Gel Electrophoresis for Biomarker Discovery: Latest Insights
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gel Electrophoresis Evolution and Objectives
Gel electrophoresis has been a cornerstone technique in molecular biology since its inception in the 1930s. Initially developed for protein separation, it has evolved significantly over the decades to become an indispensable tool in biomarker discovery. The technique's evolution has been marked by several key milestones, each enhancing its precision, efficiency, and applicability in various fields of biological research.
In the 1950s, the introduction of polyacrylamide gels revolutionized the field, allowing for better resolution of proteins based on their molecular weight. This advancement paved the way for more sophisticated applications in protein analysis and biomarker identification. The 1970s saw the development of two-dimensional gel electrophoresis, which dramatically increased the resolving power of the technique, enabling researchers to separate complex protein mixtures with unprecedented clarity.
The advent of capillary electrophoresis in the 1980s further expanded the capabilities of gel electrophoresis, offering higher resolution, faster analysis times, and the ability to work with smaller sample volumes. This innovation proved particularly valuable in the emerging field of proteomics and biomarker discovery, where sample quantity is often limited.
Recent years have witnessed the integration of gel electrophoresis with advanced detection methods, such as mass spectrometry and fluorescence imaging. These combinations have significantly enhanced the sensitivity and specificity of biomarker detection, allowing researchers to identify and quantify proteins present in extremely low concentrations.
The primary objectives of gel electrophoresis in biomarker discovery are multifaceted. Firstly, it aims to provide a high-resolution separation of complex biological samples, enabling the identification of potential biomarkers that may be indicative of specific diseases or physiological states. Secondly, it seeks to offer reproducible and quantifiable results, crucial for validating biomarkers across different studies and laboratories.
Another key objective is to improve the detection of low-abundance proteins, which often represent the most promising biomarkers. This goal has driven the development of more sensitive staining techniques and the integration of pre-fractionation methods to enrich samples for proteins of interest.
Furthermore, gel electrophoresis techniques are continually evolving to address the challenges of analyzing post-translational modifications, which play a critical role in protein function and can serve as important biomarkers. The ability to detect and characterize these modifications is becoming increasingly important in the field of personalized medicine and targeted therapies.
As we look to the future, the objectives of gel electrophoresis in biomarker discovery are expanding to include greater automation, higher throughput, and improved integration with other analytical techniques. These advancements aim to accelerate the biomarker discovery process, reduce variability, and enhance the translation of findings from the laboratory to clinical applications.
In the 1950s, the introduction of polyacrylamide gels revolutionized the field, allowing for better resolution of proteins based on their molecular weight. This advancement paved the way for more sophisticated applications in protein analysis and biomarker identification. The 1970s saw the development of two-dimensional gel electrophoresis, which dramatically increased the resolving power of the technique, enabling researchers to separate complex protein mixtures with unprecedented clarity.
The advent of capillary electrophoresis in the 1980s further expanded the capabilities of gel electrophoresis, offering higher resolution, faster analysis times, and the ability to work with smaller sample volumes. This innovation proved particularly valuable in the emerging field of proteomics and biomarker discovery, where sample quantity is often limited.
Recent years have witnessed the integration of gel electrophoresis with advanced detection methods, such as mass spectrometry and fluorescence imaging. These combinations have significantly enhanced the sensitivity and specificity of biomarker detection, allowing researchers to identify and quantify proteins present in extremely low concentrations.
The primary objectives of gel electrophoresis in biomarker discovery are multifaceted. Firstly, it aims to provide a high-resolution separation of complex biological samples, enabling the identification of potential biomarkers that may be indicative of specific diseases or physiological states. Secondly, it seeks to offer reproducible and quantifiable results, crucial for validating biomarkers across different studies and laboratories.
Another key objective is to improve the detection of low-abundance proteins, which often represent the most promising biomarkers. This goal has driven the development of more sensitive staining techniques and the integration of pre-fractionation methods to enrich samples for proteins of interest.
Furthermore, gel electrophoresis techniques are continually evolving to address the challenges of analyzing post-translational modifications, which play a critical role in protein function and can serve as important biomarkers. The ability to detect and characterize these modifications is becoming increasingly important in the field of personalized medicine and targeted therapies.
As we look to the future, the objectives of gel electrophoresis in biomarker discovery are expanding to include greater automation, higher throughput, and improved integration with other analytical techniques. These advancements aim to accelerate the biomarker discovery process, reduce variability, and enhance the translation of findings from the laboratory to clinical applications.
Biomarker Discovery Market Analysis
The biomarker discovery market has experienced significant growth in recent years, driven by the increasing demand for personalized medicine and early disease detection. This market segment is closely tied to the broader field of molecular diagnostics and precision medicine, with gel electrophoresis playing a crucial role in the identification and analysis of potential biomarkers.
The global biomarker discovery market was valued at approximately $25 billion in 2020 and is projected to reach $58 billion by 2026, growing at a compound annual growth rate (CAGR) of 15.2% during the forecast period. This robust growth is attributed to factors such as the rising prevalence of chronic diseases, advancements in proteomics and genomics technologies, and increased funding for biomarker research.
Gel electrophoresis, particularly in its advanced forms like 2D gel electrophoresis and capillary electrophoresis, remains a cornerstone technique in biomarker discovery. The market for gel electrophoresis equipment and consumables is expected to grow steadily, with a CAGR of 5.8% from 2021 to 2026, reaching a value of $1.9 billion by the end of the forecast period.
The biomarker discovery market is segmented based on technology, application, and end-user. Gel electrophoresis falls under the technology segment, which also includes mass spectrometry, next-generation sequencing, and other techniques. In terms of applications, oncology remains the largest segment, accounting for 35% of the market share, followed by cardiovascular diseases and neurological disorders.
Geographically, North America dominates the biomarker discovery market, holding a 40% market share, followed by Europe and Asia-Pacific. The Asia-Pacific region is expected to witness the highest growth rate in the coming years due to increasing research activities and healthcare investments in countries like China and India.
Key players in the biomarker discovery market include Thermo Fisher Scientific, Bio-Rad Laboratories, Agilent Technologies, and Merck KGaA, among others. These companies are actively investing in research and development to enhance their gel electrophoresis technologies and maintain their competitive edge in the biomarker discovery space.
The market is also witnessing a trend towards the integration of artificial intelligence and machine learning in biomarker discovery processes, including the analysis of gel electrophoresis data. This integration is expected to accelerate the identification of novel biomarkers and improve the accuracy of diagnostic and prognostic tools.
The global biomarker discovery market was valued at approximately $25 billion in 2020 and is projected to reach $58 billion by 2026, growing at a compound annual growth rate (CAGR) of 15.2% during the forecast period. This robust growth is attributed to factors such as the rising prevalence of chronic diseases, advancements in proteomics and genomics technologies, and increased funding for biomarker research.
Gel electrophoresis, particularly in its advanced forms like 2D gel electrophoresis and capillary electrophoresis, remains a cornerstone technique in biomarker discovery. The market for gel electrophoresis equipment and consumables is expected to grow steadily, with a CAGR of 5.8% from 2021 to 2026, reaching a value of $1.9 billion by the end of the forecast period.
The biomarker discovery market is segmented based on technology, application, and end-user. Gel electrophoresis falls under the technology segment, which also includes mass spectrometry, next-generation sequencing, and other techniques. In terms of applications, oncology remains the largest segment, accounting for 35% of the market share, followed by cardiovascular diseases and neurological disorders.
Geographically, North America dominates the biomarker discovery market, holding a 40% market share, followed by Europe and Asia-Pacific. The Asia-Pacific region is expected to witness the highest growth rate in the coming years due to increasing research activities and healthcare investments in countries like China and India.
Key players in the biomarker discovery market include Thermo Fisher Scientific, Bio-Rad Laboratories, Agilent Technologies, and Merck KGaA, among others. These companies are actively investing in research and development to enhance their gel electrophoresis technologies and maintain their competitive edge in the biomarker discovery space.
The market is also witnessing a trend towards the integration of artificial intelligence and machine learning in biomarker discovery processes, including the analysis of gel electrophoresis data. This integration is expected to accelerate the identification of novel biomarkers and improve the accuracy of diagnostic and prognostic tools.
Current Gel Electrophoresis Techniques and Limitations
Gel electrophoresis remains a cornerstone technique in biomarker discovery, offering valuable insights into protein and nucleic acid separation. Current techniques encompass a range of methodologies, each with its own strengths and limitations.
One-dimensional gel electrophoresis (1-DE) continues to be widely used for its simplicity and cost-effectiveness. This technique separates proteins or nucleic acids based on their molecular weight, providing a quick overview of sample composition. However, 1-DE is limited in its resolution, particularly when dealing with complex mixtures containing numerous biomolecules with similar molecular weights.
Two-dimensional gel electrophoresis (2-DE) addresses some of the limitations of 1-DE by separating proteins based on two properties: isoelectric point and molecular weight. This technique offers higher resolution and can resolve thousands of proteins simultaneously. Nevertheless, 2-DE faces challenges in reproducibility, labor-intensiveness, and difficulties in detecting low-abundance proteins.
Capillary gel electrophoresis (CGE) has gained popularity due to its high resolution, automation capabilities, and minimal sample requirements. CGE excels in separating DNA fragments and proteins with high efficiency. However, it may struggle with larger biomolecules and can be more expensive to implement compared to traditional gel-based methods.
Pulsed-field gel electrophoresis (PFGE) has become indispensable for separating large DNA molecules, such as entire chromosomes. While PFGE offers unparalleled resolution for large DNA fragments, it is time-consuming and requires specialized equipment, limiting its widespread adoption in routine biomarker discovery workflows.
Gradient gel electrophoresis techniques, including native PAGE and SDS-PAGE with gradient gels, provide enhanced resolution across a wide range of molecular weights. These methods are particularly useful for separating proteins with similar molecular masses. However, they can be challenging to optimize and may require more complex gel preparation procedures.
Despite these advancements, gel electrophoresis techniques face several common limitations. Sample preparation remains a critical challenge, as protein solubility issues and potential loss of post-translational modifications can affect results. Additionally, the detection of low-abundance biomarkers continues to be problematic, often requiring complementary techniques such as Western blotting or mass spectrometry for validation.
Quantification and reproducibility also present ongoing challenges. Gel-to-gel variations can complicate comparative analyses, necessitating careful standardization and the use of internal controls. Furthermore, the dynamic range of protein detection in gels is limited, potentially obscuring important biomarkers present at low concentrations.
One-dimensional gel electrophoresis (1-DE) continues to be widely used for its simplicity and cost-effectiveness. This technique separates proteins or nucleic acids based on their molecular weight, providing a quick overview of sample composition. However, 1-DE is limited in its resolution, particularly when dealing with complex mixtures containing numerous biomolecules with similar molecular weights.
Two-dimensional gel electrophoresis (2-DE) addresses some of the limitations of 1-DE by separating proteins based on two properties: isoelectric point and molecular weight. This technique offers higher resolution and can resolve thousands of proteins simultaneously. Nevertheless, 2-DE faces challenges in reproducibility, labor-intensiveness, and difficulties in detecting low-abundance proteins.
Capillary gel electrophoresis (CGE) has gained popularity due to its high resolution, automation capabilities, and minimal sample requirements. CGE excels in separating DNA fragments and proteins with high efficiency. However, it may struggle with larger biomolecules and can be more expensive to implement compared to traditional gel-based methods.
Pulsed-field gel electrophoresis (PFGE) has become indispensable for separating large DNA molecules, such as entire chromosomes. While PFGE offers unparalleled resolution for large DNA fragments, it is time-consuming and requires specialized equipment, limiting its widespread adoption in routine biomarker discovery workflows.
Gradient gel electrophoresis techniques, including native PAGE and SDS-PAGE with gradient gels, provide enhanced resolution across a wide range of molecular weights. These methods are particularly useful for separating proteins with similar molecular masses. However, they can be challenging to optimize and may require more complex gel preparation procedures.
Despite these advancements, gel electrophoresis techniques face several common limitations. Sample preparation remains a critical challenge, as protein solubility issues and potential loss of post-translational modifications can affect results. Additionally, the detection of low-abundance biomarkers continues to be problematic, often requiring complementary techniques such as Western blotting or mass spectrometry for validation.
Quantification and reproducibility also present ongoing challenges. Gel-to-gel variations can complicate comparative analyses, necessitating careful standardization and the use of internal controls. Furthermore, the dynamic range of protein detection in gels is limited, potentially obscuring important biomarkers present at low concentrations.
Advanced Gel Electrophoresis Methods
01 Gel electrophoresis techniques for biomarker separation
Various gel electrophoresis techniques are used for separating and identifying biomarkers. These methods involve the use of different gel compositions, electric fields, and buffer systems to achieve optimal separation of biomolecules based on their size, charge, or other properties. The techniques can be applied to proteins, nucleic acids, and other potential biomarkers.- Gel electrophoresis techniques for biomarker separation: Various gel electrophoresis techniques are employed for separating and identifying biomarkers. These methods utilize different gel compositions and electric fields to separate biomolecules based on their size, charge, or other properties. Improved gel formulations and electrophoresis conditions enhance the resolution and detection of potential biomarkers.
- Integration of gel electrophoresis with mass spectrometry: Combining gel electrophoresis with mass spectrometry techniques allows for more comprehensive biomarker discovery. This integration enables the separation of complex biological samples followed by precise identification and characterization of potential biomarkers, improving the overall efficiency and accuracy of the discovery process.
- Microfluidic devices for gel electrophoresis-based biomarker analysis: Microfluidic devices are developed to perform gel electrophoresis on a miniaturized scale. These devices offer advantages such as reduced sample volume, faster analysis times, and the potential for high-throughput screening of biomarkers. Integration of multiple analytical steps on a single chip enhances the efficiency of biomarker discovery.
- Two-dimensional gel electrophoresis for biomarker discovery: Two-dimensional gel electrophoresis is utilized to separate complex protein mixtures based on two different properties, typically isoelectric point and molecular weight. This technique allows for improved resolution and identification of potential biomarkers in biological samples, enabling the discovery of novel disease-associated proteins.
- Data analysis and bioinformatics tools for biomarker identification: Advanced data analysis and bioinformatics tools are developed to process and interpret the results obtained from gel electrophoresis experiments. These tools employ statistical methods, machine learning algorithms, and database comparisons to identify potential biomarkers from complex electrophoresis data, facilitating the discovery of clinically relevant molecules.
02 Two-dimensional gel electrophoresis for complex biomarker analysis
Two-dimensional gel electrophoresis is a powerful technique for separating complex mixtures of proteins or other biomolecules. This method combines two separation techniques, typically isoelectric focusing followed by SDS-PAGE, to achieve high-resolution separation of potential biomarkers based on both their isoelectric point and molecular weight.Expand Specific Solutions03 Capillary electrophoresis for biomarker discovery
Capillary electrophoresis is a high-resolution separation technique used for biomarker discovery. It offers advantages such as small sample volume requirements, rapid analysis times, and high separation efficiency. Various modes of capillary electrophoresis can be employed for different types of biomarkers, including proteins, peptides, and small molecules.Expand Specific Solutions04 Integration of gel electrophoresis with mass spectrometry
Combining gel electrophoresis with mass spectrometry enhances biomarker discovery by providing both separation and identification capabilities. This approach allows for the isolation of potential biomarkers through gel electrophoresis, followed by their characterization and identification using mass spectrometry techniques such as MALDI-TOF or LC-MS/MS.Expand Specific Solutions05 Microfluidic devices for gel electrophoresis-based biomarker analysis
Microfluidic devices are being developed for miniaturized gel electrophoresis systems, enabling rapid and high-throughput biomarker analysis. These devices integrate sample preparation, separation, and detection steps into a single platform, offering advantages such as reduced sample consumption, faster analysis times, and potential for automation in biomarker discovery workflows.Expand Specific Solutions
Key Players in Biomarker Discovery Tools
The gel electrophoresis market for biomarker discovery is in a growth phase, driven by increasing demand for personalized medicine and advancements in proteomics research. The global market size is projected to expand significantly in the coming years, with a compound annual growth rate exceeding 5%. Technologically, gel electrophoresis is mature but continues to evolve with innovations in automation and high-throughput capabilities. Key players like Bio-Rad Laboratories, Thermo Fisher Scientific (through its Life Technologies and Applied Biosystems brands), and Beckman Coulter are leading the field with advanced systems and reagents. Emerging companies such as Helena Laboratories and NeoGenomics are also making notable contributions, particularly in specialized applications and integrated solutions for biomarker analysis.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad has developed advanced gel electrophoresis systems for biomarker discovery, including their innovative PROTEAN i12 IEF System. This system utilizes isoelectric focusing (IEF) for high-resolution protein separation, enabling the detection of low-abundance biomarkers[1]. Their technology incorporates a patented electrode design that ensures uniform electric field distribution, resulting in improved reproducibility and resolution[2]. Bio-Rad's gel electrophoresis solutions also feature integrated image analysis software for quantitative biomarker assessment, streamlining the discovery process[3]. The company has recently introduced automated gel loading systems, reducing human error and increasing throughput for large-scale biomarker studies[4].
Strengths: High-resolution protein separation, improved reproducibility, integrated analysis software. Weaknesses: Relatively high cost, requires specialized training for optimal use.
Cytiva Sweden AB
Technical Solution: Cytiva (formerly GE Healthcare Life Sciences) has developed the Amersham ECL Gel system, a novel approach to gel electrophoresis for biomarker discovery. This system utilizes a unique polyacrylamide gel matrix with covalently bound fluorescent compounds, enabling direct fluorescence detection of proteins without the need for staining or western blotting[5]. The technology offers a linear dynamic range of up to five orders of magnitude, allowing for accurate quantification of both high and low abundance biomarkers in a single run[6]. Cytiva's system also incorporates automated image capture and analysis software, streamlining the biomarker discovery workflow[7]. Recent advancements include the integration of machine learning algorithms for improved biomarker identification and validation[8].
Strengths: Wide dynamic range, simplified workflow, high sensitivity. Weaknesses: Limited to fluorescence-based detection, may require specialized equipment.
Innovative Gel Electrophoresis Patents
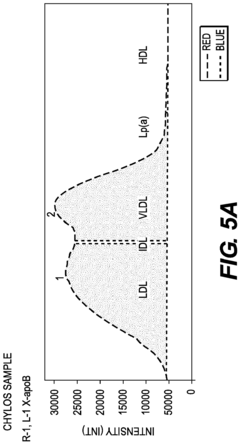
Fluorescent in-SITU detection of lipid particle apolipoproteins within primary electrophoretic matrix
PatentActiveEP2962108A1
Innovation
- A gel electrophoresis system and method using fluorescently tagged antibodies that bind to lipoprotein particles, allowing in-situ detection and differentiation of specific apolipoproteins and lipoprotein particles within a single electrophoretic matrix, eliminating the need for transfer protocols and non-specific staining.
Fluorescent in-situ detection of lipid particle apolipoproteins within primary electrophoretic matrix
PatentInactiveEP3438663A1
Innovation
- A gel electrophoresis system that uses fluorescently tagged antibodies to detect specific apolipoproteins and lipoprotein particles directly in the electrophoretic matrix, allowing for simultaneous detection of multiple particles with distinguishable signals, eliminating the need for transfer protocols and non-specific protein staining.
Regulatory Considerations for Biomarker Discovery Tools
Regulatory considerations play a crucial role in the development and implementation of biomarker discovery tools, including gel electrophoresis techniques. As these tools are used to identify potential biomarkers for various diseases and conditions, they must adhere to stringent regulatory guidelines to ensure their reliability, accuracy, and safety.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of biomarker discovery tools. The FDA has established specific guidelines for the validation and qualification of biomarkers, which must be followed when using gel electrophoresis for biomarker discovery. These guidelines emphasize the importance of analytical validation, clinical validation, and utility assessment of biomarkers.
The European Medicines Agency (EMA) also provides regulatory guidance for biomarker discovery tools within the European Union. The EMA's guidelines focus on the qualification of novel methodologies for medicine development, including biomarker discovery techniques. Researchers and developers must demonstrate the robustness and reproducibility of their gel electrophoresis methods to meet these regulatory requirements.
International standards, such as those set by the International Conference on Harmonisation (ICH), provide a framework for the global harmonization of regulatory requirements. The ICH guidelines address various aspects of biomarker discovery, including quality control, validation procedures, and data integrity, which are all relevant to gel electrophoresis techniques.
Regulatory bodies also emphasize the importance of Good Laboratory Practice (GLP) and Good Clinical Practice (GCP) in biomarker discovery research. These practices ensure the quality and integrity of non-clinical and clinical studies, respectively, and are essential for the development of reliable biomarkers using gel electrophoresis.
Data privacy and protection regulations, such as the General Data Protection Regulation (GDPR) in the European Union and the Health Insurance Portability and Accountability Act (HIPAA) in the United States, must also be considered when handling patient samples and data in biomarker discovery research. These regulations impact the collection, storage, and analysis of biological samples used in gel electrophoresis studies.
As the field of biomarker discovery continues to evolve, regulatory agencies are adapting their guidelines to keep pace with technological advancements. This includes the development of specific regulations for novel biomarker discovery platforms and the integration of artificial intelligence and machine learning in data analysis.
Researchers and developers working with gel electrophoresis for biomarker discovery must stay informed about these regulatory considerations and ensure compliance throughout the research and development process. This includes maintaining detailed documentation, implementing robust quality control measures, and adhering to ethical standards in sample collection and data management.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of biomarker discovery tools. The FDA has established specific guidelines for the validation and qualification of biomarkers, which must be followed when using gel electrophoresis for biomarker discovery. These guidelines emphasize the importance of analytical validation, clinical validation, and utility assessment of biomarkers.
The European Medicines Agency (EMA) also provides regulatory guidance for biomarker discovery tools within the European Union. The EMA's guidelines focus on the qualification of novel methodologies for medicine development, including biomarker discovery techniques. Researchers and developers must demonstrate the robustness and reproducibility of their gel electrophoresis methods to meet these regulatory requirements.
International standards, such as those set by the International Conference on Harmonisation (ICH), provide a framework for the global harmonization of regulatory requirements. The ICH guidelines address various aspects of biomarker discovery, including quality control, validation procedures, and data integrity, which are all relevant to gel electrophoresis techniques.
Regulatory bodies also emphasize the importance of Good Laboratory Practice (GLP) and Good Clinical Practice (GCP) in biomarker discovery research. These practices ensure the quality and integrity of non-clinical and clinical studies, respectively, and are essential for the development of reliable biomarkers using gel electrophoresis.
Data privacy and protection regulations, such as the General Data Protection Regulation (GDPR) in the European Union and the Health Insurance Portability and Accountability Act (HIPAA) in the United States, must also be considered when handling patient samples and data in biomarker discovery research. These regulations impact the collection, storage, and analysis of biological samples used in gel electrophoresis studies.
As the field of biomarker discovery continues to evolve, regulatory agencies are adapting their guidelines to keep pace with technological advancements. This includes the development of specific regulations for novel biomarker discovery platforms and the integration of artificial intelligence and machine learning in data analysis.
Researchers and developers working with gel electrophoresis for biomarker discovery must stay informed about these regulatory considerations and ensure compliance throughout the research and development process. This includes maintaining detailed documentation, implementing robust quality control measures, and adhering to ethical standards in sample collection and data management.
Data Analysis in Gel Electrophoresis-Based Biomarker Discovery
Data analysis in gel electrophoresis-based biomarker discovery plays a crucial role in extracting meaningful information from complex biological samples. The process involves several key steps, each requiring sophisticated analytical techniques and software tools.
Image acquisition and preprocessing form the initial stage of data analysis. High-resolution digital imaging systems capture gel images, which are then subjected to various preprocessing algorithms. These algorithms enhance image quality, remove background noise, and correct for any distortions or irregularities in the gel.
Lane and band detection algorithms are employed to identify individual lanes and protein bands within the gel image. Machine learning techniques, such as convolutional neural networks, have significantly improved the accuracy and efficiency of this process, enabling the detection of faint or overlapping bands that may be missed by traditional methods.
Quantification of protein bands is a critical step in biomarker discovery. Densitometry techniques measure the intensity of each band, correlating it with protein abundance. Advanced software packages offer various normalization methods to account for variations in sample loading and staining efficiency, ensuring accurate comparisons across different gel runs.
Statistical analysis of the quantified data is essential for identifying potential biomarkers. Multivariate analysis techniques, such as principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA), are commonly used to detect patterns and differences between sample groups. These methods help researchers identify proteins that show significant changes in abundance between healthy and diseased states.
Machine learning algorithms have revolutionized the field of biomarker discovery in gel electrophoresis. Supervised learning methods, such as support vector machines and random forests, can be trained on labeled datasets to classify samples and predict disease states based on protein expression patterns. Unsupervised learning techniques, like clustering algorithms, can reveal hidden structures in the data and group samples with similar protein profiles.
Data integration is becoming increasingly important as researchers combine gel electrophoresis results with other omics data. Bioinformatics tools enable the integration of proteomic data with genomic, transcriptomic, and metabolomic information, providing a more comprehensive understanding of biological systems and potential biomarkers.
Visualization tools play a crucial role in presenting and interpreting complex gel electrophoresis data. Heat maps, volcano plots, and network diagrams help researchers identify trends, outliers, and relationships between proteins. Interactive visualization platforms allow for dynamic exploration of the data, facilitating hypothesis generation and decision-making in biomarker discovery projects.
Image acquisition and preprocessing form the initial stage of data analysis. High-resolution digital imaging systems capture gel images, which are then subjected to various preprocessing algorithms. These algorithms enhance image quality, remove background noise, and correct for any distortions or irregularities in the gel.
Lane and band detection algorithms are employed to identify individual lanes and protein bands within the gel image. Machine learning techniques, such as convolutional neural networks, have significantly improved the accuracy and efficiency of this process, enabling the detection of faint or overlapping bands that may be missed by traditional methods.
Quantification of protein bands is a critical step in biomarker discovery. Densitometry techniques measure the intensity of each band, correlating it with protein abundance. Advanced software packages offer various normalization methods to account for variations in sample loading and staining efficiency, ensuring accurate comparisons across different gel runs.
Statistical analysis of the quantified data is essential for identifying potential biomarkers. Multivariate analysis techniques, such as principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA), are commonly used to detect patterns and differences between sample groups. These methods help researchers identify proteins that show significant changes in abundance between healthy and diseased states.
Machine learning algorithms have revolutionized the field of biomarker discovery in gel electrophoresis. Supervised learning methods, such as support vector machines and random forests, can be trained on labeled datasets to classify samples and predict disease states based on protein expression patterns. Unsupervised learning techniques, like clustering algorithms, can reveal hidden structures in the data and group samples with similar protein profiles.
Data integration is becoming increasingly important as researchers combine gel electrophoresis results with other omics data. Bioinformatics tools enable the integration of proteomic data with genomic, transcriptomic, and metabolomic information, providing a more comprehensive understanding of biological systems and potential biomarkers.
Visualization tools play a crucial role in presenting and interpreting complex gel electrophoresis data. Heat maps, volcano plots, and network diagrams help researchers identify trends, outliers, and relationships between proteins. Interactive visualization platforms allow for dynamic exploration of the data, facilitating hypothesis generation and decision-making in biomarker discovery projects.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!