Conductive Polymer Inks in Pharmaceutical Packaging
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Conductive Polymer Inks Background and Objectives
Conductive polymer inks represent a significant technological advancement in the field of printed electronics, emerging from the convergence of materials science and electronic engineering. Since the discovery of conductive polymers in the 1970s, these materials have evolved from laboratory curiosities to commercially viable alternatives to traditional metallic conductors. The development trajectory has accelerated particularly in the last decade, with formulations achieving conductivity levels approaching those of conventional metals while maintaining the flexibility and processability inherent to polymers.
The pharmaceutical packaging sector has traditionally relied on conventional materials and technologies that primarily focus on product protection and shelf-life extension. However, the industry is now experiencing a paradigm shift toward smart packaging solutions that can monitor product conditions, authenticate medications, and enhance patient compliance. This evolution is driven by increasing regulatory requirements for drug safety, growing concerns about counterfeit pharmaceuticals, and the need for improved supply chain visibility.
Conductive polymer inks offer a promising technological platform to address these emerging needs in pharmaceutical packaging. Unlike traditional metallic inks, polymer-based formulations can be applied using standard printing techniques at lower temperatures, making them compatible with heat-sensitive pharmaceutical packaging materials. Their inherent flexibility allows application on curved or flexible packaging surfaces without compromising electrical performance.
The primary technical objectives for conductive polymer inks in pharmaceutical packaging applications include achieving stable electrical conductivity under varying environmental conditions, ensuring biocompatibility and non-toxicity for pharmaceutical contact, developing formulations with extended shelf life, and creating cost-effective manufacturing processes for large-scale implementation. Additionally, there is a critical need to develop inks that maintain functionality when exposed to sterilization processes commonly used in pharmaceutical packaging.
Current research is focused on several key areas: optimizing polymer structures to enhance conductivity while maintaining processability, developing environmentally friendly formulations that minimize the use of toxic solvents, creating hybrid systems that combine polymers with metallic nanoparticles for enhanced performance, and engineering ink compositions that adhere effectively to pharmaceutical packaging substrates including plastics, glass, and paperboard.
The technological trajectory suggests a move toward multi-functional conductive polymer systems that not only provide electrical conductivity but also incorporate sensing capabilities for temperature, humidity, and tampering detection. This evolution aligns with the pharmaceutical industry's increasing emphasis on product integrity verification and real-time monitoring throughout the supply chain, ultimately contributing to enhanced medication safety and efficacy.
The pharmaceutical packaging sector has traditionally relied on conventional materials and technologies that primarily focus on product protection and shelf-life extension. However, the industry is now experiencing a paradigm shift toward smart packaging solutions that can monitor product conditions, authenticate medications, and enhance patient compliance. This evolution is driven by increasing regulatory requirements for drug safety, growing concerns about counterfeit pharmaceuticals, and the need for improved supply chain visibility.
Conductive polymer inks offer a promising technological platform to address these emerging needs in pharmaceutical packaging. Unlike traditional metallic inks, polymer-based formulations can be applied using standard printing techniques at lower temperatures, making them compatible with heat-sensitive pharmaceutical packaging materials. Their inherent flexibility allows application on curved or flexible packaging surfaces without compromising electrical performance.
The primary technical objectives for conductive polymer inks in pharmaceutical packaging applications include achieving stable electrical conductivity under varying environmental conditions, ensuring biocompatibility and non-toxicity for pharmaceutical contact, developing formulations with extended shelf life, and creating cost-effective manufacturing processes for large-scale implementation. Additionally, there is a critical need to develop inks that maintain functionality when exposed to sterilization processes commonly used in pharmaceutical packaging.
Current research is focused on several key areas: optimizing polymer structures to enhance conductivity while maintaining processability, developing environmentally friendly formulations that minimize the use of toxic solvents, creating hybrid systems that combine polymers with metallic nanoparticles for enhanced performance, and engineering ink compositions that adhere effectively to pharmaceutical packaging substrates including plastics, glass, and paperboard.
The technological trajectory suggests a move toward multi-functional conductive polymer systems that not only provide electrical conductivity but also incorporate sensing capabilities for temperature, humidity, and tampering detection. This evolution aligns with the pharmaceutical industry's increasing emphasis on product integrity verification and real-time monitoring throughout the supply chain, ultimately contributing to enhanced medication safety and efficacy.
Market Analysis for Smart Pharmaceutical Packaging
The global smart pharmaceutical packaging market is experiencing robust growth, driven by increasing demand for enhanced medication adherence, drug authenticity verification, and real-time monitoring capabilities. Current market valuations indicate the smart pharmaceutical packaging sector reached approximately 3.8 billion USD in 2022, with projections suggesting a compound annual growth rate (CAGR) of 8.2% through 2030. This growth trajectory significantly outpaces traditional pharmaceutical packaging segments, reflecting the industry's shift toward more sophisticated solutions.
Consumer behavior analysis reveals mounting concerns regarding medication safety and authenticity, particularly in regions with prevalent counterfeit drug circulation. Market research indicates that 65% of patients report improved medication adherence when using smart packaging solutions, creating a compelling value proposition for healthcare providers and pharmaceutical companies alike. Additionally, regulatory bodies worldwide are implementing stricter requirements for drug traceability and authentication, further catalyzing market expansion.
Conductive polymer inks represent a critical enabling technology within this market landscape, offering the potential to transform conventional packaging into interactive smart systems. The integration of these materials allows for the development of printed electronics directly on packaging substrates, enabling features such as temperature monitoring, tampering detection, and patient engagement functionalities at significantly lower costs than traditional electronic components.
Regional market analysis shows North America currently dominating with approximately 38% market share, followed by Europe at 29% and Asia-Pacific at 24%. However, the Asia-Pacific region demonstrates the highest growth potential, with an anticipated CAGR of 10.5% through 2030, driven by expanding pharmaceutical manufacturing capabilities and increasing healthcare expenditure in countries like China and India.
Market segmentation reveals distinct application categories for conductive polymer ink technologies in pharmaceutical packaging: compliance monitoring (42% of applications), anti-counterfeiting measures (31%), and environmental condition monitoring (27%). The compliance monitoring segment shows particular promise, addressing the estimated 125 billion USD annual cost of medication non-adherence in developed economies.
Key market drivers include aging global populations requiring complex medication regimens, increasing prevalence of chronic diseases necessitating long-term medication management, and pharmaceutical companies seeking product differentiation in competitive markets. Technological advancements in printing techniques and material sciences are simultaneously reducing implementation costs, making smart packaging solutions increasingly accessible to mid-tier pharmaceutical manufacturers.
Consumer behavior analysis reveals mounting concerns regarding medication safety and authenticity, particularly in regions with prevalent counterfeit drug circulation. Market research indicates that 65% of patients report improved medication adherence when using smart packaging solutions, creating a compelling value proposition for healthcare providers and pharmaceutical companies alike. Additionally, regulatory bodies worldwide are implementing stricter requirements for drug traceability and authentication, further catalyzing market expansion.
Conductive polymer inks represent a critical enabling technology within this market landscape, offering the potential to transform conventional packaging into interactive smart systems. The integration of these materials allows for the development of printed electronics directly on packaging substrates, enabling features such as temperature monitoring, tampering detection, and patient engagement functionalities at significantly lower costs than traditional electronic components.
Regional market analysis shows North America currently dominating with approximately 38% market share, followed by Europe at 29% and Asia-Pacific at 24%. However, the Asia-Pacific region demonstrates the highest growth potential, with an anticipated CAGR of 10.5% through 2030, driven by expanding pharmaceutical manufacturing capabilities and increasing healthcare expenditure in countries like China and India.
Market segmentation reveals distinct application categories for conductive polymer ink technologies in pharmaceutical packaging: compliance monitoring (42% of applications), anti-counterfeiting measures (31%), and environmental condition monitoring (27%). The compliance monitoring segment shows particular promise, addressing the estimated 125 billion USD annual cost of medication non-adherence in developed economies.
Key market drivers include aging global populations requiring complex medication regimens, increasing prevalence of chronic diseases necessitating long-term medication management, and pharmaceutical companies seeking product differentiation in competitive markets. Technological advancements in printing techniques and material sciences are simultaneously reducing implementation costs, making smart packaging solutions increasingly accessible to mid-tier pharmaceutical manufacturers.
Technical Challenges in Conductive Polymer Applications
Despite significant advancements in conductive polymer technology, several technical challenges persist in their application for pharmaceutical packaging. The inherent trade-off between conductivity and processability remains a fundamental obstacle. As conductivity increases through higher loading of conductive materials, the ink's viscosity often increases disproportionately, creating processing difficulties during printing operations. This balance becomes particularly critical in pharmaceutical packaging where precise deposition is essential for functionality.
Material stability presents another significant challenge, especially in pharmaceutical environments where exposure to various chemicals, sterilization processes, and temperature fluctuations is common. Conductive polymers often exhibit degradation when exposed to oxygen, moisture, UV radiation, and certain pharmaceutical compounds, resulting in diminished electrical performance over time. This instability can compromise the integrity of smart packaging features designed to monitor drug quality or authenticity.
Adhesion issues between conductive polymer inks and pharmaceutical packaging substrates frequently occur due to surface energy mismatches. Poor adhesion leads to delamination, cracking, and ultimately circuit failure. The diverse range of packaging materials used in pharmaceuticals—from glass to various polymers—compounds this challenge, requiring specialized formulations for different substrate types.
Scalability and manufacturing integration pose substantial hurdles for widespread adoption. Current laboratory-scale production methods for high-performance conductive polymer inks often involve complex synthesis procedures that are difficult to scale up while maintaining consistent electrical properties. The pharmaceutical industry's stringent quality control requirements further complicate this transition from research to commercial implementation.
Biocompatibility and regulatory compliance represent critical barriers specific to pharmaceutical applications. Conductive polymers must not only perform their electronic functions but also meet strict safety standards to ensure they don't leach harmful substances into medications or interact negatively with active pharmaceutical ingredients. The regulatory pathway for novel electronic components in pharmaceutical packaging remains complex and poorly defined in many jurisdictions.
Resolution limitations affect the development of miniaturized circuits necessary for advanced pharmaceutical packaging applications. Current printing technologies struggle to consistently achieve the fine feature sizes required for sophisticated sensing and monitoring functions while maintaining electrical performance across production batches.
Cross-contamination concerns arise from the potential migration of conductive materials into drug formulations, particularly for sensitive biopharmaceuticals. This risk necessitates additional barrier layers or specialized ink formulations, adding complexity and cost to packaging designs.
Material stability presents another significant challenge, especially in pharmaceutical environments where exposure to various chemicals, sterilization processes, and temperature fluctuations is common. Conductive polymers often exhibit degradation when exposed to oxygen, moisture, UV radiation, and certain pharmaceutical compounds, resulting in diminished electrical performance over time. This instability can compromise the integrity of smart packaging features designed to monitor drug quality or authenticity.
Adhesion issues between conductive polymer inks and pharmaceutical packaging substrates frequently occur due to surface energy mismatches. Poor adhesion leads to delamination, cracking, and ultimately circuit failure. The diverse range of packaging materials used in pharmaceuticals—from glass to various polymers—compounds this challenge, requiring specialized formulations for different substrate types.
Scalability and manufacturing integration pose substantial hurdles for widespread adoption. Current laboratory-scale production methods for high-performance conductive polymer inks often involve complex synthesis procedures that are difficult to scale up while maintaining consistent electrical properties. The pharmaceutical industry's stringent quality control requirements further complicate this transition from research to commercial implementation.
Biocompatibility and regulatory compliance represent critical barriers specific to pharmaceutical applications. Conductive polymers must not only perform their electronic functions but also meet strict safety standards to ensure they don't leach harmful substances into medications or interact negatively with active pharmaceutical ingredients. The regulatory pathway for novel electronic components in pharmaceutical packaging remains complex and poorly defined in many jurisdictions.
Resolution limitations affect the development of miniaturized circuits necessary for advanced pharmaceutical packaging applications. Current printing technologies struggle to consistently achieve the fine feature sizes required for sophisticated sensing and monitoring functions while maintaining electrical performance across production batches.
Cross-contamination concerns arise from the potential migration of conductive materials into drug formulations, particularly for sensitive biopharmaceuticals. This risk necessitates additional barrier layers or specialized ink formulations, adding complexity and cost to packaging designs.
Current Solutions for Pharmaceutical Packaging Integration
01 Conductive polymer compositions for printable electronics
Conductive polymer inks can be formulated with specific polymers like PEDOT:PSS, polyaniline, or polythiophene derivatives to create printable electronic components. These formulations typically include solvents, binders, and additives that enhance conductivity while maintaining printability. The resulting inks can be used for flexible circuits, displays, and sensors with tunable electrical properties.- Conductive polymer compositions for printable electronics: Conductive polymer inks can be formulated with specific polymers like PEDOT:PSS, polyaniline, or polythiophene derivatives to create printable electronic components. These formulations typically include solvents, binders, and additives that enhance conductivity while maintaining printability. The resulting inks can be used for flexible circuits, displays, and sensors with tunable electrical properties.
- Carbon-based additives for enhanced conductivity: Incorporating carbon-based materials such as graphene, carbon nanotubes, or carbon black into polymer inks significantly improves their electrical conductivity. These additives create conductive networks within the polymer matrix, allowing for lower resistance pathways. The dispersion quality and loading amount of these carbon materials directly impact the final conductivity and printing performance of the ink.
- Metal nanoparticle incorporation techniques: Metal nanoparticles, particularly silver, gold, and copper, can be incorporated into polymer inks to achieve high conductivity. These particles require surface modification to prevent aggregation and ensure stable dispersion in the ink formulation. Sintering processes after printing allow the metal particles to form continuous conductive pathways, significantly enhancing the electrical performance of printed structures.
- Processing methods for improved ink performance: Various processing techniques can optimize conductive polymer ink performance, including solvent selection, viscosity modification, and surface tension adjustment. Post-processing methods such as thermal annealing, photonic sintering, or chemical treatments can significantly enhance conductivity after printing. These processes influence the morphology and crystallinity of the conductive components, directly affecting electrical properties.
- Application-specific ink formulations: Conductive polymer inks can be tailored for specific applications such as transparent electrodes, RFID tags, or biomedical sensors. These specialized formulations may include additives for adhesion to particular substrates, environmental stability, or biocompatibility. The rheological properties can be adjusted to suit different printing methods including screen printing, inkjet printing, or roll-to-roll processing, enabling diverse manufacturing approaches for printed electronics.
02 Nanoparticle-enhanced conductive polymer inks
Incorporating metallic or carbon-based nanoparticles into polymer ink formulations significantly improves conductivity while maintaining flexibility. These hybrid inks combine the processability of polymers with the high conductivity of nanomaterials such as silver nanoparticles, carbon nanotubes, or graphene. The nanoparticles create conductive networks within the polymer matrix, resulting in enhanced electrical performance for printed electronics applications.Expand Specific Solutions03 Processing techniques for conductive polymer inks
Various processing methods can be applied to optimize the performance of conductive polymer inks, including thermal annealing, solvent engineering, and post-deposition treatments. These techniques help to remove excess solvents, improve polymer chain alignment, and enhance inter-chain charge transport. Proper processing can significantly reduce sheet resistance and improve adhesion to various substrates, making the inks suitable for industrial-scale printed electronics.Expand Specific Solutions04 Substrate compatibility and adhesion enhancement
Formulating conductive polymer inks with specific adhesion promoters and surface modifiers enables compatibility with diverse substrates including flexible plastics, paper, textiles, and glass. These additives improve wetting characteristics and interfacial bonding between the conductive polymer and substrate surface. Proper substrate treatment and ink formulation ensure durability against mechanical stress, bending, and environmental factors while maintaining electrical performance.Expand Specific Solutions05 Environmental stability and encapsulation methods
Enhancing the environmental stability of conductive polymer inks involves incorporating stabilizers, antioxidants, and UV protectants into the formulation. Additionally, various encapsulation methods can be employed to protect the printed conductive patterns from moisture, oxygen, and other degradation factors. These approaches extend the functional lifetime of printed electronic devices and maintain consistent electrical performance under various environmental conditions.Expand Specific Solutions
Leading Companies in Conductive Polymer Ink Industry
The conductive polymer inks market in pharmaceutical packaging is in an early growth phase, characterized by increasing adoption driven by smart packaging innovations. The global market size is expanding rapidly, with projections indicating significant growth as pharmaceutical companies seek enhanced product security and patient compliance solutions. Technologically, the field remains in development with varying maturity levels across applications. Leading players include LG Chem and Henkel AG, who leverage their chemical expertise to develop specialized formulations, while research institutions like Xiamen University and Clemson University contribute fundamental innovations. Companies like Vorbeck Materials and InkTec are advancing commercialization with specialized conductive ink formulations, while electronics manufacturers such as Samsung Electro-Mechanics are exploring integration opportunities for smart pharmaceutical packaging applications.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced conductive polymer ink formulations specifically designed for pharmaceutical packaging applications. Their technology incorporates PEDOT:PSS (poly(3,4-ethylenedioxythiophene):polystyrene sulfonate) polymers modified with proprietary additives to enhance conductivity while maintaining biocompatibility. The company's approach involves a water-based formulation that achieves sheet resistance values below 100 ohms/square while eliminating toxic solvents typically found in conventional conductive inks. LG Chem's pharmaceutical packaging solutions integrate these inks into anti-counterfeiting systems that can be detected through simple electrical resistance measurements or RFID-based verification methods, allowing for real-time authentication and tracking throughout the supply chain. Their manufacturing process employs roll-to-roll printing techniques that enable mass production while maintaining consistent electrical properties across batches.
Strengths: Superior conductivity-to-transparency ratio compared to competitors; environmentally friendly water-based formulation; excellent adhesion to pharmaceutical packaging substrates; compatibility with existing high-speed printing equipment. Weaknesses: Higher cost compared to conventional non-conductive packaging materials; limited shelf life of conductive properties in certain environmental conditions; requires specialized equipment for quality control testing.
Vorbeck Materials Corp.
Technical Solution: Vorbeck Materials has pioneered graphene-based conductive polymer inks specifically engineered for pharmaceutical packaging applications. Their proprietary Vor-ink™ technology incorporates single-layer graphene sheets dispersed in a polymer matrix that can be printed on various packaging substrates. The company's formulation achieves conductivity levels of 10-15 S/cm while maintaining flexibility and durability required for pharmaceutical packaging. Vorbeck's approach enables the creation of printed electronic circuits directly on packaging materials that can monitor environmental conditions such as temperature and humidity during transport and storage. Their technology also incorporates tamper-evident features that irreversibly change electrical properties when packages are compromised, providing an additional layer of security for sensitive pharmaceutical products. The inks are compatible with standard flexographic and gravure printing processes, allowing pharmaceutical manufacturers to implement the technology without significant equipment modifications.
Strengths: Industry-leading conductivity due to graphene incorporation; exceptional mechanical durability with resistance to cracking and abrasion; compatibility with existing printing infrastructure; ability to function as both conductive traces and sensors. Weaknesses: Higher material costs compared to traditional carbon-based conductive inks; requires careful process control to ensure consistent graphene dispersion; limited color options which may impact packaging aesthetics.
Key Patents and Innovations in Conductive Polymer Inks
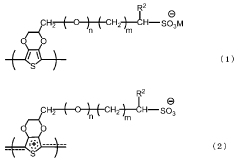
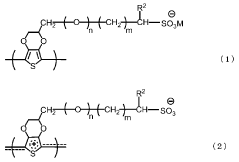
Conductive polymer ink composition
PatentWO2015182954A1
Innovation
- A conductive polymer ink composition comprising PEDOT:PSS aqueous dispersion, dimethyl sulfoxide, a solvent, a surfactant, and a phosphate compound, optimized with specific weight percentages to enhance conductivity and processability, including the use of deionized water and polyhydric alcohols like propylene glycol, which improves dispersibility and conductivity.
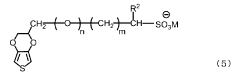
Conductive polymer-containing ink and application thereof
PatentActiveJP2019151814A
Innovation
- A conductive polymer-containing ink with a water content of 40% by weight or less, comprising specific solvents and resins, achieving appropriate viscosity and surface tension, allowing stable dissolution and formation of a conductive polymer film with high durability and hole-transporting properties.
Safety and Biocompatibility Considerations
The integration of conductive polymer inks into pharmaceutical packaging introduces critical safety and biocompatibility considerations that must be thoroughly addressed before widespread implementation. These materials come into direct or indirect contact with medications and ultimately with patients, necessitating rigorous evaluation of their potential health impacts.
Primary concerns revolve around leachable compounds and extractables from polymer inks that might migrate into drug formulations. Studies have shown that certain conductive polymers, particularly those containing metal nanoparticles or organic solvents, can release trace elements over time. Recent research by the FDA and European Medicines Agency has established threshold limits for various compounds, with particular attention to heavy metals like silver and copper commonly used in conductive applications.
Regulatory frameworks worldwide have evolved to address these emerging materials. The FDA's Guidance for Industry on Container Closure Systems for Packaging Human Drugs and Biologics specifically addresses electronic components in packaging, while the European Union's Regulation (EC) No 1935/2004 covers materials intended to contact medicinal products. Compliance with these regulations requires extensive toxicological profiling and migration studies.
Biocompatibility testing protocols for conductive polymer inks typically include cytotoxicity assessments, sensitization studies, and genotoxicity evaluations. Recent innovations have focused on developing "bio-benign" conductive polymers using naturally derived materials. For instance, PEDOT:PSS formulations modified with biocompatible stabilizers have demonstrated significantly reduced cytotoxicity while maintaining electrical performance.
The pharmaceutical industry has established a tiered risk assessment approach for these materials. This includes chemical characterization, extraction studies under various conditions (temperature, pH, time), and toxicological evaluation of extractables. Advanced analytical techniques such as LC-MS/MS and ICP-MS are employed to detect trace contaminants at parts-per-billion levels.
Environmental considerations also factor into safety assessments, as end-of-life disposal of smart pharmaceutical packaging must not introduce harmful substances into ecosystems. Biodegradable conductive polymers represent a promising research direction, with polythiophene derivatives showing particular potential for environmentally responsible applications.
Industry-academic collaborations have accelerated the development of safety standards specific to conductive materials in pharmaceutical contexts. The International Pharmaceutical Excipients Council has recently established a working group focused on electronic packaging components, aiming to develop harmonized testing protocols and acceptance criteria for these novel materials.
Primary concerns revolve around leachable compounds and extractables from polymer inks that might migrate into drug formulations. Studies have shown that certain conductive polymers, particularly those containing metal nanoparticles or organic solvents, can release trace elements over time. Recent research by the FDA and European Medicines Agency has established threshold limits for various compounds, with particular attention to heavy metals like silver and copper commonly used in conductive applications.
Regulatory frameworks worldwide have evolved to address these emerging materials. The FDA's Guidance for Industry on Container Closure Systems for Packaging Human Drugs and Biologics specifically addresses electronic components in packaging, while the European Union's Regulation (EC) No 1935/2004 covers materials intended to contact medicinal products. Compliance with these regulations requires extensive toxicological profiling and migration studies.
Biocompatibility testing protocols for conductive polymer inks typically include cytotoxicity assessments, sensitization studies, and genotoxicity evaluations. Recent innovations have focused on developing "bio-benign" conductive polymers using naturally derived materials. For instance, PEDOT:PSS formulations modified with biocompatible stabilizers have demonstrated significantly reduced cytotoxicity while maintaining electrical performance.
The pharmaceutical industry has established a tiered risk assessment approach for these materials. This includes chemical characterization, extraction studies under various conditions (temperature, pH, time), and toxicological evaluation of extractables. Advanced analytical techniques such as LC-MS/MS and ICP-MS are employed to detect trace contaminants at parts-per-billion levels.
Environmental considerations also factor into safety assessments, as end-of-life disposal of smart pharmaceutical packaging must not introduce harmful substances into ecosystems. Biodegradable conductive polymers represent a promising research direction, with polythiophene derivatives showing particular potential for environmentally responsible applications.
Industry-academic collaborations have accelerated the development of safety standards specific to conductive materials in pharmaceutical contexts. The International Pharmaceutical Excipients Council has recently established a working group focused on electronic packaging components, aiming to develop harmonized testing protocols and acceptance criteria for these novel materials.
Regulatory Framework for Smart Pharmaceutical Packaging
The regulatory landscape for smart pharmaceutical packaging incorporating conductive polymer inks presents a complex framework that manufacturers must navigate carefully. At the international level, organizations such as the International Conference on Harmonisation (ICH) and the World Health Organization (WHO) provide overarching guidelines that influence regional and national regulations. These guidelines emphasize patient safety, product efficacy, and quality assurance throughout the pharmaceutical supply chain.
In the United States, the Food and Drug Administration (FDA) has established specific requirements for pharmaceutical packaging materials through 21 CFR Parts 210 and 211, with additional guidance on novel packaging technologies under the FDA's Emerging Technology Program. For conductive polymer inks specifically, manufacturers must demonstrate that these materials do not migrate into drug products or alter their chemical composition, efficacy, or safety profiles.
The European Union operates under the European Medicines Agency (EMA) framework, which includes Directive 2001/83/EC and Regulation (EU) 2017/745 for medical devices. The EU has also implemented specific requirements for food contact materials through Regulation (EC) No 1935/2004, which has implications for pharmaceutical packaging materials. Additionally, the EU's REACH regulation (Registration, Evaluation, Authorization and Restriction of Chemicals) imposes strict controls on chemical substances used in manufacturing processes.
In Asia, regulatory frameworks vary significantly by country. Japan's Pharmaceutical and Medical Devices Agency (PMDA) has established stringent requirements for packaging materials, while China's National Medical Products Administration (NMPA) continues to evolve its regulatory approach to novel packaging technologies.
Beyond regional regulations, industry standards such as ISO 15378:2017 provide specific requirements for primary packaging materials for medicinal products. These standards help ensure consistency and quality across the global pharmaceutical supply chain.
For conductive polymer inks specifically, regulatory considerations extend to electronic components and potential electromagnetic interference. The International Electrotechnical Commission (IEC) standards and electromagnetic compatibility (EMC) regulations must be considered when developing smart packaging solutions that incorporate electronic elements.
Environmental regulations also impact the development and deployment of conductive polymer inks in pharmaceutical packaging. The EU's Waste Electrical and Electronic Equipment (WEEE) Directive and Restriction of Hazardous Substances (RoHS) Directive impose restrictions on certain materials and mandate recycling requirements that may affect smart packaging designs.
In the United States, the Food and Drug Administration (FDA) has established specific requirements for pharmaceutical packaging materials through 21 CFR Parts 210 and 211, with additional guidance on novel packaging technologies under the FDA's Emerging Technology Program. For conductive polymer inks specifically, manufacturers must demonstrate that these materials do not migrate into drug products or alter their chemical composition, efficacy, or safety profiles.
The European Union operates under the European Medicines Agency (EMA) framework, which includes Directive 2001/83/EC and Regulation (EU) 2017/745 for medical devices. The EU has also implemented specific requirements for food contact materials through Regulation (EC) No 1935/2004, which has implications for pharmaceutical packaging materials. Additionally, the EU's REACH regulation (Registration, Evaluation, Authorization and Restriction of Chemicals) imposes strict controls on chemical substances used in manufacturing processes.
In Asia, regulatory frameworks vary significantly by country. Japan's Pharmaceutical and Medical Devices Agency (PMDA) has established stringent requirements for packaging materials, while China's National Medical Products Administration (NMPA) continues to evolve its regulatory approach to novel packaging technologies.
Beyond regional regulations, industry standards such as ISO 15378:2017 provide specific requirements for primary packaging materials for medicinal products. These standards help ensure consistency and quality across the global pharmaceutical supply chain.
For conductive polymer inks specifically, regulatory considerations extend to electronic components and potential electromagnetic interference. The International Electrotechnical Commission (IEC) standards and electromagnetic compatibility (EMC) regulations must be considered when developing smart packaging solutions that incorporate electronic elements.
Environmental regulations also impact the development and deployment of conductive polymer inks in pharmaceutical packaging. The EU's Waste Electrical and Electronic Equipment (WEEE) Directive and Restriction of Hazardous Substances (RoHS) Directive impose restrictions on certain materials and mandate recycling requirements that may affect smart packaging designs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!