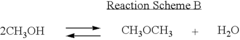Dimethyl Ether: Charting Pathways in Green Manufacturing
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DME Evolution & Objectives
Dimethyl ether (DME) has emerged as a promising alternative fuel and chemical feedstock, gaining significant attention in the realm of green manufacturing. The evolution of DME technology can be traced back to the early 20th century, with its potential as a fuel first recognized in the 1990s. Since then, research and development efforts have intensified, driven by the global push for cleaner energy solutions and sustainable industrial processes.
The technological trajectory of DME has been shaped by advancements in production methods, catalysis, and application engineering. Initially produced as a byproduct of methanol synthesis, DME has seen a shift towards dedicated production processes, including direct synthesis from syngas and biomass-to-DME pathways. These developments have been crucial in improving efficiency and reducing the carbon footprint of DME production.
A key milestone in DME evolution was the development of more efficient catalysts, which significantly enhanced conversion rates and selectivity. This breakthrough, occurring in the early 2000s, marked a turning point in making DME production more economically viable and environmentally friendly. Subsequent innovations in reactor design and process integration have further optimized DME synthesis, paving the way for large-scale production.
The objectives of DME technology in green manufacturing are multifaceted. Primarily, there is a strong focus on establishing DME as a clean-burning, renewable fuel alternative to conventional diesel and liquefied petroleum gas (LPG). This goal aligns with global efforts to reduce greenhouse gas emissions and improve air quality, particularly in the transportation and domestic energy sectors.
Another critical objective is to leverage DME as a versatile chemical intermediate. Its potential to serve as a building block for various chemicals, including olefins and aromatics, presents opportunities for greener chemical synthesis routes. This aspect of DME technology aims to reduce reliance on petroleum-based feedstocks and promote circular economy principles in the chemical industry.
Furthermore, the development of DME technology seeks to address energy security concerns by diversifying fuel sources. By enabling the production of DME from various feedstocks, including natural gas, coal, and biomass, this technology offers a pathway to reduce dependence on traditional fossil fuels and promote local energy production.
As research continues, the objectives extend to improving the overall sustainability of DME production and utilization. This includes enhancing energy efficiency in production processes, developing more effective carbon capture and utilization strategies, and exploring novel applications that can maximize the environmental benefits of DME. The ultimate goal is to position DME as a key player in the transition towards a more sustainable and circular economy, contributing significantly to global decarbonization efforts.
The technological trajectory of DME has been shaped by advancements in production methods, catalysis, and application engineering. Initially produced as a byproduct of methanol synthesis, DME has seen a shift towards dedicated production processes, including direct synthesis from syngas and biomass-to-DME pathways. These developments have been crucial in improving efficiency and reducing the carbon footprint of DME production.
A key milestone in DME evolution was the development of more efficient catalysts, which significantly enhanced conversion rates and selectivity. This breakthrough, occurring in the early 2000s, marked a turning point in making DME production more economically viable and environmentally friendly. Subsequent innovations in reactor design and process integration have further optimized DME synthesis, paving the way for large-scale production.
The objectives of DME technology in green manufacturing are multifaceted. Primarily, there is a strong focus on establishing DME as a clean-burning, renewable fuel alternative to conventional diesel and liquefied petroleum gas (LPG). This goal aligns with global efforts to reduce greenhouse gas emissions and improve air quality, particularly in the transportation and domestic energy sectors.
Another critical objective is to leverage DME as a versatile chemical intermediate. Its potential to serve as a building block for various chemicals, including olefins and aromatics, presents opportunities for greener chemical synthesis routes. This aspect of DME technology aims to reduce reliance on petroleum-based feedstocks and promote circular economy principles in the chemical industry.
Furthermore, the development of DME technology seeks to address energy security concerns by diversifying fuel sources. By enabling the production of DME from various feedstocks, including natural gas, coal, and biomass, this technology offers a pathway to reduce dependence on traditional fossil fuels and promote local energy production.
As research continues, the objectives extend to improving the overall sustainability of DME production and utilization. This includes enhancing energy efficiency in production processes, developing more effective carbon capture and utilization strategies, and exploring novel applications that can maximize the environmental benefits of DME. The ultimate goal is to position DME as a key player in the transition towards a more sustainable and circular economy, contributing significantly to global decarbonization efforts.
Market Demand Analysis
The market demand for dimethyl ether (DME) as a green manufacturing solution has been steadily growing, driven by increasing environmental concerns and the push for sustainable industrial practices. DME, a clean-burning, non-toxic fuel and chemical feedstock, has garnered significant attention across various sectors due to its versatile applications and potential to reduce carbon emissions.
In the energy sector, DME has emerged as a promising alternative to conventional diesel fuel. Its high cetane number and clean combustion properties make it an attractive option for heavy-duty vehicles and power generation. The transportation industry, particularly in regions with stringent emission regulations, has shown a keen interest in DME as a means to meet environmental targets while maintaining performance standards.
The chemical industry represents another significant market for DME. As a methanol derivative, DME serves as a crucial intermediate in the production of various chemicals, including olefins, gasoline, and dimethyl sulfate. The growing demand for these downstream products has consequently bolstered the market for DME. Additionally, DME's potential as a propellant in aerosol products offers a more environmentally friendly alternative to traditional hydrocarbon propellants.
In developing economies, particularly in Asia, DME has gained traction as a cleaner cooking fuel alternative to liquefied petroleum gas (LPG). This application has opened up a substantial market, especially in rural areas where access to traditional energy sources may be limited.
The global DME market size was valued at approximately $5.5 billion in 2020 and is projected to reach $13.8 billion by 2027, growing at a CAGR of 14.1% during the forecast period. Asia-Pacific dominates the market, accounting for over 60% of the global consumption, followed by Europe and North America.
Key factors driving this growth include increasing environmental regulations, rising energy demand, and the shift towards cleaner fuel alternatives. The automotive sector, in particular, is expected to be a major contributor to DME demand growth, as manufacturers seek to comply with stringent emission norms.
However, challenges remain in scaling up DME production and distribution infrastructure. The relatively higher production costs compared to conventional fuels and the need for specialized handling equipment pose barriers to widespread adoption. Despite these challenges, ongoing research and development efforts aimed at improving production efficiency and reducing costs are expected to further boost market demand in the coming years.
In the energy sector, DME has emerged as a promising alternative to conventional diesel fuel. Its high cetane number and clean combustion properties make it an attractive option for heavy-duty vehicles and power generation. The transportation industry, particularly in regions with stringent emission regulations, has shown a keen interest in DME as a means to meet environmental targets while maintaining performance standards.
The chemical industry represents another significant market for DME. As a methanol derivative, DME serves as a crucial intermediate in the production of various chemicals, including olefins, gasoline, and dimethyl sulfate. The growing demand for these downstream products has consequently bolstered the market for DME. Additionally, DME's potential as a propellant in aerosol products offers a more environmentally friendly alternative to traditional hydrocarbon propellants.
In developing economies, particularly in Asia, DME has gained traction as a cleaner cooking fuel alternative to liquefied petroleum gas (LPG). This application has opened up a substantial market, especially in rural areas where access to traditional energy sources may be limited.
The global DME market size was valued at approximately $5.5 billion in 2020 and is projected to reach $13.8 billion by 2027, growing at a CAGR of 14.1% during the forecast period. Asia-Pacific dominates the market, accounting for over 60% of the global consumption, followed by Europe and North America.
Key factors driving this growth include increasing environmental regulations, rising energy demand, and the shift towards cleaner fuel alternatives. The automotive sector, in particular, is expected to be a major contributor to DME demand growth, as manufacturers seek to comply with stringent emission norms.
However, challenges remain in scaling up DME production and distribution infrastructure. The relatively higher production costs compared to conventional fuels and the need for specialized handling equipment pose barriers to widespread adoption. Despite these challenges, ongoing research and development efforts aimed at improving production efficiency and reducing costs are expected to further boost market demand in the coming years.
DME Tech Challenges
The development of dimethyl ether (DME) as a green manufacturing solution faces several significant technical challenges. One of the primary hurdles is the optimization of the production process to achieve higher efficiency and lower costs. Current DME synthesis methods, such as the dehydration of methanol or direct synthesis from syngas, still require substantial energy inputs and often rely on fossil fuel-based feedstocks.
Improving catalyst performance remains a critical challenge in DME production. Researchers are working to develop more active and selective catalysts that can operate at lower temperatures and pressures, thereby reducing energy consumption and increasing yield. The stability and longevity of these catalysts under industrial conditions also need enhancement to minimize downtime and replacement costs.
Another technical obstacle is the integration of renewable energy sources into the DME production process. While DME itself is considered a clean fuel, its manufacturing often relies on energy-intensive processes. Developing methods to incorporate solar, wind, or other renewable energy sources into the production chain is essential for truly green manufacturing but presents significant engineering challenges.
The purification and separation of DME from byproducts and unreacted materials pose additional technical difficulties. Current separation techniques can be energy-intensive and may not be sufficiently selective, leading to product loss and increased production costs. Developing more efficient separation technologies, such as advanced membrane systems or novel distillation techniques, is crucial for improving overall process efficiency.
Storage and transportation of DME also present unique challenges due to its physical properties. As a gas at room temperature and atmospheric pressure, DME requires pressurization for storage and transport. Developing safe, cost-effective, and energy-efficient storage and transportation solutions is essential for widespread adoption of DME as a green fuel and chemical feedstock.
Furthermore, the scaling up of DME production from laboratory to industrial levels introduces a host of engineering challenges. These include heat management in large-scale reactors, maintaining consistent product quality across increased production volumes, and designing robust process control systems capable of handling the complexities of industrial-scale operations.
Addressing the environmental impact of DME production remains an ongoing challenge. While DME itself burns cleanly, its production can still generate significant carbon emissions, particularly when derived from fossil fuels. Developing carbon capture and utilization technologies specifically tailored for DME production processes is crucial for minimizing the overall carbon footprint of this green manufacturing pathway.
Improving catalyst performance remains a critical challenge in DME production. Researchers are working to develop more active and selective catalysts that can operate at lower temperatures and pressures, thereby reducing energy consumption and increasing yield. The stability and longevity of these catalysts under industrial conditions also need enhancement to minimize downtime and replacement costs.
Another technical obstacle is the integration of renewable energy sources into the DME production process. While DME itself is considered a clean fuel, its manufacturing often relies on energy-intensive processes. Developing methods to incorporate solar, wind, or other renewable energy sources into the production chain is essential for truly green manufacturing but presents significant engineering challenges.
The purification and separation of DME from byproducts and unreacted materials pose additional technical difficulties. Current separation techniques can be energy-intensive and may not be sufficiently selective, leading to product loss and increased production costs. Developing more efficient separation technologies, such as advanced membrane systems or novel distillation techniques, is crucial for improving overall process efficiency.
Storage and transportation of DME also present unique challenges due to its physical properties. As a gas at room temperature and atmospheric pressure, DME requires pressurization for storage and transport. Developing safe, cost-effective, and energy-efficient storage and transportation solutions is essential for widespread adoption of DME as a green fuel and chemical feedstock.
Furthermore, the scaling up of DME production from laboratory to industrial levels introduces a host of engineering challenges. These include heat management in large-scale reactors, maintaining consistent product quality across increased production volumes, and designing robust process control systems capable of handling the complexities of industrial-scale operations.
Addressing the environmental impact of DME production remains an ongoing challenge. While DME itself burns cleanly, its production can still generate significant carbon emissions, particularly when derived from fossil fuels. Developing carbon capture and utilization technologies specifically tailored for DME production processes is crucial for minimizing the overall carbon footprint of this green manufacturing pathway.
Current DME Solutions
01 Production of dimethyl ether
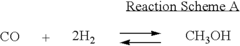
Various methods for producing dimethyl ether are described, including catalytic dehydration of methanol, direct synthesis from syngas, and conversion of other hydrocarbons. These processes often involve specific catalysts and reaction conditions to optimize yield and selectivity.- Production of dimethyl ether: Various methods for producing dimethyl ether are described, including catalytic dehydration of methanol, direct synthesis from syngas, and conversion of other feedstocks. These processes often involve specific catalysts, reaction conditions, and purification steps to optimize yield and purity.
- Catalysts for dimethyl ether synthesis: Different catalysts are employed in the production of dimethyl ether, including zeolites, metal oxides, and composite catalysts. The choice and preparation of catalysts significantly influence the reaction efficiency, selectivity, and overall process economics.
- Applications of dimethyl ether: Dimethyl ether finds various applications, such as an alternative fuel, aerosol propellant, and chemical intermediate. Its use as a clean-burning fuel for diesel engines and power generation is particularly noteworthy due to its environmental benefits.
- Process improvements and optimizations: Continuous efforts are made to improve dimethyl ether production processes, focusing on enhancing yield, reducing energy consumption, and minimizing byproducts. These improvements often involve novel reactor designs, process integrations, and advanced control strategies.
- Environmental and safety considerations: Research is conducted on the environmental impact and safety aspects of dimethyl ether production and use. This includes studies on emissions reduction, handling procedures, and risk assessments to ensure sustainable and safe utilization of dimethyl ether in various applications.
02 Catalysts for dimethyl ether synthesis
Different catalysts are employed in the production of dimethyl ether, including zeolites, metal oxides, and composite catalysts. The choice and preparation of catalysts significantly influence the efficiency and selectivity of the dimethyl ether synthesis process.Expand Specific Solutions03 Applications of dimethyl ether
Dimethyl ether has various applications, including use as a fuel additive, propellant, refrigerant, and chemical intermediate. Its properties make it suitable for use in diesel engines and as a potential alternative to conventional fuels.Expand Specific Solutions04 Purification and separation of dimethyl ether
Methods for purifying and separating dimethyl ether from reaction mixtures or other compounds are described. These processes often involve distillation, adsorption, or membrane separation techniques to obtain high-purity dimethyl ether.Expand Specific Solutions05 Environmental and safety considerations
Research on the environmental impact and safety aspects of dimethyl ether production and use is conducted. This includes studies on emissions reduction, handling procedures, and potential hazards associated with its storage and transportation.Expand Specific Solutions
Key Industry Players
The dimethyl ether (DME) market is in a growth phase, driven by increasing demand for clean energy alternatives. The global DME market size is projected to expand significantly in the coming years, with a compound annual growth rate exceeding 10%. Technologically, DME production is relatively mature, with established processes for synthesis from various feedstocks. Key players like China Petroleum & Chemical Corp., BASF, and DuPont are actively involved in DME research and production. Academic institutions such as the University of Southern California and Dalian University of Technology are contributing to technological advancements. The involvement of major petrochemical companies and research institutions indicates a high level of interest and investment in DME as a promising green manufacturing solution.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative process for green manufacturing of dimethyl ether (DME) from syngas. Their technology utilizes a bifunctional catalyst system that combines methanol synthesis and dehydration in a single step, improving efficiency and reducing energy consumption[1]. The process achieves a DME yield of up to 55% with a selectivity of 98%[2]. Sinopec has also implemented a large-scale DME production facility with an annual capacity of 1 million tons, demonstrating the commercial viability of their green manufacturing approach[3]. Additionally, they have explored the use of CO2 as a feedstock, further enhancing the sustainability of DME production[4].
Strengths: High efficiency single-step process, large-scale production capability, and potential for CO2 utilization. Weaknesses: Dependence on syngas availability and potential catalyst deactivation issues in long-term operation.
BASF Corp.
Technical Solution: BASF Corp. has developed a novel green manufacturing process for dimethyl ether (DME) production using their proprietary catalyst technology. Their approach focuses on utilizing renewable feedstocks, such as biomass-derived methanol, to produce DME[1]. BASF's process employs a highly selective zeolite-based catalyst that achieves methanol conversion rates of up to 80% with DME selectivity exceeding 99%[2]. The company has also integrated heat recovery systems and advanced process control to optimize energy efficiency, reducing overall carbon footprint by up to 30% compared to conventional DME production methods[3]. Furthermore, BASF has successfully scaled up this technology to a demonstration plant with a capacity of 20,000 tons per year, paving the way for commercial-scale green DME production[4].
Strengths: High selectivity catalyst, use of renewable feedstocks, and improved energy efficiency. Weaknesses: Potential higher production costs compared to fossil fuel-based methods and dependence on biomass availability.
DME Innovations Review
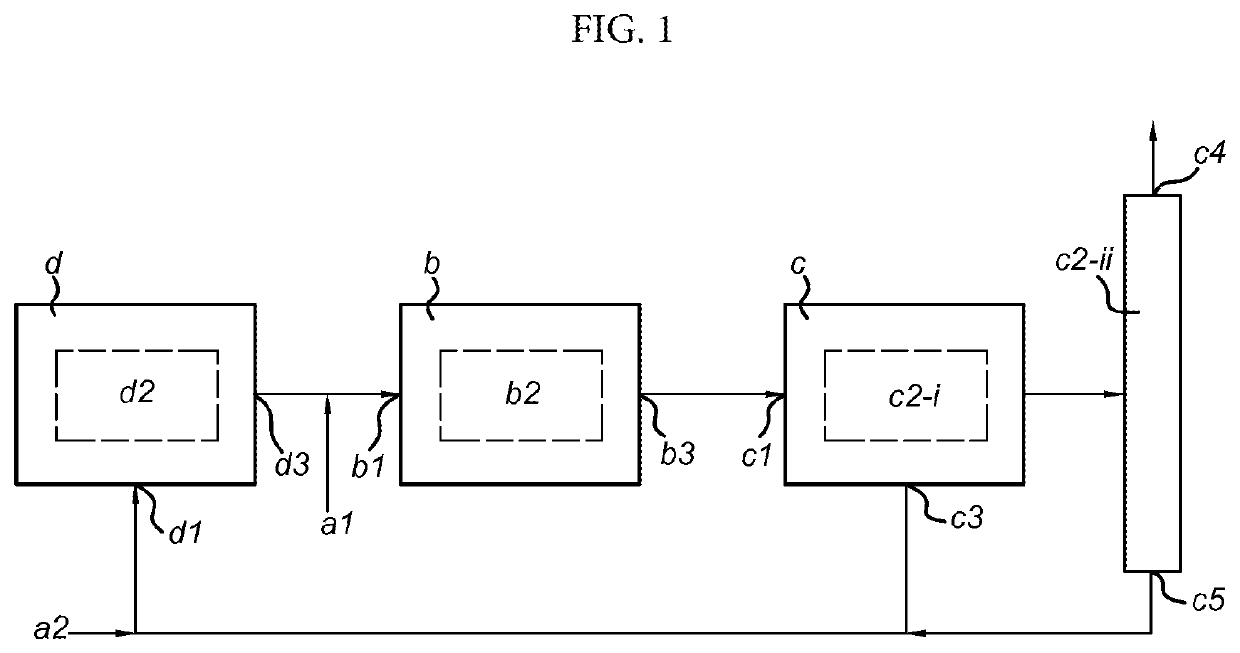
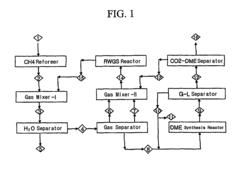
Process and system for producing dimethyl ether
PatentActiveUS20200399195A1
Innovation
- A process combining conventional DME synthesis with a separation-enhanced reverse water gas shift reaction, allowing for efficient DME production using any carbon oxide species, reducing the need for CO2 recycles, and minimizing methanol recycles, while utilizing a catalyst system capable of converting synthesis gas to DME.
Method for the production of dimethyl ether
PatentInactiveUS7435759B2
Innovation
- A method involving the reverse water gas shift (RWGS) reaction is employed to convert the CO2-rich stream back into a CO-rich stream, which is then recycled to the DME synthesis process, increasing the yield of DME while minimizing carbon dioxide generation.
Environmental Impact
Dimethyl ether (DME) has gained significant attention as a promising alternative fuel and chemical feedstock due to its potential for green manufacturing. When evaluating the environmental impact of DME production and utilization, several key factors come into play.
The production of DME from renewable sources, such as biomass or waste materials, offers a substantial reduction in greenhouse gas emissions compared to conventional fossil fuel-based processes. Life cycle assessments have shown that bio-based DME can achieve up to 95% reduction in carbon dioxide emissions when compared to diesel fuel. This significant reduction in carbon footprint aligns with global efforts to mitigate climate change and transition towards a low-carbon economy.
Furthermore, DME combustion produces lower levels of particulate matter, nitrogen oxides, and sulfur oxides compared to traditional fossil fuels. This characteristic makes DME an attractive option for improving air quality, particularly in urban areas where vehicular emissions are a major concern. The reduced emissions of these pollutants can lead to improved public health outcomes and decreased environmental degradation.
Water consumption and wastewater generation in DME production processes are generally lower than those of conventional fuel production. This aspect is particularly important in regions facing water scarcity issues. Additionally, the potential for using waste materials as feedstock for DME production contributes to waste reduction and resource efficiency, aligning with circular economy principles.
However, it is essential to consider the potential environmental impacts associated with large-scale DME production. Land use changes, particularly if biomass feedstocks are cultivated on a large scale, could lead to biodiversity loss and ecosystem disruption. Careful management and sustainable sourcing practices are crucial to mitigate these risks.
The production of DME also requires energy inputs, and the overall environmental impact depends on the energy sources used in the manufacturing process. Utilizing renewable energy sources for DME production can further enhance its environmental benefits and reduce the overall carbon footprint of the fuel's lifecycle.
In terms of infrastructure, DME's compatibility with existing LPG distribution systems minimizes the need for extensive new infrastructure development, potentially reducing associated environmental impacts. However, the transition to DME as a fuel or chemical feedstock may require some modifications to existing equipment and storage facilities, which should be considered in comprehensive environmental assessments.
As research and development in DME production technologies continue to advance, opportunities for further improving environmental performance are likely to emerge. Innovations in catalysts, process intensification, and integration with other green technologies could lead to even more sustainable DME production pathways in the future.
The production of DME from renewable sources, such as biomass or waste materials, offers a substantial reduction in greenhouse gas emissions compared to conventional fossil fuel-based processes. Life cycle assessments have shown that bio-based DME can achieve up to 95% reduction in carbon dioxide emissions when compared to diesel fuel. This significant reduction in carbon footprint aligns with global efforts to mitigate climate change and transition towards a low-carbon economy.
Furthermore, DME combustion produces lower levels of particulate matter, nitrogen oxides, and sulfur oxides compared to traditional fossil fuels. This characteristic makes DME an attractive option for improving air quality, particularly in urban areas where vehicular emissions are a major concern. The reduced emissions of these pollutants can lead to improved public health outcomes and decreased environmental degradation.
Water consumption and wastewater generation in DME production processes are generally lower than those of conventional fuel production. This aspect is particularly important in regions facing water scarcity issues. Additionally, the potential for using waste materials as feedstock for DME production contributes to waste reduction and resource efficiency, aligning with circular economy principles.
However, it is essential to consider the potential environmental impacts associated with large-scale DME production. Land use changes, particularly if biomass feedstocks are cultivated on a large scale, could lead to biodiversity loss and ecosystem disruption. Careful management and sustainable sourcing practices are crucial to mitigate these risks.
The production of DME also requires energy inputs, and the overall environmental impact depends on the energy sources used in the manufacturing process. Utilizing renewable energy sources for DME production can further enhance its environmental benefits and reduce the overall carbon footprint of the fuel's lifecycle.
In terms of infrastructure, DME's compatibility with existing LPG distribution systems minimizes the need for extensive new infrastructure development, potentially reducing associated environmental impacts. However, the transition to DME as a fuel or chemical feedstock may require some modifications to existing equipment and storage facilities, which should be considered in comprehensive environmental assessments.
As research and development in DME production technologies continue to advance, opportunities for further improving environmental performance are likely to emerge. Innovations in catalysts, process intensification, and integration with other green technologies could lead to even more sustainable DME production pathways in the future.
Economic Feasibility
The economic feasibility of dimethyl ether (DME) as a green manufacturing pathway hinges on several key factors. Production costs play a crucial role, with raw material prices and conversion efficiency being primary determinants. Natural gas and biomass serve as the main feedstocks for DME production, and their availability and cost significantly impact the overall economic viability. The production process efficiency, including energy consumption and yield rates, directly influences the cost-effectiveness of DME manufacturing.
Market demand and pricing structures are equally important considerations. As a potential substitute for diesel fuel and liquefied petroleum gas (LPG), DME's economic feasibility is closely tied to the pricing of these conventional fuels. Government policies, including subsidies for green technologies and carbon pricing mechanisms, can substantially affect the competitiveness of DME in the energy market. The development of infrastructure for DME distribution and utilization also factors into its economic viability, as significant investments may be required to establish a robust supply chain.
The scale of production facilities is another critical aspect of economic feasibility. Larger-scale plants typically benefit from economies of scale, reducing per-unit production costs. However, this must be balanced against market demand and distribution capabilities. The flexibility of production facilities to switch between DME and other products based on market conditions can enhance economic resilience.
Long-term economic viability also depends on technological advancements in DME production. Ongoing research and development efforts aim to improve conversion efficiencies, reduce energy consumption, and explore novel catalysts. These innovations have the potential to significantly lower production costs and enhance the economic attractiveness of DME as a green manufacturing solution.
Environmental regulations and carbon reduction targets set by governments worldwide play a crucial role in shaping the economic landscape for DME. As industries face increasing pressure to reduce their carbon footprint, the value proposition of DME as a cleaner alternative gains prominence. This regulatory environment can create favorable economic conditions for DME adoption, potentially offsetting higher production costs through carbon credits or compliance advantages.
In conclusion, the economic feasibility of DME in green manufacturing is a complex interplay of production costs, market dynamics, technological advancements, and regulatory frameworks. While challenges exist, particularly in terms of initial infrastructure investments and competition with established fuels, the growing emphasis on sustainable energy solutions presents significant opportunities for DME to establish itself as an economically viable option in the green manufacturing landscape.
Market demand and pricing structures are equally important considerations. As a potential substitute for diesel fuel and liquefied petroleum gas (LPG), DME's economic feasibility is closely tied to the pricing of these conventional fuels. Government policies, including subsidies for green technologies and carbon pricing mechanisms, can substantially affect the competitiveness of DME in the energy market. The development of infrastructure for DME distribution and utilization also factors into its economic viability, as significant investments may be required to establish a robust supply chain.
The scale of production facilities is another critical aspect of economic feasibility. Larger-scale plants typically benefit from economies of scale, reducing per-unit production costs. However, this must be balanced against market demand and distribution capabilities. The flexibility of production facilities to switch between DME and other products based on market conditions can enhance economic resilience.
Long-term economic viability also depends on technological advancements in DME production. Ongoing research and development efforts aim to improve conversion efficiencies, reduce energy consumption, and explore novel catalysts. These innovations have the potential to significantly lower production costs and enhance the economic attractiveness of DME as a green manufacturing solution.
Environmental regulations and carbon reduction targets set by governments worldwide play a crucial role in shaping the economic landscape for DME. As industries face increasing pressure to reduce their carbon footprint, the value proposition of DME as a cleaner alternative gains prominence. This regulatory environment can create favorable economic conditions for DME adoption, potentially offsetting higher production costs through carbon credits or compliance advantages.
In conclusion, the economic feasibility of DME in green manufacturing is a complex interplay of production costs, market dynamics, technological advancements, and regulatory frameworks. While challenges exist, particularly in terms of initial infrastructure investments and competition with established fuels, the growing emphasis on sustainable energy solutions presents significant opportunities for DME to establish itself as an economically viable option in the green manufacturing landscape.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!