Lepidolite in biomedical applications for controlled drug delivery
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lepidolite in Biomedicine: Background and Objectives
Lepidolite, a lithium-rich mica mineral, has recently garnered significant attention in the biomedical field, particularly for its potential applications in controlled drug delivery systems. This emerging area of research represents a convergence of materials science, pharmacology, and nanotechnology, aiming to revolutionize therapeutic approaches and enhance patient outcomes.
The journey of lepidolite in biomedicine began with the recognition of its unique physicochemical properties. Its layered structure, combined with the presence of lithium ions, offers intriguing possibilities for drug encapsulation and controlled release. As researchers delved deeper into its characteristics, the potential for lepidolite to address longstanding challenges in drug delivery became increasingly apparent.
The primary objective of this research is to explore and develop innovative drug delivery systems utilizing lepidolite as a key component. These systems aim to overcome traditional limitations in pharmacokinetics, such as poor bioavailability, rapid drug metabolism, and undesired side effects. By leveraging lepidolite's properties, researchers seek to create more efficient, targeted, and sustained drug release mechanisms.
One of the critical goals is to enhance the therapeutic index of various drugs by improving their solubility, stability, and bioavailability. Lepidolite-based delivery systems have shown promise in protecting sensitive drug molecules from degradation in harsh biological environments, potentially expanding the range of administrable drugs and their efficacy.
Another significant objective is to achieve precise control over drug release kinetics. This involves developing lepidolite-based formulations that can respond to specific physiological triggers or external stimuli, allowing for tailored drug release profiles. Such advancements could lead to personalized treatment regimens and improved patient compliance.
The research also aims to explore lepidolite's potential in targeted drug delivery. By modifying the surface of lepidolite-based nanocarriers, researchers hope to achieve site-specific drug delivery, minimizing systemic exposure and associated side effects while maximizing therapeutic efficacy at the target site.
Furthermore, the investigation into lepidolite's biocompatibility and biodegradability forms a crucial part of this research. Ensuring the safety and efficacy of lepidolite-based drug delivery systems is paramount for their eventual clinical translation and regulatory approval.
As this field evolves, researchers are also focusing on scalable and cost-effective production methods for lepidolite-based drug delivery systems. This objective is essential for bridging the gap between laboratory discoveries and practical, widely accessible therapeutic applications.
The journey of lepidolite in biomedicine began with the recognition of its unique physicochemical properties. Its layered structure, combined with the presence of lithium ions, offers intriguing possibilities for drug encapsulation and controlled release. As researchers delved deeper into its characteristics, the potential for lepidolite to address longstanding challenges in drug delivery became increasingly apparent.
The primary objective of this research is to explore and develop innovative drug delivery systems utilizing lepidolite as a key component. These systems aim to overcome traditional limitations in pharmacokinetics, such as poor bioavailability, rapid drug metabolism, and undesired side effects. By leveraging lepidolite's properties, researchers seek to create more efficient, targeted, and sustained drug release mechanisms.
One of the critical goals is to enhance the therapeutic index of various drugs by improving their solubility, stability, and bioavailability. Lepidolite-based delivery systems have shown promise in protecting sensitive drug molecules from degradation in harsh biological environments, potentially expanding the range of administrable drugs and their efficacy.
Another significant objective is to achieve precise control over drug release kinetics. This involves developing lepidolite-based formulations that can respond to specific physiological triggers or external stimuli, allowing for tailored drug release profiles. Such advancements could lead to personalized treatment regimens and improved patient compliance.
The research also aims to explore lepidolite's potential in targeted drug delivery. By modifying the surface of lepidolite-based nanocarriers, researchers hope to achieve site-specific drug delivery, minimizing systemic exposure and associated side effects while maximizing therapeutic efficacy at the target site.
Furthermore, the investigation into lepidolite's biocompatibility and biodegradability forms a crucial part of this research. Ensuring the safety and efficacy of lepidolite-based drug delivery systems is paramount for their eventual clinical translation and regulatory approval.
As this field evolves, researchers are also focusing on scalable and cost-effective production methods for lepidolite-based drug delivery systems. This objective is essential for bridging the gap between laboratory discoveries and practical, widely accessible therapeutic applications.
Market Analysis for Controlled Drug Delivery Systems
The controlled drug delivery systems market has been experiencing significant growth due to the increasing prevalence of chronic diseases, the rising demand for targeted drug delivery, and advancements in nanotechnology. This market segment is expected to continue its upward trajectory, driven by the need for more efficient and patient-friendly drug administration methods.
The global controlled drug delivery systems market is segmented based on technology, route of administration, and therapeutic areas. Key technologies include liposomes, microspheres, nanoparticles, and implants. Among these, nanoparticle-based systems have gained considerable traction due to their ability to enhance drug bioavailability and target specific tissues.
Geographically, North America dominates the market, followed by Europe and Asia-Pacific. The United States, in particular, holds a significant market share due to its advanced healthcare infrastructure and high R&D investments. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness rapid growth in the coming years, driven by improving healthcare access and increasing pharmaceutical research activities.
The therapeutic areas with the highest demand for controlled drug delivery systems include oncology, cardiovascular diseases, diabetes, and central nervous system disorders. Oncology, in particular, has seen a surge in research and development of targeted drug delivery systems to minimize side effects and improve treatment efficacy.
Key market players in the controlled drug delivery systems sector include Johnson & Johnson, Pfizer, Novartis, and Merck & Co. These companies are investing heavily in research and development to innovate new drug delivery technologies and maintain their competitive edge.
The market is also witnessing a trend towards personalized medicine, with an increasing focus on developing drug delivery systems that can be tailored to individual patient needs. This trend is expected to drive further innovation and market growth in the coming years.
Despite the positive outlook, the market faces challenges such as high development costs, stringent regulatory requirements, and the complexity of scaling up production for novel drug delivery systems. These factors can potentially hinder market growth and pose barriers to entry for new players.
In conclusion, the controlled drug delivery systems market presents significant opportunities for growth and innovation. The integration of new materials, such as lepidolite, into biomedical applications for controlled drug delivery could potentially address some of the existing challenges and open up new avenues for market expansion.
The global controlled drug delivery systems market is segmented based on technology, route of administration, and therapeutic areas. Key technologies include liposomes, microspheres, nanoparticles, and implants. Among these, nanoparticle-based systems have gained considerable traction due to their ability to enhance drug bioavailability and target specific tissues.
Geographically, North America dominates the market, followed by Europe and Asia-Pacific. The United States, in particular, holds a significant market share due to its advanced healthcare infrastructure and high R&D investments. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness rapid growth in the coming years, driven by improving healthcare access and increasing pharmaceutical research activities.
The therapeutic areas with the highest demand for controlled drug delivery systems include oncology, cardiovascular diseases, diabetes, and central nervous system disorders. Oncology, in particular, has seen a surge in research and development of targeted drug delivery systems to minimize side effects and improve treatment efficacy.
Key market players in the controlled drug delivery systems sector include Johnson & Johnson, Pfizer, Novartis, and Merck & Co. These companies are investing heavily in research and development to innovate new drug delivery technologies and maintain their competitive edge.
The market is also witnessing a trend towards personalized medicine, with an increasing focus on developing drug delivery systems that can be tailored to individual patient needs. This trend is expected to drive further innovation and market growth in the coming years.
Despite the positive outlook, the market faces challenges such as high development costs, stringent regulatory requirements, and the complexity of scaling up production for novel drug delivery systems. These factors can potentially hinder market growth and pose barriers to entry for new players.
In conclusion, the controlled drug delivery systems market presents significant opportunities for growth and innovation. The integration of new materials, such as lepidolite, into biomedical applications for controlled drug delivery could potentially address some of the existing challenges and open up new avenues for market expansion.
Current Challenges in Lepidolite-Based Drug Delivery
Despite the promising potential of lepidolite in controlled drug delivery systems, several significant challenges currently hinder its widespread adoption and efficacy in biomedical applications. One of the primary obstacles is the complex and variable composition of lepidolite, which can lead to inconsistencies in drug loading and release profiles. The mineral's natural variability in elemental composition and crystal structure makes it difficult to achieve standardized and reproducible results across different batches.
Another major challenge lies in the optimization of lepidolite's surface properties for enhanced drug adsorption and controlled release. The interaction between lepidolite and various drug molecules is not yet fully understood, limiting the ability to fine-tune the release kinetics for specific therapeutic needs. This lack of precise control over drug-mineral interactions can result in suboptimal drug delivery performance and potentially compromise treatment efficacy.
The biocompatibility and long-term safety of lepidolite-based drug delivery systems remain areas of concern. While initial studies have shown promising results, comprehensive in vivo studies are still needed to fully assess the potential toxicity and immunogenicity of lepidolite particles in the human body. The fate and biodegradation of lepidolite after drug release also require further investigation to ensure its safe elimination from the body.
Scale-up and manufacturing challenges present significant hurdles in the commercialization of lepidolite-based drug delivery systems. Current production methods often result in heterogeneous particle sizes and morphologies, which can affect the drug loading capacity and release profiles. Developing cost-effective and scalable production techniques that maintain consistent quality and performance is crucial for the successful translation of this technology from laboratory to clinical applications.
The regulatory landscape for mineral-based drug delivery systems, including those utilizing lepidolite, is still evolving. The lack of established guidelines and standards specific to these novel materials poses challenges in obtaining regulatory approvals and conducting clinical trials. This regulatory uncertainty can slow down the development and commercialization process for lepidolite-based drug delivery products.
Lastly, there is a need for more advanced characterization techniques to fully understand the behavior of lepidolite in biological systems. Current analytical methods may not provide sufficient resolution to elucidate the complex interactions between lepidolite, drug molecules, and biological tissues. Developing new imaging and analytical tools tailored for mineral-based drug delivery systems could significantly advance the field and address many of the current challenges.
Another major challenge lies in the optimization of lepidolite's surface properties for enhanced drug adsorption and controlled release. The interaction between lepidolite and various drug molecules is not yet fully understood, limiting the ability to fine-tune the release kinetics for specific therapeutic needs. This lack of precise control over drug-mineral interactions can result in suboptimal drug delivery performance and potentially compromise treatment efficacy.
The biocompatibility and long-term safety of lepidolite-based drug delivery systems remain areas of concern. While initial studies have shown promising results, comprehensive in vivo studies are still needed to fully assess the potential toxicity and immunogenicity of lepidolite particles in the human body. The fate and biodegradation of lepidolite after drug release also require further investigation to ensure its safe elimination from the body.
Scale-up and manufacturing challenges present significant hurdles in the commercialization of lepidolite-based drug delivery systems. Current production methods often result in heterogeneous particle sizes and morphologies, which can affect the drug loading capacity and release profiles. Developing cost-effective and scalable production techniques that maintain consistent quality and performance is crucial for the successful translation of this technology from laboratory to clinical applications.
The regulatory landscape for mineral-based drug delivery systems, including those utilizing lepidolite, is still evolving. The lack of established guidelines and standards specific to these novel materials poses challenges in obtaining regulatory approvals and conducting clinical trials. This regulatory uncertainty can slow down the development and commercialization process for lepidolite-based drug delivery products.
Lastly, there is a need for more advanced characterization techniques to fully understand the behavior of lepidolite in biological systems. Current analytical methods may not provide sufficient resolution to elucidate the complex interactions between lepidolite, drug molecules, and biological tissues. Developing new imaging and analytical tools tailored for mineral-based drug delivery systems could significantly advance the field and address many of the current challenges.
Existing Lepidolite Drug Delivery Mechanisms
01 Lepidolite-based drug delivery systems
Lepidolite, a lithium-rich mineral, is being explored for its potential in drug delivery systems. Its unique properties may allow for controlled release of medications, improving therapeutic efficacy and reducing side effects. Research is focused on developing novel formulations that incorporate lepidolite as a carrier or matrix for various drugs.- Lepidolite-based drug delivery systems: Lepidolite, a lithium-rich mineral, is being explored as a novel material for drug delivery systems. Its unique properties, including ion exchange capabilities and biocompatibility, make it a promising candidate for controlled release formulations. These systems can potentially enhance drug efficacy and reduce side effects by providing targeted and sustained delivery of therapeutic agents.
- Nanoparticle formulations incorporating lepidolite: Researchers are developing nanoparticle formulations that incorporate lepidolite for improved drug delivery. These nanoparticles can be engineered to encapsulate various drugs and provide controlled release profiles. The use of lepidolite in nanoparticle formulations may enhance drug stability, increase bioavailability, and allow for targeted delivery to specific tissues or organs.
- Transdermal drug delivery using lepidolite-based patches: Lepidolite is being investigated for use in transdermal drug delivery patches. The mineral's properties may allow for sustained release of drugs through the skin, providing an alternative to oral or injectable medications. This approach could be particularly beneficial for drugs that undergo significant first-pass metabolism or require consistent blood levels over extended periods.
- Lepidolite-based oral drug delivery systems: Oral drug delivery systems incorporating lepidolite are being developed to improve the bioavailability and efficacy of various medications. These systems may utilize lepidolite's ion exchange properties to control drug release in the gastrointestinal tract, potentially enhancing absorption and reducing dosing frequency. This approach could be particularly useful for drugs with poor solubility or stability in the digestive environment.
- Combination of lepidolite with other materials for enhanced drug delivery: Researchers are exploring combinations of lepidolite with other materials, such as polymers or bioactive compounds, to create advanced drug delivery systems. These hybrid materials may offer synergistic effects, improving drug loading capacity, release kinetics, and targeting capabilities. Such combinations could lead to more effective and versatile drug delivery platforms for a wide range of therapeutic applications.
02 Nanoparticle formulations with lepidolite
Researchers are investigating the use of lepidolite in nanoparticle formulations for drug delivery. These nanoparticles may enhance drug solubility, stability, and targeted delivery. The incorporation of lepidolite into nanostructures could potentially improve the bioavailability and efficacy of various therapeutic agents.Expand Specific Solutions03 Transdermal drug delivery using lepidolite
Lepidolite is being studied for its potential in transdermal drug delivery systems. Its properties may allow for enhanced skin penetration and controlled release of drugs through the skin. This approach could offer advantages for certain medications, providing a non-invasive alternative to traditional drug administration methods.Expand Specific Solutions04 Lepidolite-based implantable drug delivery devices
The development of implantable drug delivery devices incorporating lepidolite is an emerging area of research. These devices could provide long-term, controlled release of medications for chronic conditions. The unique properties of lepidolite may allow for the creation of biocompatible and effective implantable systems.Expand Specific Solutions05 Combination of lepidolite with other materials for drug delivery
Researchers are exploring the combination of lepidolite with other materials to create advanced drug delivery systems. These hybrid formulations may leverage the properties of multiple components to achieve improved drug release profiles, targeting capabilities, or biocompatibility. Such combinations could lead to more effective and versatile drug delivery platforms.Expand Specific Solutions
Key Players in Lepidolite Biomedical Research
The research on lepidolite in biomedical applications for controlled drug delivery is in an early developmental stage, with a growing market potential as the pharmaceutical industry seeks innovative drug delivery systems. The technology's maturity is still evolving, with several key players contributing to its advancement. Companies like PolyPid Ltd. and Intarcia Therapeutics, Inc. are pioneering controlled release technologies, while research institutions such as Massachusetts Institute of Technology and Fudan University are driving fundamental research. The involvement of established pharmaceutical companies like Merck Patent GmbH and Becton, Dickinson & Co. indicates growing industry interest, suggesting a competitive landscape poised for significant developments in the coming years.
PolyPid Ltd.
Technical Solution: PolyPid has developed a novel drug delivery platform called PLEX (Polymer-Lipid Encapsulation matriX) for controlled release of various therapeutic compounds. This technology encapsulates drugs within a proprietary matrix of lipids and polymers, allowing for customized release profiles over extended periods. For lepidolite-based drug delivery, PolyPid could potentially incorporate lepidolite nanoparticles into their PLEX system, leveraging the mineral's unique properties for sustained and targeted drug release. The company has demonstrated extended release periods of up to several months in some applications[1], which could be particularly beneficial for chronic conditions requiring long-term treatment.
Strengths: Highly customizable release profiles, extended drug delivery periods, and potential for local administration. Weaknesses: May require additional research to optimize lepidolite integration and ensure biocompatibility with the PLEX system.
Intarcia Therapeutics, Inc.
Technical Solution: Intarcia Therapeutics has developed the Medici Drug Delivery System, a subdermal osmotic pump technology for long-term drug delivery. This platform could be adapted to incorporate lepidolite-based formulations for controlled release applications. The Medici system consists of a small titanium device implanted under the skin, capable of delivering medication for up to one year[3]. By leveraging lepidolite's properties, Intarcia could potentially enhance the stability and release characteristics of drugs within their osmotic pump system. The company has focused on treatments for chronic diseases such as diabetes and obesity, areas where lepidolite-based drug delivery could offer new therapeutic approaches.
Strengths: Long-term drug delivery capability, minimally invasive administration. Weaknesses: Limited to subdermal application, may require significant research to integrate lepidolite effectively.
Innovative Lepidolite Formulations for Drug Release
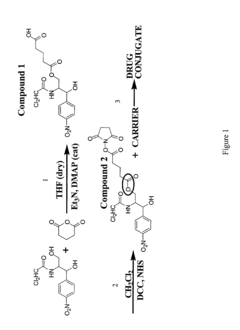
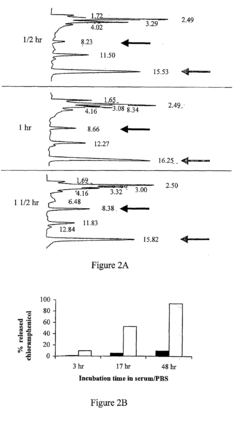
Targeted drug-carrying bacteriophages
PatentInactiveUS20080057038A1
Innovation
- Development of bacteriophage-drug conjugates where drugs are linked to the outer surface of bacteriophages, either directly or via linkers, with optional targeting moieties, allowing for high drug loading capacity and targeted delivery to specific cells, utilizing genetically modified bacteriophages or linkers for controlled release.
Targeted drug-carrying bacteriophages
PatentWO2006095345A2
Innovation
- Development of bacteriophage-drug conjugates where drugs are linked to the outer surface of bacteriophages, either directly or via linkers, with optional targeting moieties, allowing for high drug loading capacity and targeted delivery to specific cells, including those with broad host spectra and controlled drug release mechanisms.
Regulatory Framework for Novel Drug Delivery Systems
The regulatory framework for novel drug delivery systems involving lepidolite in biomedical applications is a complex and evolving landscape. As lepidolite-based controlled drug delivery systems represent an emerging technology, regulatory agencies worldwide are adapting their guidelines to address the unique characteristics and potential risks associated with these innovative approaches.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating novel drug delivery systems. The FDA's Center for Drug Evaluation and Research (CDER) is responsible for evaluating the safety and efficacy of new drug delivery technologies, including those incorporating lepidolite. The regulatory pathway for these systems typically falls under the New Drug Application (NDA) process, which requires extensive preclinical and clinical data to demonstrate safety, efficacy, and quality.
The European Medicines Agency (EMA) oversees the regulation of novel drug delivery systems in the European Union. The EMA has established specific guidelines for nanomedicine and advanced therapy medicinal products, which may be applicable to lepidolite-based drug delivery systems. These guidelines emphasize the importance of characterizing the physicochemical properties of the delivery system and assessing its potential impact on drug pharmacokinetics and biodistribution.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) is responsible for regulating novel drug delivery systems. The PMDA has implemented a regulatory framework that focuses on the evaluation of quality, safety, and efficacy of new drug delivery technologies, including those utilizing innovative materials like lepidolite.
Regulatory agencies worldwide are increasingly adopting a risk-based approach to the evaluation of novel drug delivery systems. This approach considers the unique characteristics of the delivery system, such as the use of lepidolite, and assesses potential risks associated with its application in controlled drug delivery. Key considerations include the biocompatibility of lepidolite, its potential for accumulation in tissues, and any long-term effects on human health.
International harmonization efforts, such as those led by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), are working to establish consistent regulatory standards for novel drug delivery systems across different regions. These efforts aim to streamline the development and approval processes for innovative technologies while ensuring patient safety and product efficacy.
As research on lepidolite in biomedical applications for controlled drug delivery progresses, regulatory agencies are likely to refine their guidelines and requirements. Developers of lepidolite-based drug delivery systems should engage in early and frequent communication with regulatory authorities to navigate the evolving regulatory landscape and ensure compliance with current and emerging standards.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating novel drug delivery systems. The FDA's Center for Drug Evaluation and Research (CDER) is responsible for evaluating the safety and efficacy of new drug delivery technologies, including those incorporating lepidolite. The regulatory pathway for these systems typically falls under the New Drug Application (NDA) process, which requires extensive preclinical and clinical data to demonstrate safety, efficacy, and quality.
The European Medicines Agency (EMA) oversees the regulation of novel drug delivery systems in the European Union. The EMA has established specific guidelines for nanomedicine and advanced therapy medicinal products, which may be applicable to lepidolite-based drug delivery systems. These guidelines emphasize the importance of characterizing the physicochemical properties of the delivery system and assessing its potential impact on drug pharmacokinetics and biodistribution.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) is responsible for regulating novel drug delivery systems. The PMDA has implemented a regulatory framework that focuses on the evaluation of quality, safety, and efficacy of new drug delivery technologies, including those utilizing innovative materials like lepidolite.
Regulatory agencies worldwide are increasingly adopting a risk-based approach to the evaluation of novel drug delivery systems. This approach considers the unique characteristics of the delivery system, such as the use of lepidolite, and assesses potential risks associated with its application in controlled drug delivery. Key considerations include the biocompatibility of lepidolite, its potential for accumulation in tissues, and any long-term effects on human health.
International harmonization efforts, such as those led by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), are working to establish consistent regulatory standards for novel drug delivery systems across different regions. These efforts aim to streamline the development and approval processes for innovative technologies while ensuring patient safety and product efficacy.
As research on lepidolite in biomedical applications for controlled drug delivery progresses, regulatory agencies are likely to refine their guidelines and requirements. Developers of lepidolite-based drug delivery systems should engage in early and frequent communication with regulatory authorities to navigate the evolving regulatory landscape and ensure compliance with current and emerging standards.
Biocompatibility and Safety Assessments of Lepidolite
Lepidolite, a lithium-rich mineral, has garnered significant attention in biomedical applications, particularly for controlled drug delivery systems. The biocompatibility and safety assessments of lepidolite are crucial aspects that require thorough investigation before its widespread adoption in medical applications.
Initial studies on the biocompatibility of lepidolite have shown promising results. In vitro experiments using various cell lines have demonstrated that lepidolite nanoparticles exhibit low cytotoxicity at concentrations relevant for drug delivery applications. These findings suggest that lepidolite-based drug carriers may be well-tolerated by living cells, a fundamental requirement for any biomaterial.
Further research has focused on the potential inflammatory responses triggered by lepidolite particles. Preliminary data indicate that lepidolite does not induce significant pro-inflammatory cytokine production in immune cells, suggesting a favorable immunological profile. However, more comprehensive studies are needed to fully characterize the long-term immune responses to lepidolite-based drug delivery systems.
The biodegradability of lepidolite in physiological conditions is another critical factor under investigation. Initial findings suggest that lepidolite undergoes slow degradation in simulated body fluids, which could be advantageous for sustained drug release applications. However, the fate of degradation products and their potential impact on organ systems require further elucidation.
Safety assessments of lepidolite have also examined its potential for accumulation in various tissues. Preliminary in vivo studies in animal models have shown that lepidolite nanoparticles are primarily cleared through hepatic and renal pathways, with no significant accumulation observed in major organs. These results are encouraging, but long-term studies are necessary to confirm the absence of chronic toxicity or unexpected bioaccumulation.
The potential for lepidolite to interact with biological membranes and cellular components is another area of active research. Studies have shown that lepidolite nanoparticles can be internalized by cells through various endocytic pathways, but the extent and consequences of these interactions need further investigation to ensure that they do not disrupt normal cellular functions.
Genotoxicity assessments of lepidolite have yielded mixed results. While some studies report no significant DNA damage in standard genotoxicity assays, others have observed minor effects at higher concentrations. These conflicting findings underscore the need for more rigorous and standardized testing protocols to conclusively determine the genotoxic potential of lepidolite-based materials.
As research progresses, it is crucial to establish standardized protocols for the synthesis and characterization of lepidolite nanoparticles to ensure reproducibility and reliability in biocompatibility and safety assessments. Additionally, regulatory guidelines specific to lepidolite-based biomaterials may need to be developed to facilitate their translation from bench to bedside.
Initial studies on the biocompatibility of lepidolite have shown promising results. In vitro experiments using various cell lines have demonstrated that lepidolite nanoparticles exhibit low cytotoxicity at concentrations relevant for drug delivery applications. These findings suggest that lepidolite-based drug carriers may be well-tolerated by living cells, a fundamental requirement for any biomaterial.
Further research has focused on the potential inflammatory responses triggered by lepidolite particles. Preliminary data indicate that lepidolite does not induce significant pro-inflammatory cytokine production in immune cells, suggesting a favorable immunological profile. However, more comprehensive studies are needed to fully characterize the long-term immune responses to lepidolite-based drug delivery systems.
The biodegradability of lepidolite in physiological conditions is another critical factor under investigation. Initial findings suggest that lepidolite undergoes slow degradation in simulated body fluids, which could be advantageous for sustained drug release applications. However, the fate of degradation products and their potential impact on organ systems require further elucidation.
Safety assessments of lepidolite have also examined its potential for accumulation in various tissues. Preliminary in vivo studies in animal models have shown that lepidolite nanoparticles are primarily cleared through hepatic and renal pathways, with no significant accumulation observed in major organs. These results are encouraging, but long-term studies are necessary to confirm the absence of chronic toxicity or unexpected bioaccumulation.
The potential for lepidolite to interact with biological membranes and cellular components is another area of active research. Studies have shown that lepidolite nanoparticles can be internalized by cells through various endocytic pathways, but the extent and consequences of these interactions need further investigation to ensure that they do not disrupt normal cellular functions.
Genotoxicity assessments of lepidolite have yielded mixed results. While some studies report no significant DNA damage in standard genotoxicity assays, others have observed minor effects at higher concentrations. These conflicting findings underscore the need for more rigorous and standardized testing protocols to conclusively determine the genotoxic potential of lepidolite-based materials.
As research progresses, it is crucial to establish standardized protocols for the synthesis and characterization of lepidolite nanoparticles to ensure reproducibility and reliability in biocompatibility and safety assessments. Additionally, regulatory guidelines specific to lepidolite-based biomaterials may need to be developed to facilitate their translation from bench to bedside.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!