This not only increases operational costs but also raises concerns about the sustainability of large-scale lepidolite extraction in these areas.
Furthermore, the variability in lepidolite composition across different deposits presents challenges in developing standardized extraction processes. Each deposit may require tailored extraction methods, making it difficult to achieve economies of scale and consistent product quality across different production sites.
Lastly, the development of efficient recycling methods for lepidolite-derived lithium batteries remains a significant challenge. As the demand for medical batteries grows, the ability to recover and reuse lithium from spent batteries becomes increasingly important for sustainability and resource conservation. However, the complex chemistry of lepidolite-derived lithium batteries makes recycling more challenging compared to other lithium battery types.
The conventional extraction process for lepidolite involves roasting the mineral at temperatures exceeding 800°C, followed by acid leaching. This energy-intensive procedure not only increases production costs but also raises environmental concerns due to high carbon emissions. Moreover, the use of strong acids in the leaching stage poses potential risks to worker safety and environmental integrity, requiring stringent control measures.
Another significant challenge lies in the relatively low lithium content of lepidolite compared to other lithium sources. Typical lepidolite ores contain only 1-1.5% lithium by weight, necessitating the processing of large volumes of material to obtain commercially viable quantities of lithium. This low yield increases the overall extraction costs and generates substantial amounts of waste material, further complicating the environmental management aspects of lepidolite processing.
The presence of impurities in lepidolite, such as rubidium, cesium, and fluorine, also complicates the extraction process. These elements must be carefully separated from the lithium to meet the high purity standards required for medical battery applications. The additional purification steps add complexity to the extraction process and can significantly impact the final product's cost and quality.
Water consumption is another critical challenge in lepidolite extraction. The process requires substantial amounts of water for mineral processing and acid leaching, which can be problematic in water-scarce regions where many lepidolite deposits are located. This not only increases operational costs but also raises concerns about the sustainability of large-scale lepidolite extraction in these areas.
Furthermore, the variability in lepidolite composition across different deposits presents challenges in developing standardized extraction processes. Each deposit may require tailored extraction methods, making it difficult to achieve economies of scale and consistent product quality across different production sites.
Lastly, the development of efficient recycling methods for lepidolite-derived lithium batteries remains a significant challenge. As the demand for medical batteries grows, the ability to recover and reuse lithium from spent batteries becomes increasingly important for sustainability and resource conservation. However, the complex chemistry of lepidolite-derived lithium batteries makes recycling more challenging compared to other lithium battery types.
Current Lepidolite Processing Methods
01 Extraction methods for lepidolite-derived lithium
Various extraction methods are employed to obtain lithium from lepidolite, including acid leaching, roasting, and hydrometallurgical processes. These techniques aim to efficiently separate lithium from other minerals present in lepidolite ore, optimizing the recovery of this valuable element for use in batteries and other applications.- Extraction methods for lepidolite-derived lithium: Various extraction methods are employed to obtain lithium from lepidolite, including acid leaching, roasting, and hydrometallurgical processes. These techniques aim to efficiently separate lithium from other minerals present in lepidolite ore, optimizing the recovery of this valuable element for industrial applications.
- Purification and processing of lepidolite-derived lithium: After extraction, lepidolite-derived lithium undergoes purification and processing steps to meet industry standards. This may involve ion exchange, crystallization, or membrane separation techniques to remove impurities and increase the concentration of lithium compounds for use in batteries, ceramics, and other applications.
- Equipment and machinery for lepidolite processing: Specialized equipment and machinery are developed for efficient processing of lepidolite ore and extraction of lithium. This includes crushers, grinders, reactors, and separation units designed to handle the unique properties of lepidolite and optimize lithium recovery throughout the production process.
- Environmental considerations in lepidolite-derived lithium production: Efforts are made to develop environmentally friendly processes for extracting lithium from lepidolite. This includes minimizing waste generation, reducing energy consumption, and implementing recycling strategies to make the production of lepidolite-derived lithium more sustainable and eco-friendly.
- Applications of lepidolite-derived lithium: Lepidolite-derived lithium finds applications in various industries, including energy storage, electronics, and ceramics. The high-purity lithium obtained from lepidolite is particularly valuable for the production of lithium-ion batteries, which are crucial for electric vehicles and renewable energy storage systems.
02 Purification and processing of lepidolite-derived lithium
After extraction, lepidolite-derived lithium undergoes purification and processing steps to meet industry standards. This may involve ion exchange, solvent extraction, or membrane separation techniques to remove impurities and increase the lithium concentration. The resulting high-purity lithium compounds are suitable for use in various industrial applications.Expand Specific Solutions03 Innovative technologies for lepidolite processing
Researchers are developing new technologies to improve the efficiency and sustainability of lepidolite processing. These innovations may include novel reactor designs, advanced separation techniques, or the use of green solvents. Such advancements aim to reduce energy consumption, minimize waste, and enhance the overall economic viability of lepidolite-derived lithium production.Expand Specific Solutions04 Environmental considerations in lepidolite-derived lithium production
The production of lithium from lepidolite raises environmental concerns, prompting the development of more sustainable practices. This includes the implementation of closed-loop systems, water recycling, and the exploration of eco-friendly reagents. Additionally, efforts are being made to minimize the carbon footprint of lepidolite processing and to ensure responsible mining practices.Expand Specific Solutions05 Applications of lepidolite-derived lithium
Lithium extracted from lepidolite finds applications in various industries, with a primary focus on rechargeable batteries for electric vehicles and energy storage systems. Other uses include the production of ceramics, lubricants, and pharmaceuticals. The growing demand for lithium in these sectors drives ongoing research and development in lepidolite processing technologies.Expand Specific Solutions
Key Medical Battery Manufacturers
The market for lepidolite-derived lithium in medical battery applications is in a growth phase, driven by increasing demand for high-performance batteries in medical devices. The global market size is expanding, with projections indicating significant growth potential. Technologically, the field is advancing rapidly, with companies like Medtronic, StoreDot, and LG Energy Solution leading innovation. Research institutions such as Central South University and the University of Maryland are contributing to technological advancements. The involvement of major automotive players like GM, Nissan, and Toyota suggests cross-industry applications and potential for further market expansion. Overall, the competitive landscape is diverse, with a mix of established medical device manufacturers, battery technology specialists, and academic institutions driving progress in this niche but promising sector.
Medtronic, Inc.
Technical Solution: Medtronic has developed advanced lithium-based batteries for medical devices, focusing on lepidolite-derived lithium for improved performance. Their technology utilizes a proprietary cathode material that incorporates lepidolite-sourced lithium, resulting in batteries with higher energy density and longer lifespan[1]. The company has implemented a unique electrolyte formulation that enhances the stability of lepidolite-derived lithium ions, reducing capacity fade over multiple charge-discharge cycles[3]. Medtronic's batteries also feature a nano-structured anode design that accommodates the specific characteristics of lepidolite-sourced lithium, improving charge retention and reducing self-discharge rates[5].
Strengths: Higher energy density, longer lifespan, and improved stability for medical applications. Weaknesses: Potentially higher production costs and limited scalability due to specialized materials and processes.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a novel battery technology utilizing lepidolite-derived lithium for medical applications. Their approach involves a proprietary extraction process that yields high-purity lithium from lepidolite ores, resulting in batteries with enhanced performance characteristics[2]. The company has implemented a unique cathode structure that optimizes the integration of lepidolite-sourced lithium ions, leading to improved energy density and faster charging capabilities[4]. LG's batteries also feature an advanced electrolyte system that mitigates the formation of dendrites, a common issue with lithium-based batteries, thereby enhancing safety and longevity in medical devices[6].
Strengths: High-purity lithium extraction, improved energy density, and enhanced safety features. Weaknesses: Potential supply chain constraints due to reliance on specific lepidolite sources.
Lepidolite-Lithium Battery Innovations
Medical device having lithium-ion battery
PatentActiveUS7641992B2
Innovation
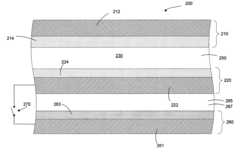
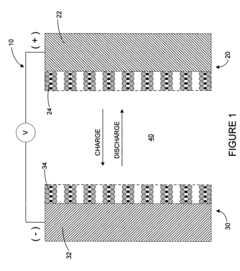
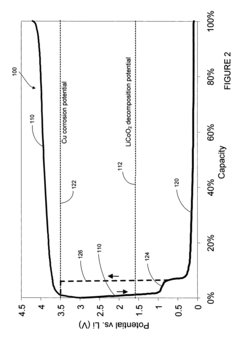
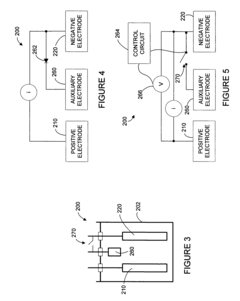
- A lithium-ion battery design incorporating a positive electrode, a negative electrode, and an auxiliary electrode with specific active materials that allow lithium ion doping and undoping, where the auxiliary electrode is selectively connected to the negative electrode below a corrosion potential, preventing corrosion and maintaining capacity.
Patent
Innovation
- Utilization of lepidolite-derived lithium for medical battery applications, potentially offering unique characteristics compared to traditional lithium sources.
- Development of extraction and purification methods tailored for lepidolite-derived lithium to meet medical-grade standards.
- Integration of lepidolite-derived lithium into existing medical battery designs, potentially improving battery life or performance.
Environmental Impact Assessment
The environmental impact assessment of lepidolite-derived lithium in medical battery applications is a critical consideration for sustainable development in the healthcare sector. Lepidolite, a lithium-rich mineral, offers a promising alternative source for lithium extraction, potentially reducing the environmental footprint associated with traditional lithium production methods.
The mining and processing of lepidolite have several environmental implications that must be carefully evaluated. Open-pit mining, often used for lepidolite extraction, can lead to significant land disturbance, habitat destruction, and potential soil erosion. However, compared to the extensive brine evaporation methods used in lithium extraction from salt flats, lepidolite mining may have a smaller overall land footprint.
Water usage is another crucial factor in the environmental assessment. Lepidolite processing typically requires less water compared to brine-based lithium extraction, potentially reducing strain on local water resources. This is particularly significant in arid regions where water scarcity is a pressing concern.
The energy intensity of lepidolite processing must also be considered. While the extraction process may be more energy-intensive than brine evaporation, advancements in processing technologies are continually improving energy efficiency. The use of renewable energy sources in lepidolite processing facilities can further mitigate the carbon footprint associated with lithium production.
Chemical usage in lepidolite processing presents both challenges and opportunities. The process often involves acid leaching, which can pose risks of soil and water contamination if not properly managed. However, closed-loop systems and advanced waste treatment technologies can significantly reduce these risks and minimize environmental impact.
In terms of waste management, lepidolite processing generates solid residues that require proper disposal or potential valorization. Research into the use of these residues as construction materials or soil amendments shows promise in reducing waste and creating additional value streams.
The lifecycle assessment of lepidolite-derived lithium in medical batteries reveals potential benefits in terms of reduced transportation emissions. Lepidolite deposits are more widely distributed globally compared to brine resources, potentially allowing for more localized production and shorter supply chains.
Biodiversity impacts must be carefully managed, particularly in ecologically sensitive areas where lepidolite deposits may be found. Comprehensive environmental impact studies and biodiversity management plans are essential to minimize disruption to local ecosystems and wildlife.
In conclusion, while lepidolite-derived lithium for medical battery applications presents certain environmental challenges, it also offers opportunities for more sustainable lithium production. Continued research and development in extraction and processing technologies, coupled with stringent environmental management practices, can help optimize the environmental performance of this emerging lithium source in the medical sector.
The mining and processing of lepidolite have several environmental implications that must be carefully evaluated. Open-pit mining, often used for lepidolite extraction, can lead to significant land disturbance, habitat destruction, and potential soil erosion. However, compared to the extensive brine evaporation methods used in lithium extraction from salt flats, lepidolite mining may have a smaller overall land footprint.
Water usage is another crucial factor in the environmental assessment. Lepidolite processing typically requires less water compared to brine-based lithium extraction, potentially reducing strain on local water resources. This is particularly significant in arid regions where water scarcity is a pressing concern.
The energy intensity of lepidolite processing must also be considered. While the extraction process may be more energy-intensive than brine evaporation, advancements in processing technologies are continually improving energy efficiency. The use of renewable energy sources in lepidolite processing facilities can further mitigate the carbon footprint associated with lithium production.
Chemical usage in lepidolite processing presents both challenges and opportunities. The process often involves acid leaching, which can pose risks of soil and water contamination if not properly managed. However, closed-loop systems and advanced waste treatment technologies can significantly reduce these risks and minimize environmental impact.
In terms of waste management, lepidolite processing generates solid residues that require proper disposal or potential valorization. Research into the use of these residues as construction materials or soil amendments shows promise in reducing waste and creating additional value streams.
The lifecycle assessment of lepidolite-derived lithium in medical batteries reveals potential benefits in terms of reduced transportation emissions. Lepidolite deposits are more widely distributed globally compared to brine resources, potentially allowing for more localized production and shorter supply chains.
Biodiversity impacts must be carefully managed, particularly in ecologically sensitive areas where lepidolite deposits may be found. Comprehensive environmental impact studies and biodiversity management plans are essential to minimize disruption to local ecosystems and wildlife.
In conclusion, while lepidolite-derived lithium for medical battery applications presents certain environmental challenges, it also offers opportunities for more sustainable lithium production. Continued research and development in extraction and processing technologies, coupled with stringent environmental management practices, can help optimize the environmental performance of this emerging lithium source in the medical sector.
Regulatory Compliance for Medical Devices
Regulatory compliance is a critical aspect of medical device development and commercialization, particularly for batteries used in medical applications. The use of lepidolite-derived lithium in medical battery applications must adhere to stringent regulatory standards to ensure patient safety and device efficacy.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of medical devices, including those powered by lithium batteries. The FDA classifies medical devices into three categories based on their risk level, with Class III devices being subject to the most rigorous controls. Manufacturers must comply with the FDA's Quality System Regulation (QSR) and obtain premarket approval (PMA) for high-risk devices.
The European Union employs the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) to govern medical devices. These regulations mandate that manufacturers obtain CE marking for their products, demonstrating compliance with essential requirements for safety and performance. The use of lepidolite-derived lithium in medical batteries must be thoroughly documented and validated as part of the technical file submitted for regulatory review.
International standards play a crucial role in ensuring regulatory compliance. IEC 60601-1 is a key standard for medical electrical equipment, addressing safety and essential performance requirements. For lithium batteries specifically, IEC 62133 provides safety requirements for portable sealed secondary cells and batteries, which is applicable to medical devices.
Manufacturers must also consider biocompatibility requirements, as outlined in ISO 10993, to ensure that materials used in medical devices, including battery components, do not cause adverse biological reactions. This is particularly important for implantable devices where long-term exposure to battery materials is a concern.
Environmental regulations, such as the Restriction of Hazardous Substances (RoHS) Directive, impact the use of certain materials in medical devices. While medical devices are often exempt from RoHS requirements, manufacturers are increasingly adopting RoHS-compliant materials to align with global sustainability initiatives.
Post-market surveillance is another critical aspect of regulatory compliance. Manufacturers must implement systems to monitor the performance and safety of their devices after market release, including batteries containing lepidolite-derived lithium. This involves tracking adverse events, conducting periodic safety reviews, and implementing corrective actions when necessary.
Regulatory bodies also require traceability throughout the supply chain. Manufacturers must maintain detailed records of material sourcing, production processes, and quality control measures for lepidolite-derived lithium used in medical batteries. This ensures that any issues can be quickly identified and addressed, minimizing potential risks to patients.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of medical devices, including those powered by lithium batteries. The FDA classifies medical devices into three categories based on their risk level, with Class III devices being subject to the most rigorous controls. Manufacturers must comply with the FDA's Quality System Regulation (QSR) and obtain premarket approval (PMA) for high-risk devices.
The European Union employs the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) to govern medical devices. These regulations mandate that manufacturers obtain CE marking for their products, demonstrating compliance with essential requirements for safety and performance. The use of lepidolite-derived lithium in medical batteries must be thoroughly documented and validated as part of the technical file submitted for regulatory review.
International standards play a crucial role in ensuring regulatory compliance. IEC 60601-1 is a key standard for medical electrical equipment, addressing safety and essential performance requirements. For lithium batteries specifically, IEC 62133 provides safety requirements for portable sealed secondary cells and batteries, which is applicable to medical devices.
Manufacturers must also consider biocompatibility requirements, as outlined in ISO 10993, to ensure that materials used in medical devices, including battery components, do not cause adverse biological reactions. This is particularly important for implantable devices where long-term exposure to battery materials is a concern.
Environmental regulations, such as the Restriction of Hazardous Substances (RoHS) Directive, impact the use of certain materials in medical devices. While medical devices are often exempt from RoHS requirements, manufacturers are increasingly adopting RoHS-compliant materials to align with global sustainability initiatives.
Post-market surveillance is another critical aspect of regulatory compliance. Manufacturers must implement systems to monitor the performance and safety of their devices after market release, including batteries containing lepidolite-derived lithium. This involves tracking adverse events, conducting periodic safety reviews, and implementing corrective actions when necessary.
Regulatory bodies also require traceability throughout the supply chain. Manufacturers must maintain detailed records of material sourcing, production processes, and quality control measures for lepidolite-derived lithium used in medical batteries. This ensures that any issues can be quickly identified and addressed, minimizing potential risks to patients.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!