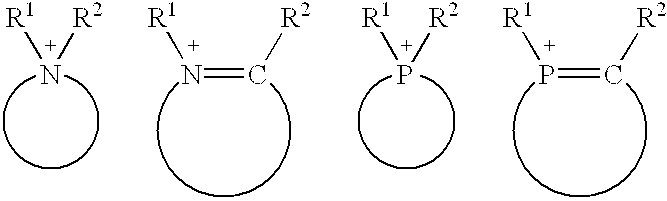
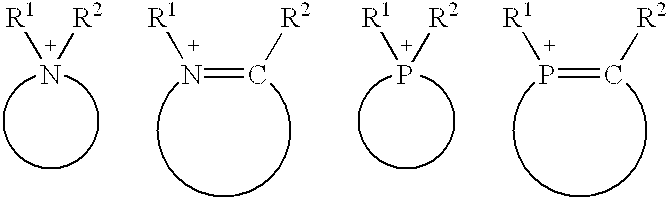
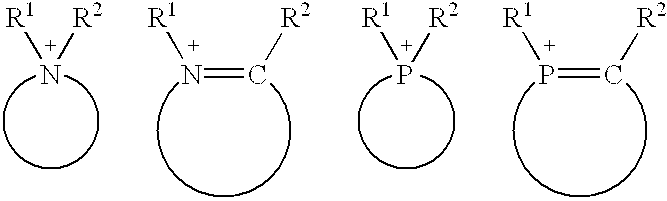
Dodecane's Role for Catalytic Research
JUL 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Dodecane Catalysis Background and Objectives
Dodecane, a linear alkane with twelve carbon atoms, has emerged as a significant compound in catalytic research, playing a crucial role in advancing our understanding of hydrocarbon reactions and catalyst development. The evolution of dodecane's application in catalysis can be traced back to the early days of petroleum refining, where it served as a model compound for studying cracking reactions.
Over the years, the focus on dodecane in catalytic research has expanded beyond its initial use in petroleum processing. Researchers have recognized its potential as a representative molecule for long-chain hydrocarbons, making it an ideal candidate for investigating various catalytic processes. This shift in perspective has led to a broader exploration of dodecane's behavior under different catalytic conditions, contributing to advancements in fields such as biofuel production, fine chemical synthesis, and environmental remediation.
The current technological landscape surrounding dodecane catalysis is characterized by a growing emphasis on sustainable and efficient chemical processes. As global concerns about environmental impact and resource depletion intensify, researchers are increasingly turning to dodecane as a model compound for developing greener catalytic technologies. This trend aligns with the broader goals of reducing carbon footprints and minimizing waste in industrial chemical processes.
One of the primary objectives in dodecane catalysis research is to enhance selectivity and yield in hydrocarbon conversion reactions. Scientists aim to design catalysts that can precisely control the transformation of dodecane into desired products while minimizing unwanted side reactions. This pursuit has led to the exploration of novel catalyst materials, including nanostructured metals, zeolites, and metal-organic frameworks, each offering unique properties for manipulating dodecane's reactivity.
Another critical goal in this field is to improve the energy efficiency of catalytic processes involving dodecane. Researchers are investigating ways to lower reaction temperatures and pressures without compromising conversion rates or product quality. This objective is particularly relevant in the context of sustainable chemistry, where reducing energy consumption is paramount.
Furthermore, the study of dodecane catalysis aims to bridge the gap between fundamental research and practical applications. By understanding the mechanistic details of dodecane reactions on various catalytic surfaces, scientists hope to develop predictive models that can accelerate the discovery and optimization of new catalytic systems. This approach not only enhances our theoretical knowledge but also paves the way for more rational catalyst design in industrial settings.
In conclusion, the background and objectives of dodecane catalysis research reflect a dynamic and evolving field at the intersection of chemistry, materials science, and sustainable technology. As we continue to push the boundaries of catalytic science, dodecane remains a valuable tool for unlocking new possibilities in chemical transformations and advancing our pursuit of more efficient, environmentally friendly industrial processes.
Over the years, the focus on dodecane in catalytic research has expanded beyond its initial use in petroleum processing. Researchers have recognized its potential as a representative molecule for long-chain hydrocarbons, making it an ideal candidate for investigating various catalytic processes. This shift in perspective has led to a broader exploration of dodecane's behavior under different catalytic conditions, contributing to advancements in fields such as biofuel production, fine chemical synthesis, and environmental remediation.
The current technological landscape surrounding dodecane catalysis is characterized by a growing emphasis on sustainable and efficient chemical processes. As global concerns about environmental impact and resource depletion intensify, researchers are increasingly turning to dodecane as a model compound for developing greener catalytic technologies. This trend aligns with the broader goals of reducing carbon footprints and minimizing waste in industrial chemical processes.
One of the primary objectives in dodecane catalysis research is to enhance selectivity and yield in hydrocarbon conversion reactions. Scientists aim to design catalysts that can precisely control the transformation of dodecane into desired products while minimizing unwanted side reactions. This pursuit has led to the exploration of novel catalyst materials, including nanostructured metals, zeolites, and metal-organic frameworks, each offering unique properties for manipulating dodecane's reactivity.
Another critical goal in this field is to improve the energy efficiency of catalytic processes involving dodecane. Researchers are investigating ways to lower reaction temperatures and pressures without compromising conversion rates or product quality. This objective is particularly relevant in the context of sustainable chemistry, where reducing energy consumption is paramount.
Furthermore, the study of dodecane catalysis aims to bridge the gap between fundamental research and practical applications. By understanding the mechanistic details of dodecane reactions on various catalytic surfaces, scientists hope to develop predictive models that can accelerate the discovery and optimization of new catalytic systems. This approach not only enhances our theoretical knowledge but also paves the way for more rational catalyst design in industrial settings.
In conclusion, the background and objectives of dodecane catalysis research reflect a dynamic and evolving field at the intersection of chemistry, materials science, and sustainable technology. As we continue to push the boundaries of catalytic science, dodecane remains a valuable tool for unlocking new possibilities in chemical transformations and advancing our pursuit of more efficient, environmentally friendly industrial processes.
Market Analysis for Dodecane-based Catalytic Processes
The market for dodecane-based catalytic processes has shown significant growth potential in recent years, driven by increasing demand for efficient and sustainable chemical production methods. Dodecane, a straight-chain alkane with twelve carbon atoms, serves as an important model compound for catalytic research, particularly in the petroleum and petrochemical industries.
The global market for catalytic processes involving dodecane is closely tied to the broader hydrocarbon processing industry. As of 2023, the global catalysts market was valued at over $30 billion, with a projected compound annual growth rate (CAGR) of 4.5% through 2028. Within this market, the segment for petroleum refining catalysts, which includes dodecane-based processes, accounts for a substantial portion, estimated at around 35% of the total market share.
Key drivers for the growth of dodecane-based catalytic processes include the increasing demand for cleaner fuels, stringent environmental regulations, and the need for more efficient conversion of heavy hydrocarbons into valuable products. The automotive industry's shift towards higher-performance and lower-emission engines has also contributed to the demand for improved catalytic processes involving model compounds like dodecane.
In the petrochemical sector, dodecane-based catalytic research has led to advancements in the production of specialty chemicals and polymers. This has opened up new market opportunities, particularly in the manufacturing of high-performance materials for aerospace, electronics, and automotive applications. The market for these specialty products is expected to grow at a CAGR of 5.5% over the next five years.
Geographically, North America and Europe currently dominate the market for advanced catalytic processes, including those involving dodecane. However, rapid industrialization and increasing investments in research and development in Asia-Pacific countries, particularly China and India, are expected to drive significant market growth in this region. The Asia-Pacific market for catalytic processes is projected to grow at a CAGR of 6.2% through 2028, outpacing other regions.
The market landscape for dodecane-based catalytic processes is characterized by intense competition and continuous innovation. Major players in this space include multinational chemical companies, specialized catalyst manufacturers, and research institutions. These entities are investing heavily in research and development to improve catalyst efficiency, selectivity, and longevity, which are crucial factors in the commercial viability of catalytic processes.
Looking ahead, the market for dodecane-based catalytic processes is expected to benefit from the growing focus on circular economy principles and sustainable chemistry. As industries seek to reduce waste and improve resource efficiency, catalytic processes that can effectively convert and upgrade hydrocarbons like dodecane are likely to see increased adoption. This trend is further supported by government initiatives and funding for green chemistry research in many countries.
The global market for catalytic processes involving dodecane is closely tied to the broader hydrocarbon processing industry. As of 2023, the global catalysts market was valued at over $30 billion, with a projected compound annual growth rate (CAGR) of 4.5% through 2028. Within this market, the segment for petroleum refining catalysts, which includes dodecane-based processes, accounts for a substantial portion, estimated at around 35% of the total market share.
Key drivers for the growth of dodecane-based catalytic processes include the increasing demand for cleaner fuels, stringent environmental regulations, and the need for more efficient conversion of heavy hydrocarbons into valuable products. The automotive industry's shift towards higher-performance and lower-emission engines has also contributed to the demand for improved catalytic processes involving model compounds like dodecane.
In the petrochemical sector, dodecane-based catalytic research has led to advancements in the production of specialty chemicals and polymers. This has opened up new market opportunities, particularly in the manufacturing of high-performance materials for aerospace, electronics, and automotive applications. The market for these specialty products is expected to grow at a CAGR of 5.5% over the next five years.
Geographically, North America and Europe currently dominate the market for advanced catalytic processes, including those involving dodecane. However, rapid industrialization and increasing investments in research and development in Asia-Pacific countries, particularly China and India, are expected to drive significant market growth in this region. The Asia-Pacific market for catalytic processes is projected to grow at a CAGR of 6.2% through 2028, outpacing other regions.
The market landscape for dodecane-based catalytic processes is characterized by intense competition and continuous innovation. Major players in this space include multinational chemical companies, specialized catalyst manufacturers, and research institutions. These entities are investing heavily in research and development to improve catalyst efficiency, selectivity, and longevity, which are crucial factors in the commercial viability of catalytic processes.
Looking ahead, the market for dodecane-based catalytic processes is expected to benefit from the growing focus on circular economy principles and sustainable chemistry. As industries seek to reduce waste and improve resource efficiency, catalytic processes that can effectively convert and upgrade hydrocarbons like dodecane are likely to see increased adoption. This trend is further supported by government initiatives and funding for green chemistry research in many countries.
Current Challenges in Dodecane Catalytic Research
Despite the significant advancements in dodecane catalytic research, several challenges persist in this field, hindering its full potential for industrial applications. One of the primary obstacles is the development of highly selective catalysts for specific reactions involving dodecane. The complex molecular structure of dodecane often leads to multiple reaction pathways, resulting in unwanted by-products and reduced efficiency.
Another major challenge lies in the stability of catalysts under the harsh reaction conditions typically required for dodecane conversion. High temperatures and pressures can cause catalyst deactivation through sintering, coking, or structural changes, necessitating frequent regeneration or replacement. This not only increases operational costs but also impacts the overall process efficiency.
The optimization of reaction kinetics and thermodynamics presents another significant hurdle. Researchers struggle to find the ideal balance between reaction rate and selectivity, particularly in processes such as hydrocracking and isomerization of dodecane. Achieving high conversion rates while maintaining product selectivity remains a key focus area for improvement.
Environmental concerns also pose challenges in dodecane catalytic research. The need for more sustainable and eco-friendly processes has led to increased efforts in developing green catalysts and reaction conditions. However, finding alternatives that match the efficiency of traditional catalytic systems while reducing environmental impact is proving to be a complex task.
Furthermore, the scalability of laboratory-proven catalytic systems to industrial-scale operations presents significant engineering challenges. Issues such as heat and mass transfer limitations, catalyst lifetime, and process integration become more pronounced at larger scales, requiring innovative reactor designs and process optimizations.
The heterogeneity of feedstocks in real-world applications adds another layer of complexity. Variations in dodecane purity and the presence of contaminants can significantly affect catalyst performance, necessitating the development of more robust and versatile catalytic systems capable of handling diverse feed compositions.
Lastly, the economic viability of dodecane catalytic processes remains a critical challenge. The high costs associated with catalyst development, production, and regeneration need to be balanced against the value of the products obtained. This economic pressure drives the need for catalysts with improved activity, selectivity, and longevity to enhance the overall process economics.
Another major challenge lies in the stability of catalysts under the harsh reaction conditions typically required for dodecane conversion. High temperatures and pressures can cause catalyst deactivation through sintering, coking, or structural changes, necessitating frequent regeneration or replacement. This not only increases operational costs but also impacts the overall process efficiency.
The optimization of reaction kinetics and thermodynamics presents another significant hurdle. Researchers struggle to find the ideal balance between reaction rate and selectivity, particularly in processes such as hydrocracking and isomerization of dodecane. Achieving high conversion rates while maintaining product selectivity remains a key focus area for improvement.
Environmental concerns also pose challenges in dodecane catalytic research. The need for more sustainable and eco-friendly processes has led to increased efforts in developing green catalysts and reaction conditions. However, finding alternatives that match the efficiency of traditional catalytic systems while reducing environmental impact is proving to be a complex task.
Furthermore, the scalability of laboratory-proven catalytic systems to industrial-scale operations presents significant engineering challenges. Issues such as heat and mass transfer limitations, catalyst lifetime, and process integration become more pronounced at larger scales, requiring innovative reactor designs and process optimizations.
The heterogeneity of feedstocks in real-world applications adds another layer of complexity. Variations in dodecane purity and the presence of contaminants can significantly affect catalyst performance, necessitating the development of more robust and versatile catalytic systems capable of handling diverse feed compositions.
Lastly, the economic viability of dodecane catalytic processes remains a critical challenge. The high costs associated with catalyst development, production, and regeneration need to be balanced against the value of the products obtained. This economic pressure drives the need for catalysts with improved activity, selectivity, and longevity to enhance the overall process economics.
Existing Dodecane Catalytic Solutions
01 Synthesis and purification of dodecane
Dodecane can be synthesized through various chemical processes, including catalytic hydrogenation of long-chain olefins or decarboxylation of fatty acids. Purification methods such as distillation or chromatography are often employed to obtain high-purity dodecane for industrial and research applications.- Synthesis and production of dodecane: Dodecane can be synthesized through various chemical processes, including the hydrogenation of long-chain alkenes or the Fischer-Tropsch process. It is also produced as a byproduct in petroleum refining. The synthesis methods often involve catalysts and specific reaction conditions to achieve high purity and yield.
- Applications in cosmetics and personal care products: Dodecane is used in cosmetics and personal care products as an emollient, solvent, and carrier for active ingredients. It can improve the texture and spreadability of formulations, enhance skin feel, and contribute to the stability of the product. Its low viscosity and non-greasy nature make it suitable for various cosmetic applications.
- Use in fuel and lubricant formulations: Dodecane is an important component in fuel and lubricant formulations. It can be used as a fuel additive to improve combustion efficiency and reduce emissions. In lubricants, it serves as a base oil or additive to enhance performance characteristics such as viscosity and thermal stability.
- Application in chemical and industrial processes: Dodecane finds applications in various chemical and industrial processes. It can be used as a solvent in organic synthesis, as a standard in chromatography, and as a heat transfer fluid in industrial applications. Its properties make it suitable for use in the production of polymers, resins, and other chemical compounds.
- Environmental and safety considerations: The use and handling of dodecane require consideration of environmental and safety aspects. It is important to implement proper storage, handling, and disposal procedures to minimize environmental impact and ensure worker safety. Research is ongoing to develop more sustainable production methods and to assess the long-term effects of dodecane on the environment and human health.
02 Use of dodecane in cosmetic and personal care products
Dodecane is utilized in cosmetic and personal care formulations as an emollient, solvent, or carrier for active ingredients. It can improve the texture, spreadability, and skin feel of various products such as creams, lotions, and hair care items.Expand Specific Solutions03 Application of dodecane in fuel and lubricant industries
Dodecane is an important component in the fuel and lubricant industries. It is used as a reference compound for diesel fuel testing, as well as a base oil in lubricant formulations. Its properties make it suitable for improving the performance and efficiency of various engines and machinery.Expand Specific Solutions04 Dodecane as a solvent in chemical processes
Dodecane serves as an effective solvent in various chemical processes, including extraction, separation, and reaction media. Its non-polar nature and low reactivity make it suitable for use in organic synthesis, analytical chemistry, and industrial applications.Expand Specific Solutions05 Environmental and safety considerations of dodecane
The use and handling of dodecane require careful consideration of environmental and safety aspects. This includes proper storage, transportation, and disposal methods to prevent environmental contamination. Safety measures such as appropriate ventilation and personal protective equipment are necessary when working with dodecane due to its flammability and potential health effects.Expand Specific Solutions
Key Players in Dodecane Catalytic Research
The competitive landscape for dodecane's role in catalytic research is characterized by a mature market with established players and ongoing innovation. The industry is in a growth phase, driven by increasing demand for efficient catalytic processes in various sectors. Market size is substantial, with major chemical and petrochemical companies investing heavily in research and development. Technologically, the field is moderately mature, with continuous advancements being made. Key players like BASF, Chevron Phillips Chemical, and China Petroleum & Chemical Corp. are at the forefront, leveraging their extensive R&D capabilities. Universities and research institutions, such as the Council of Scientific & Industrial Research and King Fahd University of Petroleum & Minerals, contribute significantly to advancing the technology through collaborative efforts with industry partners.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced catalytic processes utilizing dodecane for petrochemical research. Their approach involves using dodecane as a model compound for studying catalytic cracking and hydrocracking reactions. Sinopec's researchers have implemented a dual-function catalyst system, combining acidic and metallic sites, to enhance the conversion of dodecane into valuable lighter hydrocarbons[1]. This process achieves high selectivity towards desired products like propylene and butylene. Additionally, Sinopec has explored the use of zeolite-based catalysts modified with rare earth elements to improve the stability and activity in dodecane conversion[3]. Their research also extends to the application of dodecane in the development of more efficient and environmentally friendly lubricants for industrial machinery[5].
Strengths: Extensive experience in petrochemical processes, access to large-scale testing facilities, and strong integration with refinery operations. Weaknesses: Potential focus on traditional fossil fuel applications may limit exploration of alternative uses for dodecane.
BASF Corp.
Technical Solution: BASF Corp. has leveraged dodecane in its catalytic research to develop innovative solutions for the chemical industry. Their approach focuses on using dodecane as a key component in studying heterogeneous catalysis for alkane activation. BASF researchers have developed novel metal-organic framework (MOF) catalysts that show exceptional activity in the selective oxidation of dodecane to valuable oxygenates[2]. This process achieves high conversion rates and selectivity towards industrially important products like dodecanol and dodecanone. Furthermore, BASF has explored the use of dodecane in the development of more efficient Fischer-Tropsch catalysts, aiming to improve the production of synthetic fuels and chemicals[4]. Their research also extends to the application of dodecane-based model systems for studying catalyst deactivation mechanisms, leading to the design of more robust catalysts for long-term industrial use[6].
Strengths: Strong R&D capabilities, diverse chemical expertise, and global presence for technology implementation. Weaknesses: High research costs and potential challenges in scaling up novel catalytic processes.
Innovative Dodecane Catalysis Techniques
Composition of catalyst and solvent and catalysis processes using this composition
PatentInactiveUS7256152B2
Innovation
- A composition of at least one Bronsted acid dissolved in an ionic liquid with a non-aqueous, ionic nature serves as both a catalyst and solvent, enhancing acid catalysis activity and allowing for easier separation and recycling of the catalytic phase, thereby improving selectivity and reducing waste.
method for OBTAINING 2,6,8,12-TETRAACETIL-2,4,6,8,10,12-HEXAAZATETRACYCLO[5,5,0,03,11,05,9]DODECANE
PatentActiveRU2015130631A
Innovation
- Utilization of spent catalyst from a previous hydrogenation step, optimizing resource efficiency and reducing waste.
- Optimized reaction conditions, including specific temperature range and reactant-to-catalyst ratio, leading to improved product yield and purity.
- Simplified process with reduced duration, achieved through careful timing of hydrogen introduction and optimized reaction parameters.
Environmental Impact of Dodecane Catalysis
The environmental impact of dodecane catalysis is a critical consideration in the broader context of catalytic research and industrial applications. Dodecane, a hydrocarbon compound, plays a significant role in various catalytic processes, particularly in the petrochemical industry. However, its use and the associated catalytic reactions can have both positive and negative effects on the environment.
One of the primary environmental benefits of dodecane catalysis is its potential to improve the efficiency of chemical processes. By enhancing reaction rates and selectivity, catalytic systems involving dodecane can reduce energy consumption and minimize waste production. This increased efficiency translates to lower greenhouse gas emissions and a reduced carbon footprint for industrial operations.
Nevertheless, the production and use of dodecane in catalytic processes are not without environmental concerns. The extraction and refining of dodecane from fossil fuel sources contribute to carbon emissions and potential ecosystem disruption. Additionally, the release of volatile organic compounds (VOCs) during dodecane-based catalytic reactions can lead to air pollution and the formation of ground-level ozone, which has adverse effects on human health and vegetation.
Water pollution is another environmental risk associated with dodecane catalysis. Accidental spills or improper disposal of dodecane and its catalytic byproducts can contaminate water sources, affecting aquatic ecosystems and potentially entering the food chain. The persistence of dodecane in the environment and its potential for bioaccumulation further exacerbate these concerns.
On the other hand, research into dodecane catalysis has led to advancements in green chemistry and sustainable practices. Scientists are exploring bio-based sources of dodecane and developing more environmentally friendly catalytic systems that minimize waste and reduce the reliance on fossil fuels. These innovations have the potential to mitigate the negative environmental impacts while maintaining the benefits of dodecane-based catalytic processes.
The lifecycle assessment of dodecane catalysis reveals a complex interplay of environmental impacts. While the immediate effects of catalytic reactions may be controlled within industrial settings, the upstream and downstream consequences require careful consideration. This includes the environmental costs of dodecane production, transportation, and eventual disposal or recycling of catalysts and reaction products.
As environmental regulations become more stringent, industries utilizing dodecane catalysis are under pressure to adopt cleaner technologies and practices. This has spurred research into catalyst recovery and recycling methods, as well as the development of alternative catalytic systems that can achieve similar or superior performance with reduced environmental impact.
In conclusion, the environmental impact of dodecane catalysis is multifaceted, encompassing both potential benefits and risks. As research in this field progresses, the focus on sustainable practices and green chemistry principles is likely to drive innovations that balance catalytic efficiency with environmental stewardship.
One of the primary environmental benefits of dodecane catalysis is its potential to improve the efficiency of chemical processes. By enhancing reaction rates and selectivity, catalytic systems involving dodecane can reduce energy consumption and minimize waste production. This increased efficiency translates to lower greenhouse gas emissions and a reduced carbon footprint for industrial operations.
Nevertheless, the production and use of dodecane in catalytic processes are not without environmental concerns. The extraction and refining of dodecane from fossil fuel sources contribute to carbon emissions and potential ecosystem disruption. Additionally, the release of volatile organic compounds (VOCs) during dodecane-based catalytic reactions can lead to air pollution and the formation of ground-level ozone, which has adverse effects on human health and vegetation.
Water pollution is another environmental risk associated with dodecane catalysis. Accidental spills or improper disposal of dodecane and its catalytic byproducts can contaminate water sources, affecting aquatic ecosystems and potentially entering the food chain. The persistence of dodecane in the environment and its potential for bioaccumulation further exacerbate these concerns.
On the other hand, research into dodecane catalysis has led to advancements in green chemistry and sustainable practices. Scientists are exploring bio-based sources of dodecane and developing more environmentally friendly catalytic systems that minimize waste and reduce the reliance on fossil fuels. These innovations have the potential to mitigate the negative environmental impacts while maintaining the benefits of dodecane-based catalytic processes.
The lifecycle assessment of dodecane catalysis reveals a complex interplay of environmental impacts. While the immediate effects of catalytic reactions may be controlled within industrial settings, the upstream and downstream consequences require careful consideration. This includes the environmental costs of dodecane production, transportation, and eventual disposal or recycling of catalysts and reaction products.
As environmental regulations become more stringent, industries utilizing dodecane catalysis are under pressure to adopt cleaner technologies and practices. This has spurred research into catalyst recovery and recycling methods, as well as the development of alternative catalytic systems that can achieve similar or superior performance with reduced environmental impact.
In conclusion, the environmental impact of dodecane catalysis is multifaceted, encompassing both potential benefits and risks. As research in this field progresses, the focus on sustainable practices and green chemistry principles is likely to drive innovations that balance catalytic efficiency with environmental stewardship.
Regulatory Framework for Catalytic Processes
The regulatory framework for catalytic processes involving dodecane plays a crucial role in ensuring safety, environmental protection, and quality control in research and industrial applications. These regulations are designed to address the unique challenges posed by the use of dodecane as a catalyst or substrate in various chemical reactions.
At the international level, organizations such as the International Organization for Standardization (ISO) and the Organisation for Economic Co-operation and Development (OECD) have established guidelines for the handling and use of hydrocarbons in catalytic processes. These guidelines often serve as a foundation for national and regional regulations, providing a harmonized approach to safety and environmental standards.
In the United States, the Environmental Protection Agency (EPA) and the Occupational Safety and Health Administration (OSHA) are the primary regulatory bodies overseeing catalytic processes involving dodecane. The EPA's Toxic Substances Control Act (TSCA) regulates the production, importation, and use of chemical substances, including dodecane, in catalytic research. OSHA's Hazard Communication Standard ensures that researchers and workers are informed about the potential hazards associated with handling dodecane and other chemicals used in catalytic processes.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to the use of dodecane in catalytic research. REACH requires companies to register chemical substances and provide safety data, ensuring a comprehensive approach to chemical management and risk assessment.
Specific regulations often address the storage, handling, and disposal of dodecane and related catalytic materials. These may include requirements for proper ventilation, personal protective equipment, and containment measures to prevent environmental contamination. Additionally, regulations may mandate regular monitoring and reporting of emissions and waste products generated during catalytic processes involving dodecane.
Research institutions and industrial facilities working with dodecane in catalytic processes must also comply with local and regional regulations. These may include zoning laws, fire safety codes, and waste management regulations specific to the jurisdiction in which the research or production is conducted.
As the field of catalytic research evolves, regulatory frameworks are continually updated to address new challenges and technologies. This includes the development of regulations for emerging areas such as nanocatalysis and biocatalysis, which may involve the use of dodecane in novel applications. Researchers and industry professionals must stay informed about these regulatory changes to ensure compliance and maintain the highest standards of safety and environmental stewardship in their work with dodecane and related catalytic processes.
At the international level, organizations such as the International Organization for Standardization (ISO) and the Organisation for Economic Co-operation and Development (OECD) have established guidelines for the handling and use of hydrocarbons in catalytic processes. These guidelines often serve as a foundation for national and regional regulations, providing a harmonized approach to safety and environmental standards.
In the United States, the Environmental Protection Agency (EPA) and the Occupational Safety and Health Administration (OSHA) are the primary regulatory bodies overseeing catalytic processes involving dodecane. The EPA's Toxic Substances Control Act (TSCA) regulates the production, importation, and use of chemical substances, including dodecane, in catalytic research. OSHA's Hazard Communication Standard ensures that researchers and workers are informed about the potential hazards associated with handling dodecane and other chemicals used in catalytic processes.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to the use of dodecane in catalytic research. REACH requires companies to register chemical substances and provide safety data, ensuring a comprehensive approach to chemical management and risk assessment.
Specific regulations often address the storage, handling, and disposal of dodecane and related catalytic materials. These may include requirements for proper ventilation, personal protective equipment, and containment measures to prevent environmental contamination. Additionally, regulations may mandate regular monitoring and reporting of emissions and waste products generated during catalytic processes involving dodecane.
Research institutions and industrial facilities working with dodecane in catalytic processes must also comply with local and regional regulations. These may include zoning laws, fire safety codes, and waste management regulations specific to the jurisdiction in which the research or production is conducted.
As the field of catalytic research evolves, regulatory frameworks are continually updated to address new challenges and technologies. This includes the development of regulations for emerging areas such as nanocatalysis and biocatalysis, which may involve the use of dodecane in novel applications. Researchers and industry professionals must stay informed about these regulatory changes to ensure compliance and maintain the highest standards of safety and environmental stewardship in their work with dodecane and related catalytic processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!