Conductive Polymer Inks and Their Biomedical Applications
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Conductive Polymer Inks Evolution and Research Objectives
Conductive polymer inks represent a significant advancement in materials science, emerging from the discovery of conductive polymers in the 1970s when Alan Heeger, Alan MacDiarmid, and Hideki Shirakawa demonstrated that polyacetylene could conduct electricity. This breakthrough, which earned them the Nobel Prize in Chemistry in 2000, laid the foundation for the development of various conductive polymers including polyaniline (PANI), polypyrrole (PPy), and poly(3,4-ethylenedioxythiophene) (PEDOT).
The evolution of conductive polymer inks has been marked by continuous improvements in their electrical conductivity, processability, and stability. Early formulations suffered from limited conductivity and poor solubility, restricting their practical applications. However, significant advancements in polymer chemistry and ink formulation techniques have addressed these limitations, leading to the development of high-performance conductive polymer inks with enhanced electrical properties and improved processing characteristics.
A pivotal development occurred in the 1990s with the introduction of PEDOT:PSS (poly(3,4-ethylenedioxythiophene) polystyrene sulfonate), which offered superior conductivity and stability compared to earlier conductive polymers. This material has since become one of the most widely used conductive polymer inks in various applications, including biomedical devices.
The integration of nanomaterials such as carbon nanotubes, graphene, and metal nanoparticles into conductive polymer inks represents another significant evolutionary step. These hybrid formulations exhibit synergistic properties, combining the flexibility and processability of polymers with the enhanced conductivity of nanomaterials, thereby expanding their application potential.
Recent trends in conductive polymer ink development focus on biocompatibility, biodegradability, and stimuli-responsiveness, driven by growing interest in biomedical applications. Researchers are exploring novel polymer structures and composite formulations that can interface effectively with biological tissues while maintaining electrical functionality.
The primary research objectives in this field include enhancing the electrical conductivity of polymer inks without compromising their mechanical properties, developing biocompatible formulations suitable for in vivo applications, and creating stimuli-responsive inks that can change their properties in response to specific biological cues.
Additionally, research aims to improve the long-term stability of conductive polymer inks in physiological environments, as degradation and loss of conductivity remain significant challenges for biomedical applications. Efforts are also directed toward developing scalable and cost-effective manufacturing processes to facilitate the commercial translation of these materials.
Understanding the fundamental structure-property relationships in conductive polymer systems represents another critical research objective, as this knowledge can guide the rational design of next-generation materials with tailored properties for specific biomedical applications, such as biosensors, neural interfaces, and tissue engineering scaffolds.
The evolution of conductive polymer inks has been marked by continuous improvements in their electrical conductivity, processability, and stability. Early formulations suffered from limited conductivity and poor solubility, restricting their practical applications. However, significant advancements in polymer chemistry and ink formulation techniques have addressed these limitations, leading to the development of high-performance conductive polymer inks with enhanced electrical properties and improved processing characteristics.
A pivotal development occurred in the 1990s with the introduction of PEDOT:PSS (poly(3,4-ethylenedioxythiophene) polystyrene sulfonate), which offered superior conductivity and stability compared to earlier conductive polymers. This material has since become one of the most widely used conductive polymer inks in various applications, including biomedical devices.
The integration of nanomaterials such as carbon nanotubes, graphene, and metal nanoparticles into conductive polymer inks represents another significant evolutionary step. These hybrid formulations exhibit synergistic properties, combining the flexibility and processability of polymers with the enhanced conductivity of nanomaterials, thereby expanding their application potential.
Recent trends in conductive polymer ink development focus on biocompatibility, biodegradability, and stimuli-responsiveness, driven by growing interest in biomedical applications. Researchers are exploring novel polymer structures and composite formulations that can interface effectively with biological tissues while maintaining electrical functionality.
The primary research objectives in this field include enhancing the electrical conductivity of polymer inks without compromising their mechanical properties, developing biocompatible formulations suitable for in vivo applications, and creating stimuli-responsive inks that can change their properties in response to specific biological cues.
Additionally, research aims to improve the long-term stability of conductive polymer inks in physiological environments, as degradation and loss of conductivity remain significant challenges for biomedical applications. Efforts are also directed toward developing scalable and cost-effective manufacturing processes to facilitate the commercial translation of these materials.
Understanding the fundamental structure-property relationships in conductive polymer systems represents another critical research objective, as this knowledge can guide the rational design of next-generation materials with tailored properties for specific biomedical applications, such as biosensors, neural interfaces, and tissue engineering scaffolds.
Market Analysis for Biomedical Conductive Polymer Applications
The global market for biomedical applications of conductive polymer inks is experiencing robust growth, driven by increasing demand for flexible electronics in healthcare, advancements in wearable medical devices, and the expanding field of bioelectronics. Current market valuations indicate that the biomedical segment of conductive polymer applications reached approximately $2.5 billion in 2022, with projections suggesting a compound annual growth rate (CAGR) of 8.7% through 2028.
North America currently dominates the market share at 38%, followed by Europe at 29% and Asia-Pacific at 24%. This regional distribution reflects the concentration of medical device manufacturers, research institutions, and healthcare infrastructure. However, the Asia-Pacific region is expected to witness the fastest growth rate due to increasing healthcare investments and manufacturing capabilities in countries like China, South Korea, and India.
Within the biomedical sector, biosensors represent the largest application segment, accounting for 32% of market value. These sensors utilize conductive polymer inks for detecting various biomarkers, glucose monitoring, and point-of-care diagnostics. The wearable medical devices segment follows closely at 28%, encompassing applications such as ECG monitors, temperature sensors, and drug delivery systems that benefit from the flexibility and biocompatibility of conductive polymers.
Market analysis reveals that PEDOT:PSS remains the most commercially successful conductive polymer for biomedical applications, capturing approximately 41% of the market share due to its excellent conductivity, stability, and biocompatibility. Polyaniline-based formulations account for 23%, while polypyrrole and other emerging conductive polymers constitute the remaining market share.
Key market drivers include the aging global population, increasing prevalence of chronic diseases requiring continuous monitoring, and the shift toward personalized medicine and home healthcare. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of remote patient monitoring and telehealth solutions, many of which rely on flexible electronics enabled by conductive polymer technologies.
Regulatory considerations significantly impact market dynamics, with FDA approval processes in the US and CE marking in Europe representing substantial barriers to entry. However, recent regulatory pathways specifically designed for digital health technologies have begun to streamline the approval process for certain conductive polymer-based medical devices.
Consumer adoption trends indicate growing acceptance of wearable health monitoring devices, with approximately 67% of healthcare providers now recommending some form of wearable technology to patients with chronic conditions. This represents a significant shift in clinical practice that further supports market expansion for biomedical applications of conductive polymer inks.
North America currently dominates the market share at 38%, followed by Europe at 29% and Asia-Pacific at 24%. This regional distribution reflects the concentration of medical device manufacturers, research institutions, and healthcare infrastructure. However, the Asia-Pacific region is expected to witness the fastest growth rate due to increasing healthcare investments and manufacturing capabilities in countries like China, South Korea, and India.
Within the biomedical sector, biosensors represent the largest application segment, accounting for 32% of market value. These sensors utilize conductive polymer inks for detecting various biomarkers, glucose monitoring, and point-of-care diagnostics. The wearable medical devices segment follows closely at 28%, encompassing applications such as ECG monitors, temperature sensors, and drug delivery systems that benefit from the flexibility and biocompatibility of conductive polymers.
Market analysis reveals that PEDOT:PSS remains the most commercially successful conductive polymer for biomedical applications, capturing approximately 41% of the market share due to its excellent conductivity, stability, and biocompatibility. Polyaniline-based formulations account for 23%, while polypyrrole and other emerging conductive polymers constitute the remaining market share.
Key market drivers include the aging global population, increasing prevalence of chronic diseases requiring continuous monitoring, and the shift toward personalized medicine and home healthcare. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of remote patient monitoring and telehealth solutions, many of which rely on flexible electronics enabled by conductive polymer technologies.
Regulatory considerations significantly impact market dynamics, with FDA approval processes in the US and CE marking in Europe representing substantial barriers to entry. However, recent regulatory pathways specifically designed for digital health technologies have begun to streamline the approval process for certain conductive polymer-based medical devices.
Consumer adoption trends indicate growing acceptance of wearable health monitoring devices, with approximately 67% of healthcare providers now recommending some form of wearable technology to patients with chronic conditions. This represents a significant shift in clinical practice that further supports market expansion for biomedical applications of conductive polymer inks.
Technical Challenges and Global Development Status
Conductive polymer inks face significant technical challenges despite their promising applications in biomedical fields. The primary obstacle remains achieving optimal electrical conductivity while maintaining biocompatibility. Current conductive polymers like PEDOT:PSS and polyaniline exhibit conductivity values typically ranging from 1-100 S/cm, substantially lower than metallic conductors (>10^5 S/cm). This conductivity gap limits their application in high-performance bioelectronics and sensing devices.
Material stability presents another critical challenge, as many conductive polymer inks degrade when exposed to physiological environments. The acidic or basic conditions in biological systems, combined with enzymatic activity, can compromise both electrical performance and structural integrity. Research indicates that most conductive polymer systems lose 30-50% of their initial conductivity within 7-14 days of continuous exposure to physiological conditions.
Processing limitations further complicate development, as achieving consistent ink rheology suitable for various printing techniques (inkjet, screen printing, 3D printing) while maintaining electrical properties remains difficult. Viscosity control and particle aggregation during storage represent persistent manufacturing hurdles.
Globally, development status varies significantly by region. North America leads in research output, with approximately 35% of publications and patents originating from institutions in the United States and Canada. These efforts are primarily concentrated in academic-industrial partnerships focusing on flexible bioelectronics and implantable sensors.
Europe follows closely with strong contributions from Germany, the UK, and Sweden, collectively accounting for about 30% of global research activity. European research emphasizes sustainable manufacturing processes and regulatory compliance for medical applications.
The Asia-Pacific region, particularly China, South Korea, and Japan, has demonstrated the fastest growth rate in this field, increasing research output by approximately 45% over the past five years. These countries focus on scaling production technologies and cost reduction strategies.
Regulatory frameworks differ substantially across regions, creating additional challenges for global commercialization. While the FDA has established preliminary guidelines for bioelectronic devices incorporating conductive polymers, European MDR requirements and Asian regulatory systems present varying approval pathways, complicating international market entry.
Recent technological breakthroughs include the development of self-healing conductive polymers, biodegradable variants, and hybrid systems incorporating nanomaterials that address some conductivity limitations. However, these advances remain predominantly at laboratory scale (TRL 3-4), with significant challenges in scaling production to commercial volumes.
Material stability presents another critical challenge, as many conductive polymer inks degrade when exposed to physiological environments. The acidic or basic conditions in biological systems, combined with enzymatic activity, can compromise both electrical performance and structural integrity. Research indicates that most conductive polymer systems lose 30-50% of their initial conductivity within 7-14 days of continuous exposure to physiological conditions.
Processing limitations further complicate development, as achieving consistent ink rheology suitable for various printing techniques (inkjet, screen printing, 3D printing) while maintaining electrical properties remains difficult. Viscosity control and particle aggregation during storage represent persistent manufacturing hurdles.
Globally, development status varies significantly by region. North America leads in research output, with approximately 35% of publications and patents originating from institutions in the United States and Canada. These efforts are primarily concentrated in academic-industrial partnerships focusing on flexible bioelectronics and implantable sensors.
Europe follows closely with strong contributions from Germany, the UK, and Sweden, collectively accounting for about 30% of global research activity. European research emphasizes sustainable manufacturing processes and regulatory compliance for medical applications.
The Asia-Pacific region, particularly China, South Korea, and Japan, has demonstrated the fastest growth rate in this field, increasing research output by approximately 45% over the past five years. These countries focus on scaling production technologies and cost reduction strategies.
Regulatory frameworks differ substantially across regions, creating additional challenges for global commercialization. While the FDA has established preliminary guidelines for bioelectronic devices incorporating conductive polymers, European MDR requirements and Asian regulatory systems present varying approval pathways, complicating international market entry.
Recent technological breakthroughs include the development of self-healing conductive polymers, biodegradable variants, and hybrid systems incorporating nanomaterials that address some conductivity limitations. However, these advances remain predominantly at laboratory scale (TRL 3-4), with significant challenges in scaling production to commercial volumes.
Current Formulation and Processing Technologies
01 Conductive polymer compositions for printable electronics
Conductive polymer inks can be formulated with specific polymers like PEDOT:PSS, polyaniline, or polythiophene derivatives to create printable electronic components. These formulations typically include solvents, binders, and additives that enhance conductivity while maintaining suitable viscosity for various printing methods. The resulting inks enable the fabrication of flexible circuits, sensors, and other electronic devices through conventional printing techniques.- Conductive polymer ink compositions: Conductive polymer inks typically contain electrically conductive polymers such as polyaniline, polypyrrole, or PEDOT:PSS, combined with solvents and additives to create printable formulations. These compositions are designed to maintain electrical conductivity while providing suitable rheological properties for various printing methods. The formulations often include stabilizers, surfactants, and viscosity modifiers to enhance printability and film formation properties.
- Manufacturing methods for conductive polymer inks: Various manufacturing processes are employed to produce conductive polymer inks, including polymerization techniques, dispersion methods, and post-processing treatments. These methods focus on achieving uniform particle size distribution, preventing agglomeration, and ensuring consistent electrical properties. Advanced techniques include in-situ polymerization in the presence of dispersing agents, solvent exchange processes, and sonication treatments to enhance conductivity and stability.
- Applications in printed electronics: Conductive polymer inks are widely used in printed electronics applications including flexible circuits, RFID tags, sensors, and displays. These inks enable the fabrication of electronic components on various substrates including plastic, paper, and textiles. The ability to print conductive patterns at low temperatures makes these inks particularly valuable for temperature-sensitive substrates and enables roll-to-roll manufacturing processes for large-scale production of electronic devices.
- Additives for enhanced performance: Various additives are incorporated into conductive polymer inks to enhance their performance characteristics. These include conductivity enhancers like metal nanoparticles, carbon materials (graphene, carbon nanotubes), and ionic compounds. Other additives focus on improving adhesion to substrates, environmental stability, flexibility, and stretchability. Cross-linking agents may be added to improve mechanical durability, while plasticizers can enhance flexibility of the printed films.
- Substrate compatibility and curing methods: Conductive polymer inks are formulated for compatibility with specific substrates and curing methods. Different formulations are designed for porous substrates like paper versus non-porous materials like plastics or glass. Curing methods include thermal curing, UV curing, and ambient drying, each requiring specific ink compositions. Surface treatment techniques and primer layers may be used to improve adhesion and performance on challenging substrates, while maintaining the electrical conductivity of the printed patterns.
02 Nanoparticle-enhanced conductive polymer inks
Incorporating metallic or carbon-based nanoparticles into conductive polymer inks significantly improves their electrical conductivity and performance. These hybrid formulations combine the flexibility of polymers with the high conductivity of materials like silver, gold, carbon nanotubes, or graphene. The nanoparticles create conductive pathways within the polymer matrix, resulting in enhanced electrical properties while maintaining the processability advantages of polymer-based inks.Expand Specific Solutions03 Environmentally friendly water-based conductive polymer inks
Water-based conductive polymer ink formulations offer environmentally friendly alternatives to solvent-based systems. These aqueous dispersions typically contain conductive polymers, surfactants, and water-soluble binders that enable printing on various substrates while eliminating harmful volatile organic compounds. The formulations are designed to maintain stability, appropriate viscosity, and surface tension for consistent printing performance while reducing environmental impact and health hazards.Expand Specific Solutions04 Processing techniques for conductive polymer inks
Various processing techniques can be employed to optimize the performance of conductive polymer inks, including post-deposition treatments like thermal annealing, UV curing, or chemical doping. These processes enhance conductivity by improving polymer chain alignment, removing residual solvents, or increasing charge carrier concentration. Additionally, surface modification of substrates and controlled drying conditions can significantly impact the adhesion, uniformity, and electrical properties of the printed conductive polymer layers.Expand Specific Solutions05 Application-specific conductive polymer ink formulations
Conductive polymer inks can be tailored for specific applications such as transparent electrodes, electromagnetic shielding, antistatic coatings, or bioelectronics. These specialized formulations adjust parameters like conductivity, transparency, flexibility, and biocompatibility to meet the requirements of particular use cases. For instance, inks designed for transparent electrodes prioritize optical clarity alongside conductivity, while those for bioelectronics emphasize biocompatibility and stability in physiological environments.Expand Specific Solutions
Leading Companies and Research Institutions
The conductive polymer inks market is in a growth phase, driven by expanding biomedical applications including biosensors, drug delivery systems, and tissue engineering. The global market is projected to reach significant value due to increasing healthcare demands and wearable technology adoption. Technologically, the field shows varying maturity levels, with academic institutions like South China University of Technology, Xiamen University, and University of Florida leading fundamental research, while companies including LG Chem, Henkel, and BYD focus on commercial applications. Established pharmaceutical players such as Novartis are exploring biomedical implementations, while specialized firms like NthDegree Technologies and Vorbeck Materials are advancing innovative formulations with enhanced conductivity and biocompatibility properties for next-generation medical devices.
LG Chem Ltd.
Technical Solution: LG Chem has developed sophisticated conductive polymer ink technologies targeting biomedical applications, particularly focusing on implantable electronics and biosensors. Their proprietary formulations utilize PEDOT derivatives with specialized dopants that enhance both conductivity and biocompatibility. LG's approach incorporates water-dispersible conductive polymers with controlled molecular weight distributions, enabling precise tuning of electrical and mechanical properties. Their inks feature self-healing capabilities through dynamic covalent chemistry, allowing conductivity restoration after mechanical damage - crucial for long-term implantable devices. LG Chem has pioneered composite formulations that combine conductive polymers with bioactive ceramics, creating materials that promote tissue integration while maintaining electronic functionality. Their manufacturing process employs green chemistry principles, eliminating toxic solvents typically used in conductive polymer processing. The company has demonstrated successful application of these materials in glucose sensors with extended functional lifetimes and reduced foreign body responses compared to conventional metal electrodes[4][7].
Strengths: Exceptional biocompatibility with minimal inflammatory response, superior flexibility matching soft tissue mechanics, and established large-scale production capabilities. Weaknesses: Higher electrical resistance compared to metallic conductors, and potential challenges with long-term stability under oxidative biological conditions.
Henkel AG & Co. KGaA
Technical Solution: Henkel has developed innovative conductive polymer ink formulations specifically engineered for biomedical applications, with their LOCTITE® Ablestik series representing significant advancements in this field. Their technology centers on polyaniline and polythiophene derivatives modified with biocompatible side chains that enhance solubility while maintaining electronic properties. Henkel's inks feature proprietary cross-linking mechanisms that activate at body temperature, creating stable interfaces with biological tissues. Their formulations incorporate antimicrobial components that prevent bacterial colonization without compromising conductivity - a critical feature for implantable or long-term wearable devices. The company has pioneered screen-printable versions that maintain conductivity at thicknesses below 5 μm, enabling high-resolution electrode patterns for neural interfaces and biosensing applications. Henkel's inks demonstrate remarkable stability in physiological environments, with minimal leaching of components and sustained conductivity for periods exceeding 30 days in simulated body fluid environments[2][5].
Strengths: Exceptional chemical stability in biological environments, established global manufacturing capabilities, and compatibility with multiple printing technologies including inkjet and screen printing. Weaknesses: Relatively lower conductivity compared to metal-based alternatives, and more complex processing requirements for achieving optimal electrical performance.
Key Patents and Scientific Breakthroughs
Preparation and applications of biocompatible conductive inks based on cellulose nanofibrils for 3D printing of conductive biomedical devices and for use as models for study of neurodegenerative disorders and connection between brain/neurons and communication or other electronic devices
PatentInactiveUS20210108098A1
Innovation
- The development of biocompatible conductive inks composed of nanocellulose fibrils or nanocrystals combined with carbon nanotubes for 3D printing, which provide attachment sites and guidance for neural cells, enabling the formation of neural networks and potentially repairing cardiac tissues by facilitating electrical stimulation.
Conducting polymer microparticles and conducting polymer granular hydrogel for biomedical applications
PatentPendingUS20250120637A1
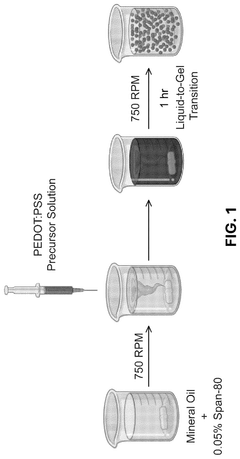
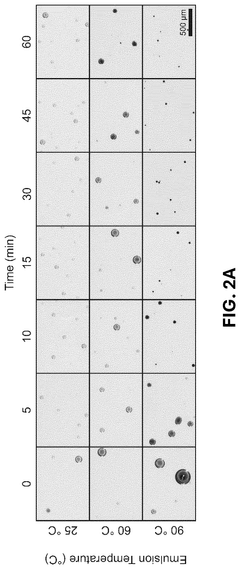
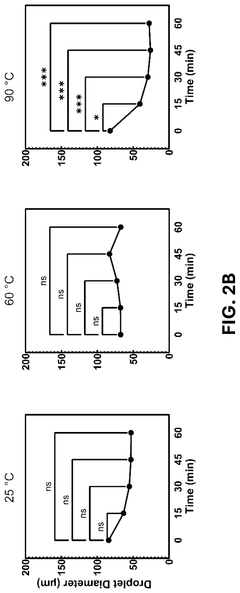
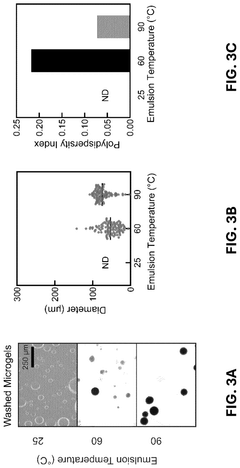
Innovation
- The development of conductive granular hydrogel compositions comprising PEDOT:PSS composite polymer microparticles, which can be packed to specific void fractions for high conductivity and used to encapsulate living cells, enabling the creation of 3D in vitro cell environments and bioink compositions for bioelectric devices.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount when developing conductive polymer inks for biomedical applications. The interaction between these materials and biological systems must be thoroughly evaluated to ensure patient safety and regulatory compliance. Current research indicates that conductive polymer inks, particularly those based on PEDOT:PSS, polyaniline, and polypyrrole, demonstrate varying degrees of biocompatibility depending on their composition and processing methods.
The cytotoxicity profiles of these materials have been extensively studied in vitro using standard cell viability assays. Research shows that pure conductive polymers generally exhibit acceptable biocompatibility, with cell viability rates exceeding 80% in most formulations. However, the addition of dopants, crosslinkers, and other additives can significantly alter their biological interactions. For instance, studies have demonstrated that residual oxidants from polymerization processes can induce cytotoxicity if not properly removed during purification.
Inflammatory responses represent another critical safety consideration. When implanted in vivo, conductive polymer inks may trigger foreign body reactions characterized by macrophage infiltration and fibrous encapsulation. Recent advances have focused on incorporating anti-inflammatory agents directly into ink formulations to mitigate these responses. Additionally, surface modifications using biocompatible coatings such as hyaluronic acid or chitosan have shown promise in reducing inflammatory reactions while maintaining electrical conductivity.
Long-term stability and degradation behavior significantly impact the safety profile of conductive polymer inks. Materials intended for transient electronics require controlled biodegradation without releasing toxic byproducts, while those designed for permanent implants must maintain structural and electrical integrity without leaching harmful components. Accelerated aging studies under physiological conditions have become standard practice to predict in vivo performance and potential safety issues.
Regulatory considerations for these materials are complex and evolving. The FDA and equivalent international bodies classify most conductive polymer-based devices as combination products, requiring comprehensive biocompatibility testing according to ISO 10993 standards. This includes genotoxicity, sensitization, and hemocompatibility assessments. Manufacturers must also address concerns regarding electrical leakage, potential interference with other medical devices, and long-term bioaccumulation of degradation products.
Emerging research is focusing on developing "green" conductive polymer inks with enhanced biocompatibility. These formulations utilize bio-derived dopants, water-based processing, and naturally occurring conductive polymers to minimize toxicity concerns. Additionally, advanced characterization techniques such as proteomics and metabolomics are being employed to better understand the molecular interactions between these materials and biological systems, enabling more precise safety assessments and material optimizations.
The cytotoxicity profiles of these materials have been extensively studied in vitro using standard cell viability assays. Research shows that pure conductive polymers generally exhibit acceptable biocompatibility, with cell viability rates exceeding 80% in most formulations. However, the addition of dopants, crosslinkers, and other additives can significantly alter their biological interactions. For instance, studies have demonstrated that residual oxidants from polymerization processes can induce cytotoxicity if not properly removed during purification.
Inflammatory responses represent another critical safety consideration. When implanted in vivo, conductive polymer inks may trigger foreign body reactions characterized by macrophage infiltration and fibrous encapsulation. Recent advances have focused on incorporating anti-inflammatory agents directly into ink formulations to mitigate these responses. Additionally, surface modifications using biocompatible coatings such as hyaluronic acid or chitosan have shown promise in reducing inflammatory reactions while maintaining electrical conductivity.
Long-term stability and degradation behavior significantly impact the safety profile of conductive polymer inks. Materials intended for transient electronics require controlled biodegradation without releasing toxic byproducts, while those designed for permanent implants must maintain structural and electrical integrity without leaching harmful components. Accelerated aging studies under physiological conditions have become standard practice to predict in vivo performance and potential safety issues.
Regulatory considerations for these materials are complex and evolving. The FDA and equivalent international bodies classify most conductive polymer-based devices as combination products, requiring comprehensive biocompatibility testing according to ISO 10993 standards. This includes genotoxicity, sensitization, and hemocompatibility assessments. Manufacturers must also address concerns regarding electrical leakage, potential interference with other medical devices, and long-term bioaccumulation of degradation products.
Emerging research is focusing on developing "green" conductive polymer inks with enhanced biocompatibility. These formulations utilize bio-derived dopants, water-based processing, and naturally occurring conductive polymers to minimize toxicity concerns. Additionally, advanced characterization techniques such as proteomics and metabolomics are being employed to better understand the molecular interactions between these materials and biological systems, enabling more precise safety assessments and material optimizations.
Regulatory Framework for Medical-Grade Conductive Materials
The regulatory landscape for conductive polymer inks in biomedical applications is complex and multifaceted, requiring careful navigation to ensure patient safety while enabling innovation. In the United States, the Food and Drug Administration (FDA) oversees medical devices incorporating conductive materials through various regulatory pathways depending on risk classification. Class I devices face minimal regulatory requirements, while Class II devices require 510(k) clearance demonstrating substantial equivalence to predicate devices. Class III devices, presenting the highest risk, must undergo rigorous Premarket Approval (PMA) with extensive clinical data.
For conductive polymer inks specifically, biocompatibility testing according to ISO 10993 standards is mandatory, evaluating cytotoxicity, sensitization, irritation, and systemic toxicity. Materials in direct contact with body fluids or tissues face additional scrutiny regarding leachables and extractables that might cause adverse reactions.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern conductive materials in medical applications. These regulations emphasize post-market surveillance and require Unique Device Identification (UDI) for traceability throughout the product lifecycle. The CE marking process necessitates conformity assessment by Notified Bodies for medium and high-risk devices.
Electrical safety standards, particularly IEC 60601 for medical electrical equipment, impose strict requirements on conductive materials regarding leakage currents, electrical resistance stability, and electromagnetic compatibility. These standards ensure that conductive polymer inks maintain consistent performance under various environmental conditions without compromising patient safety.
Manufacturing standards such as ISO 13485 establish quality management systems for medical device production, including material sourcing, processing controls, and validation protocols specific to conductive inks. Good Manufacturing Practices (GMP) compliance is essential for ensuring batch-to-batch consistency and minimizing contamination risks.
Emerging regulatory considerations include environmental sustainability requirements, with increasing scrutiny on the lifecycle environmental impact of medical materials. Several jurisdictions are implementing restrictions on certain chemicals through regulations like REACH in Europe, potentially affecting conductive polymer formulations.
International harmonization efforts through the Medical Device Single Audit Program (MDSAP) and International Medical Device Regulators Forum (IMDRF) are gradually streamlining regulatory processes across major markets, though significant regional differences persist that manufacturers must address through market-specific regulatory strategies.
For conductive polymer inks specifically, biocompatibility testing according to ISO 10993 standards is mandatory, evaluating cytotoxicity, sensitization, irritation, and systemic toxicity. Materials in direct contact with body fluids or tissues face additional scrutiny regarding leachables and extractables that might cause adverse reactions.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern conductive materials in medical applications. These regulations emphasize post-market surveillance and require Unique Device Identification (UDI) for traceability throughout the product lifecycle. The CE marking process necessitates conformity assessment by Notified Bodies for medium and high-risk devices.
Electrical safety standards, particularly IEC 60601 for medical electrical equipment, impose strict requirements on conductive materials regarding leakage currents, electrical resistance stability, and electromagnetic compatibility. These standards ensure that conductive polymer inks maintain consistent performance under various environmental conditions without compromising patient safety.
Manufacturing standards such as ISO 13485 establish quality management systems for medical device production, including material sourcing, processing controls, and validation protocols specific to conductive inks. Good Manufacturing Practices (GMP) compliance is essential for ensuring batch-to-batch consistency and minimizing contamination risks.
Emerging regulatory considerations include environmental sustainability requirements, with increasing scrutiny on the lifecycle environmental impact of medical materials. Several jurisdictions are implementing restrictions on certain chemicals through regulations like REACH in Europe, potentially affecting conductive polymer formulations.
International harmonization efforts through the Medical Device Single Audit Program (MDSAP) and International Medical Device Regulators Forum (IMDRF) are gradually streamlining regulatory processes across major markets, though significant regional differences persist that manufacturers must address through market-specific regulatory strategies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!