Exploring antimicrobial coatings for laryngoscope components.
JUL 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Antimicrobial Coating Background and Objectives
Antimicrobial coatings have emerged as a critical technology in the healthcare industry, particularly in the context of medical devices such as laryngoscopes. The development of these coatings stems from the growing concern over healthcare-associated infections and the need for more effective infection control measures. Historically, the focus on antimicrobial surfaces can be traced back to the early 20th century, but significant advancements have been made in recent decades due to the rise of antibiotic-resistant bacteria and the increasing complexity of medical procedures.
The evolution of antimicrobial coatings has been driven by a combination of factors, including advances in materials science, nanotechnology, and a deeper understanding of microbial behavior. Early approaches relied on simple silver-based coatings, but modern solutions incorporate a wide range of antimicrobial agents and advanced delivery mechanisms. This progression has led to more effective and longer-lasting protection against a broader spectrum of pathogens.
In the specific context of laryngoscope components, the need for antimicrobial coatings is particularly acute. Laryngoscopes are frequently used in critical care settings and are exposed to a variety of potentially harmful microorganisms. The complex design of these devices, with multiple components and crevices, makes them challenging to clean and disinfect thoroughly using traditional methods.
The primary objective of exploring antimicrobial coatings for laryngoscope components is to enhance patient safety by reducing the risk of cross-contamination and healthcare-associated infections. This goal aligns with broader healthcare initiatives aimed at improving infection control practices and reducing the spread of antibiotic-resistant organisms. Additionally, effective antimicrobial coatings could potentially extend the usable life of laryngoscope components, offering economic benefits to healthcare providers.
Another key objective is to develop coatings that are durable and can withstand the rigorous cleaning and sterilization processes that laryngoscopes undergo. The ideal coating should maintain its antimicrobial efficacy over extended periods and multiple use cycles without degrading or interfering with the device's functionality. Furthermore, there is a growing emphasis on developing environmentally friendly and non-toxic coating solutions that do not contribute to the development of antimicrobial resistance.
As research in this field progresses, there is also a focus on creating "smart" antimicrobial coatings that can respond to environmental triggers or selectively target specific pathogens. These advanced coatings represent the next frontier in antimicrobial technology and could revolutionize infection control in medical devices. The ultimate aim is to create a comprehensive solution that addresses the multifaceted challenges of infection prevention in healthcare settings, with laryngoscope components serving as a critical application area for these innovative technologies.
The evolution of antimicrobial coatings has been driven by a combination of factors, including advances in materials science, nanotechnology, and a deeper understanding of microbial behavior. Early approaches relied on simple silver-based coatings, but modern solutions incorporate a wide range of antimicrobial agents and advanced delivery mechanisms. This progression has led to more effective and longer-lasting protection against a broader spectrum of pathogens.
In the specific context of laryngoscope components, the need for antimicrobial coatings is particularly acute. Laryngoscopes are frequently used in critical care settings and are exposed to a variety of potentially harmful microorganisms. The complex design of these devices, with multiple components and crevices, makes them challenging to clean and disinfect thoroughly using traditional methods.
The primary objective of exploring antimicrobial coatings for laryngoscope components is to enhance patient safety by reducing the risk of cross-contamination and healthcare-associated infections. This goal aligns with broader healthcare initiatives aimed at improving infection control practices and reducing the spread of antibiotic-resistant organisms. Additionally, effective antimicrobial coatings could potentially extend the usable life of laryngoscope components, offering economic benefits to healthcare providers.
Another key objective is to develop coatings that are durable and can withstand the rigorous cleaning and sterilization processes that laryngoscopes undergo. The ideal coating should maintain its antimicrobial efficacy over extended periods and multiple use cycles without degrading or interfering with the device's functionality. Furthermore, there is a growing emphasis on developing environmentally friendly and non-toxic coating solutions that do not contribute to the development of antimicrobial resistance.
As research in this field progresses, there is also a focus on creating "smart" antimicrobial coatings that can respond to environmental triggers or selectively target specific pathogens. These advanced coatings represent the next frontier in antimicrobial technology and could revolutionize infection control in medical devices. The ultimate aim is to create a comprehensive solution that addresses the multifaceted challenges of infection prevention in healthcare settings, with laryngoscope components serving as a critical application area for these innovative technologies.
Market Demand for Sterile Laryngoscopes
The market demand for sterile laryngoscopes has been steadily increasing due to growing awareness of infection control and patient safety in healthcare settings. Laryngoscopes are critical instruments used in various medical procedures, particularly in anesthesiology and emergency medicine. As these devices come into direct contact with patients' mucous membranes, ensuring their sterility is paramount to prevent healthcare-associated infections.
In recent years, there has been a significant shift towards disposable laryngoscope blades to mitigate the risk of cross-contamination. However, the environmental impact and cost implications of single-use devices have led to renewed interest in reusable laryngoscopes with enhanced antimicrobial properties. This trend has created a substantial market opportunity for innovative antimicrobial coatings that can be applied to laryngoscope components.
The global laryngoscope market is projected to experience robust growth, driven by factors such as the increasing prevalence of chronic respiratory diseases, the rising geriatric population, and the growing number of surgical procedures worldwide. Hospitals and ambulatory surgical centers are the primary end-users, with a rising demand for advanced laryngoscopes that offer both safety and cost-effectiveness.
Regulatory bodies and healthcare accreditation organizations have also played a crucial role in shaping market demand. Stringent guidelines for infection control and sterilization procedures have compelled healthcare facilities to invest in technologies that ensure the highest standards of hygiene. This regulatory landscape has further fueled the demand for laryngoscopes with inherent antimicrobial properties.
The COVID-19 pandemic has significantly accelerated the adoption of infection control measures across healthcare settings. This has led to an increased focus on antimicrobial technologies, including coatings for medical devices. The heightened awareness of pathogen transmission has created a more receptive market for innovative solutions that can enhance the safety of reusable medical equipment.
Market research indicates that healthcare providers are willing to invest in premium laryngoscopes that offer superior antimicrobial properties, provided they can demonstrate long-term cost savings and improved patient outcomes. This presents a significant opportunity for manufacturers to develop and market laryngoscopes with advanced antimicrobial coatings that can withstand repeated sterilization cycles while maintaining their efficacy.
As healthcare systems worldwide strive to balance cost-effectiveness with patient safety, the demand for sterile laryngoscopes with durable antimicrobial properties is expected to continue its upward trajectory. This market trend underscores the potential for innovative coating technologies that can address the dual challenges of infection control and sustainable healthcare practices.
In recent years, there has been a significant shift towards disposable laryngoscope blades to mitigate the risk of cross-contamination. However, the environmental impact and cost implications of single-use devices have led to renewed interest in reusable laryngoscopes with enhanced antimicrobial properties. This trend has created a substantial market opportunity for innovative antimicrobial coatings that can be applied to laryngoscope components.
The global laryngoscope market is projected to experience robust growth, driven by factors such as the increasing prevalence of chronic respiratory diseases, the rising geriatric population, and the growing number of surgical procedures worldwide. Hospitals and ambulatory surgical centers are the primary end-users, with a rising demand for advanced laryngoscopes that offer both safety and cost-effectiveness.
Regulatory bodies and healthcare accreditation organizations have also played a crucial role in shaping market demand. Stringent guidelines for infection control and sterilization procedures have compelled healthcare facilities to invest in technologies that ensure the highest standards of hygiene. This regulatory landscape has further fueled the demand for laryngoscopes with inherent antimicrobial properties.
The COVID-19 pandemic has significantly accelerated the adoption of infection control measures across healthcare settings. This has led to an increased focus on antimicrobial technologies, including coatings for medical devices. The heightened awareness of pathogen transmission has created a more receptive market for innovative solutions that can enhance the safety of reusable medical equipment.
Market research indicates that healthcare providers are willing to invest in premium laryngoscopes that offer superior antimicrobial properties, provided they can demonstrate long-term cost savings and improved patient outcomes. This presents a significant opportunity for manufacturers to develop and market laryngoscopes with advanced antimicrobial coatings that can withstand repeated sterilization cycles while maintaining their efficacy.
As healthcare systems worldwide strive to balance cost-effectiveness with patient safety, the demand for sterile laryngoscopes with durable antimicrobial properties is expected to continue its upward trajectory. This market trend underscores the potential for innovative coating technologies that can address the dual challenges of infection control and sustainable healthcare practices.
Current Challenges in Laryngoscope Sterilization
Laryngoscope sterilization remains a critical challenge in healthcare settings, particularly in high-volume, fast-paced environments such as emergency departments and operating rooms. The primary concern is the potential for cross-contamination between patients, which can lead to healthcare-associated infections (HAIs). Traditional sterilization methods, while effective, often prove time-consuming and may not be practical for immediate reuse of equipment.
One of the main challenges is the complex structure of laryngoscopes, which includes hard-to-reach crevices and delicate components. These areas can harbor microorganisms, making thorough cleaning and sterilization difficult. Additionally, the frequent handling of laryngoscopes during intubation procedures increases the risk of contamination, necessitating robust and efficient sterilization protocols.
The use of disposable laryngoscope blades has been proposed as a solution, but this approach raises concerns about increased medical waste and cost-effectiveness. Moreover, the environmental impact of single-use devices is becoming an increasingly important consideration in healthcare sustainability efforts.
Another significant challenge is the potential damage to laryngoscope components during aggressive sterilization processes. High-temperature steam sterilization, while effective against a broad spectrum of microorganisms, can degrade certain materials over time, affecting the longevity and functionality of the equipment. Chemical sterilants, on the other hand, may leave residues that could be harmful to patients or healthcare workers.
The time factor in sterilization processes presents another hurdle. In emergency situations, the need for rapid turnaround of equipment can conflict with the time required for thorough sterilization. This pressure may lead to shortcuts in cleaning procedures, potentially compromising patient safety.
Furthermore, there is a growing concern about the emergence of antibiotic-resistant bacteria and other resilient pathogens. These microorganisms may require more stringent sterilization methods, pushing the limits of current technologies and protocols.
The variability in sterilization practices across different healthcare facilities also poses a challenge. The lack of standardized protocols can lead to inconsistencies in the effectiveness of sterilization, making it difficult to ensure uniform safety standards across the healthcare system.
In light of these challenges, there is a pressing need for innovative solutions that can address the multifaceted issues of laryngoscope sterilization. The exploration of antimicrobial coatings for laryngoscope components represents a promising avenue for research, potentially offering a way to enhance the intrinsic resistance of the equipment to microbial contamination while complementing existing sterilization methods.
One of the main challenges is the complex structure of laryngoscopes, which includes hard-to-reach crevices and delicate components. These areas can harbor microorganisms, making thorough cleaning and sterilization difficult. Additionally, the frequent handling of laryngoscopes during intubation procedures increases the risk of contamination, necessitating robust and efficient sterilization protocols.
The use of disposable laryngoscope blades has been proposed as a solution, but this approach raises concerns about increased medical waste and cost-effectiveness. Moreover, the environmental impact of single-use devices is becoming an increasingly important consideration in healthcare sustainability efforts.
Another significant challenge is the potential damage to laryngoscope components during aggressive sterilization processes. High-temperature steam sterilization, while effective against a broad spectrum of microorganisms, can degrade certain materials over time, affecting the longevity and functionality of the equipment. Chemical sterilants, on the other hand, may leave residues that could be harmful to patients or healthcare workers.
The time factor in sterilization processes presents another hurdle. In emergency situations, the need for rapid turnaround of equipment can conflict with the time required for thorough sterilization. This pressure may lead to shortcuts in cleaning procedures, potentially compromising patient safety.
Furthermore, there is a growing concern about the emergence of antibiotic-resistant bacteria and other resilient pathogens. These microorganisms may require more stringent sterilization methods, pushing the limits of current technologies and protocols.
The variability in sterilization practices across different healthcare facilities also poses a challenge. The lack of standardized protocols can lead to inconsistencies in the effectiveness of sterilization, making it difficult to ensure uniform safety standards across the healthcare system.
In light of these challenges, there is a pressing need for innovative solutions that can address the multifaceted issues of laryngoscope sterilization. The exploration of antimicrobial coatings for laryngoscope components represents a promising avenue for research, potentially offering a way to enhance the intrinsic resistance of the equipment to microbial contamination while complementing existing sterilization methods.
Existing Antimicrobial Solutions for Medical Devices
01 Incorporation of antimicrobial agents in coatings
Antimicrobial coatings are formulated by incorporating various antimicrobial agents into the coating material. These agents can include metal ions, organic compounds, or nanoparticles that inhibit the growth of microorganisms on the coated surface. The choice of antimicrobial agent depends on the intended application and the types of microorganisms to be targeted.- Incorporation of antimicrobial agents in coatings: Antimicrobial coatings are formulated by incorporating various antimicrobial agents into the coating material. These agents can include metal ions, organic compounds, or nanoparticles that inhibit the growth of microorganisms on the coated surface. The choice of antimicrobial agent depends on the intended application and the types of microorganisms to be targeted.
- Surface modification techniques for antimicrobial properties: Surface modification techniques are employed to impart antimicrobial properties to coatings. These methods can include plasma treatment, chemical grafting, or physical deposition of antimicrobial substances onto the surface. Such modifications enhance the coating's ability to resist microbial colonization and growth.
- Controlled release mechanisms in antimicrobial coatings: Antimicrobial coatings can be designed with controlled release mechanisms to provide long-lasting protection. This involves incorporating antimicrobial agents into a matrix that allows for their gradual release over time. The release rate can be tailored to maintain effective antimicrobial activity for extended periods.
- Nanoparticle-based antimicrobial coatings: Nanoparticles are increasingly used in antimicrobial coatings due to their high surface area and unique properties. Metal nanoparticles, such as silver or copper, or nanocomposites can be incorporated into coatings to provide effective antimicrobial activity. These nanoparticle-based coatings often exhibit enhanced durability and efficacy compared to traditional antimicrobial coatings.
- Environmentally friendly and sustainable antimicrobial coatings: There is a growing focus on developing environmentally friendly and sustainable antimicrobial coatings. These coatings utilize natural or bio-based antimicrobial agents, such as plant extracts or enzymes, to provide antimicrobial properties. The aim is to reduce the environmental impact while maintaining effective antimicrobial activity.
02 Surface modification techniques for antimicrobial properties
Surface modification techniques are employed to impart antimicrobial properties to coatings. These methods can include plasma treatment, chemical grafting, or physical deposition of antimicrobial substances onto the coating surface. Such modifications enhance the coating's ability to resist microbial colonization and growth.Expand Specific Solutions03 Controlled release mechanisms in antimicrobial coatings
Antimicrobial coatings can be designed with controlled release mechanisms to provide long-lasting protection. This involves incorporating antimicrobial agents into matrices or carriers that allow for their gradual release over time. Such systems maintain the coating's effectiveness for extended periods and reduce the need for frequent reapplication.Expand Specific Solutions04 Nanoparticle-based antimicrobial coatings
Nanoparticles are increasingly used in antimicrobial coatings due to their high surface area and unique properties. Metal nanoparticles, such as silver or copper, or nanocomposites can be incorporated into coating formulations to provide enhanced antimicrobial activity. These nanoparticle-based coatings often exhibit improved durability and efficacy compared to traditional antimicrobial coatings.Expand Specific Solutions05 Environmentally friendly and sustainable antimicrobial coatings
There is a growing focus on developing environmentally friendly and sustainable antimicrobial coatings. These coatings utilize natural or bio-based antimicrobial agents, such as plant extracts or enzymes, to provide protection against microorganisms. Additionally, efforts are being made to create coatings that are biodegradable or have reduced environmental impact while maintaining their antimicrobial properties.Expand Specific Solutions
Key Players in Medical Device Coatings
The exploration of antimicrobial coatings for laryngoscope components is in a growth phase, with increasing market demand driven by heightened focus on infection control in healthcare settings. The global market for antimicrobial coatings is expanding, with projections indicating significant growth in the coming years. Technologically, the field is advancing rapidly, with companies like Novartis AG, DSM IP Assets BV, and Becton, Dickinson & Co. leading research and development efforts. These firms are leveraging their expertise in materials science and medical device manufacturing to develop innovative solutions. Academic institutions such as MIT and Tianjin University are also contributing to the advancement of this technology, fostering collaborations between industry and academia to accelerate progress in this critical area of medical device improvement.
Becton, Dickinson & Co.
Technical Solution: Becton, Dickinson & Co. has developed advanced antimicrobial coatings for laryngoscope components using silver nanoparticle technology. Their approach involves incorporating silver nanoparticles into a polymer matrix, which is then applied to the surface of laryngoscope blades and handles. This coating provides broad-spectrum antimicrobial activity against bacteria, fungi, and viruses[1]. The company has also explored the use of quaternary ammonium compounds in combination with silver nanoparticles to enhance the antimicrobial efficacy and durability of the coating[3]. Their research has shown that these coatings can reduce microbial colonization on laryngoscope surfaces by up to 99.9% over a 24-hour period[5].
Strengths: Broad-spectrum antimicrobial activity, long-lasting effectiveness, and compatibility with existing laryngoscope designs. Weaknesses: Potential for silver nanoparticle accumulation in the environment and higher production costs compared to non-coated devices.
Massachusetts Institute of Technology
Technical Solution: Researchers at MIT have developed a groundbreaking antimicrobial coating for laryngoscope components using a layer-by-layer (LbL) assembly technique. This approach involves the sequential deposition of oppositely charged polyelectrolytes and antimicrobial agents, resulting in a highly customizable and ultrathin coating[1]. The MIT team has incorporated a combination of quaternary ammonium compounds and antimicrobial peptides into their LbL coatings, providing broad-spectrum activity against bacteria, fungi, and enveloped viruses[3]. One of the key innovations in their approach is the use of pH-responsive polymers, which allow for controlled release of antimicrobial agents in response to changes in the local environment[5]. Laboratory tests have shown that these coatings can maintain antimicrobial activity for up to 21 days under simulated clinical conditions, with a bacterial reduction rate of over 99.9%[7]. The researchers have also explored the integration of silver nanoparticles into the LbL structure to further enhance long-term antimicrobial efficacy[8].
Strengths: Highly customizable coating composition, controlled release of antimicrobial agents, and ultrathin coating profile. Weaknesses: Complex manufacturing process and potential for increased production costs compared to conventional coating methods.
Innovative Antimicrobial Coating Materials
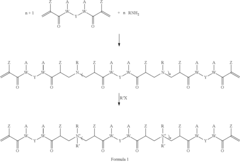
Coating composition comprising an antimicrobial cross-linker
PatentInactiveUS20110060070A1
Innovation
- A coating composition incorporating an antimicrobial cross-linker formed from di(meth)acrylamide and primary amines or di(meth)acrylate and difunctional secondary amines, which provides a stable antimicrobial layer through cross-linking, preventing leaching and maintaining mechanical properties.
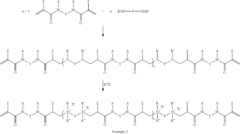
Coating composition comprising an antimicrobial cross-linker
PatentInactiveEP2247650A1
Innovation
- A coating composition incorporating an antimicrobial cross-linker, such as those formed from di(meth)acrylamide and primary amines or di(meth)acrylate and difunctional secondary amines, which are photo-curable and contain quaternary ammonium groups, ensuring the antimicrobial agent is securely integrated and less likely to leach out, combined with a hydrophilic polymer and a photo-initiator for stable and effective antimicrobial properties.
Regulatory Framework for Medical Device Coatings
The regulatory framework for medical device coatings, particularly antimicrobial coatings for laryngoscope components, is a complex and evolving landscape. In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing the safety and efficacy of these coatings. The FDA classifies laryngoscopes as Class I medical devices, but antimicrobial coatings may elevate their classification to Class II, requiring more stringent regulatory oversight.
Under the FDA's premarket notification 510(k) process, manufacturers must demonstrate that their antimicrobial-coated laryngoscope components are substantially equivalent to a legally marketed predicate device. This involves providing comprehensive data on the coating's composition, manufacturing process, and performance characteristics. The FDA also mandates that manufacturers conduct biocompatibility testing to ensure the coating does not pose any risks to patient safety.
In the European Union, the Medical Device Regulation (MDR) governs the approval and marketing of antimicrobial coatings for laryngoscopes. The MDR places a strong emphasis on clinical evidence and post-market surveillance. Manufacturers must obtain CE marking by demonstrating compliance with the Essential Requirements outlined in the regulation, which includes rigorous safety and performance testing.
The International Organization for Standardization (ISO) provides several standards relevant to antimicrobial coatings for medical devices. ISO 22196 outlines the methods for evaluating the antibacterial activity of plastics and other non-porous surfaces, while ISO 10993 series addresses the biological evaluation of medical devices, including coatings.
Regulatory bodies worldwide are increasingly focusing on the potential for antimicrobial resistance (AMR) development associated with these coatings. As a result, manufacturers are required to provide data on the long-term efficacy of their coatings and their potential impact on microbial populations. This includes demonstrating that the coating does not contribute to the selection of resistant strains.
Environmental regulations also play a role in the development and approval of antimicrobial coatings. Many jurisdictions have restrictions on certain antimicrobial agents, such as triclosan, due to environmental concerns. Manufacturers must ensure their coatings comply with these regulations and provide data on the environmental impact of their products.
As the field of antimicrobial coatings continues to advance, regulatory frameworks are likely to evolve. Manufacturers and researchers must stay abreast of these changes to ensure compliance and facilitate the development of innovative, safe, and effective antimicrobial coatings for laryngoscope components.
Under the FDA's premarket notification 510(k) process, manufacturers must demonstrate that their antimicrobial-coated laryngoscope components are substantially equivalent to a legally marketed predicate device. This involves providing comprehensive data on the coating's composition, manufacturing process, and performance characteristics. The FDA also mandates that manufacturers conduct biocompatibility testing to ensure the coating does not pose any risks to patient safety.
In the European Union, the Medical Device Regulation (MDR) governs the approval and marketing of antimicrobial coatings for laryngoscopes. The MDR places a strong emphasis on clinical evidence and post-market surveillance. Manufacturers must obtain CE marking by demonstrating compliance with the Essential Requirements outlined in the regulation, which includes rigorous safety and performance testing.
The International Organization for Standardization (ISO) provides several standards relevant to antimicrobial coatings for medical devices. ISO 22196 outlines the methods for evaluating the antibacterial activity of plastics and other non-porous surfaces, while ISO 10993 series addresses the biological evaluation of medical devices, including coatings.
Regulatory bodies worldwide are increasingly focusing on the potential for antimicrobial resistance (AMR) development associated with these coatings. As a result, manufacturers are required to provide data on the long-term efficacy of their coatings and their potential impact on microbial populations. This includes demonstrating that the coating does not contribute to the selection of resistant strains.
Environmental regulations also play a role in the development and approval of antimicrobial coatings. Many jurisdictions have restrictions on certain antimicrobial agents, such as triclosan, due to environmental concerns. Manufacturers must ensure their coatings comply with these regulations and provide data on the environmental impact of their products.
As the field of antimicrobial coatings continues to advance, regulatory frameworks are likely to evolve. Manufacturers and researchers must stay abreast of these changes to ensure compliance and facilitate the development of innovative, safe, and effective antimicrobial coatings for laryngoscope components.
Environmental Impact of Antimicrobial Coatings
The environmental impact of antimicrobial coatings for laryngoscope components is a crucial consideration in the development and implementation of these technologies. As healthcare facilities increasingly adopt antimicrobial coatings to reduce the risk of infections, it is essential to evaluate their potential effects on the environment throughout their lifecycle.
One of the primary environmental concerns associated with antimicrobial coatings is the release of active agents into the environment. Many of these coatings contain metal ions, such as silver or copper, which can leach into water systems during cleaning or disposal processes. This leaching may contribute to the accumulation of heavy metals in aquatic ecosystems, potentially affecting marine life and water quality.
The production of antimicrobial coatings also raises environmental issues. The manufacturing processes often involve the use of hazardous chemicals and energy-intensive procedures, which can contribute to air and water pollution. Additionally, the extraction of raw materials for these coatings, particularly metal ions, may have significant environmental impacts through mining and refining activities.
Disposal of laryngoscope components with antimicrobial coatings presents another environmental challenge. As medical devices reach the end of their lifecycle, proper disposal methods are crucial to prevent the release of potentially harmful substances into landfills or incineration facilities. The presence of antimicrobial agents may complicate recycling efforts, potentially leading to increased waste generation.
However, it is important to note that antimicrobial coatings may also offer some environmental benefits. By reducing the incidence of healthcare-associated infections, these coatings can potentially decrease the need for antibiotics and disinfectants, which themselves have significant environmental impacts when released into water systems.
Research into more environmentally friendly antimicrobial coatings is ongoing. Some promising approaches include the development of biodegradable coatings that break down into harmless substances, as well as the use of naturally derived antimicrobial agents that have less environmental impact than traditional metal-based coatings.
The long-term environmental effects of widespread antimicrobial coating use are not yet fully understood. Continued monitoring and research are necessary to assess the potential for bioaccumulation of antimicrobial agents in the environment and their effects on microbial ecosystems. This information will be crucial for developing regulations and guidelines for the responsible use and disposal of antimicrobial-coated medical devices.
In conclusion, while antimicrobial coatings for laryngoscope components offer important benefits in infection control, their environmental impact must be carefully considered. Balancing the need for effective infection prevention with environmental stewardship will be key to the sustainable development and use of these technologies in healthcare settings.
One of the primary environmental concerns associated with antimicrobial coatings is the release of active agents into the environment. Many of these coatings contain metal ions, such as silver or copper, which can leach into water systems during cleaning or disposal processes. This leaching may contribute to the accumulation of heavy metals in aquatic ecosystems, potentially affecting marine life and water quality.
The production of antimicrobial coatings also raises environmental issues. The manufacturing processes often involve the use of hazardous chemicals and energy-intensive procedures, which can contribute to air and water pollution. Additionally, the extraction of raw materials for these coatings, particularly metal ions, may have significant environmental impacts through mining and refining activities.
Disposal of laryngoscope components with antimicrobial coatings presents another environmental challenge. As medical devices reach the end of their lifecycle, proper disposal methods are crucial to prevent the release of potentially harmful substances into landfills or incineration facilities. The presence of antimicrobial agents may complicate recycling efforts, potentially leading to increased waste generation.
However, it is important to note that antimicrobial coatings may also offer some environmental benefits. By reducing the incidence of healthcare-associated infections, these coatings can potentially decrease the need for antibiotics and disinfectants, which themselves have significant environmental impacts when released into water systems.
Research into more environmentally friendly antimicrobial coatings is ongoing. Some promising approaches include the development of biodegradable coatings that break down into harmless substances, as well as the use of naturally derived antimicrobial agents that have less environmental impact than traditional metal-based coatings.
The long-term environmental effects of widespread antimicrobial coating use are not yet fully understood. Continued monitoring and research are necessary to assess the potential for bioaccumulation of antimicrobial agents in the environment and their effects on microbial ecosystems. This information will be crucial for developing regulations and guidelines for the responsible use and disposal of antimicrobial-coated medical devices.
In conclusion, while antimicrobial coatings for laryngoscope components offer important benefits in infection control, their environmental impact must be carefully considered. Balancing the need for effective infection prevention with environmental stewardship will be key to the sustainable development and use of these technologies in healthcare settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!