Gel Electrophoresis for Cell-Free Culture Media Analysis
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gel Electrophoresis Background and Objectives
Gel electrophoresis has been a cornerstone technique in molecular biology and biochemistry since its inception in the 1960s. This method, which separates molecules based on their size and electrical charge, has evolved significantly over the decades to become an indispensable tool in various scientific fields. In the context of cell-free culture media analysis, gel electrophoresis offers a powerful means to examine the complex mixture of proteins, nucleic acids, and other biomolecules present in these sophisticated culture systems.
The primary objective of employing gel electrophoresis for cell-free culture media analysis is to gain a comprehensive understanding of the media composition and its changes over time. This technique allows researchers to identify and quantify specific components, monitor the degradation of nutrients, and detect the accumulation of metabolic byproducts. Such insights are crucial for optimizing cell-free systems, which are increasingly used in biotechnology for protein production, metabolic engineering, and synthetic biology applications.
One of the key advantages of gel electrophoresis in this context is its ability to provide a visual representation of the molecular landscape within cell-free culture media. This visual data can be easily interpreted and compared across different experimental conditions, making it an invaluable tool for both qualitative and semi-quantitative analysis. Furthermore, the technique's versatility allows for the separation of a wide range of molecules, from small peptides to large proteins and nucleic acids, providing a comprehensive view of the media's composition.
As cell-free systems continue to gain prominence in biotechnology, the role of gel electrophoresis in their analysis is expected to expand. Researchers are now exploring advanced variations of the technique, such as two-dimensional gel electrophoresis and capillary electrophoresis, to enhance resolution and sensitivity. These advancements aim to provide even more detailed insights into the complex interactions and dynamics within cell-free culture media.
The ultimate goal of utilizing gel electrophoresis in this field is to enable the development of more efficient and productive cell-free systems. By precisely characterizing the media composition and its changes, researchers can fine-tune the nutrient balance, optimize protein expression conditions, and identify potential bottlenecks in the production process. This, in turn, can lead to improved yields, reduced costs, and the expansion of cell-free technology into new applications and industries.
The primary objective of employing gel electrophoresis for cell-free culture media analysis is to gain a comprehensive understanding of the media composition and its changes over time. This technique allows researchers to identify and quantify specific components, monitor the degradation of nutrients, and detect the accumulation of metabolic byproducts. Such insights are crucial for optimizing cell-free systems, which are increasingly used in biotechnology for protein production, metabolic engineering, and synthetic biology applications.
One of the key advantages of gel electrophoresis in this context is its ability to provide a visual representation of the molecular landscape within cell-free culture media. This visual data can be easily interpreted and compared across different experimental conditions, making it an invaluable tool for both qualitative and semi-quantitative analysis. Furthermore, the technique's versatility allows for the separation of a wide range of molecules, from small peptides to large proteins and nucleic acids, providing a comprehensive view of the media's composition.
As cell-free systems continue to gain prominence in biotechnology, the role of gel electrophoresis in their analysis is expected to expand. Researchers are now exploring advanced variations of the technique, such as two-dimensional gel electrophoresis and capillary electrophoresis, to enhance resolution and sensitivity. These advancements aim to provide even more detailed insights into the complex interactions and dynamics within cell-free culture media.
The ultimate goal of utilizing gel electrophoresis in this field is to enable the development of more efficient and productive cell-free systems. By precisely characterizing the media composition and its changes, researchers can fine-tune the nutrient balance, optimize protein expression conditions, and identify potential bottlenecks in the production process. This, in turn, can lead to improved yields, reduced costs, and the expansion of cell-free technology into new applications and industries.
Market Demand Analysis for Cell-Free Culture Media
The market demand for cell-free culture media analysis using gel electrophoresis has been steadily increasing due to the growing importance of biopharmaceutical production and research. This technique plays a crucial role in monitoring and optimizing cell-free protein synthesis systems, which are becoming increasingly popular in the biotechnology industry.
The global cell-free protein expression market, which heavily relies on gel electrophoresis for analysis, is experiencing significant growth. This growth is driven by the rising demand for personalized medicine, advancements in proteomics research, and the need for rapid protein production methods. The pharmaceutical and biotechnology sectors are the primary contributors to this market expansion, as they seek more efficient and cost-effective ways to develop and produce therapeutic proteins and vaccines.
In the academic research sector, there is a growing interest in using cell-free systems for studying complex biological processes, protein interactions, and metabolic pathways. This has led to an increased demand for analytical tools like gel electrophoresis that can provide detailed insights into the composition and quality of cell-free culture media.
The biopharmaceutical industry, in particular, has shown a strong demand for cell-free culture media analysis. As companies strive to optimize their production processes and ensure product quality, the need for robust analytical methods has become paramount. Gel electrophoresis offers a reliable and well-established technique for monitoring protein expression, purity, and degradation in cell-free systems.
Furthermore, the emergence of synthetic biology and the development of novel cell-free biosensors have created new market opportunities for gel electrophoresis in cell-free culture media analysis. These applications require precise characterization of protein components and their interactions, driving the demand for advanced analytical tools.
The market for gel electrophoresis equipment and consumables specifically tailored for cell-free culture media analysis is also expanding. Manufacturers are developing specialized kits and reagents optimized for this application, catering to the growing needs of researchers and industry professionals working with cell-free systems.
As the field of cell-free protein synthesis continues to evolve, there is an increasing demand for high-throughput and automated gel electrophoresis systems. This trend is driven by the need for faster and more efficient analysis of large numbers of samples, particularly in industrial settings where rapid product development and quality control are essential.
In conclusion, the market demand for gel electrophoresis in cell-free culture media analysis is robust and expected to grow further. This growth is fueled by advancements in biotechnology, the expansion of the biopharmaceutical industry, and the increasing adoption of cell-free protein synthesis systems across various research and industrial applications.
The global cell-free protein expression market, which heavily relies on gel electrophoresis for analysis, is experiencing significant growth. This growth is driven by the rising demand for personalized medicine, advancements in proteomics research, and the need for rapid protein production methods. The pharmaceutical and biotechnology sectors are the primary contributors to this market expansion, as they seek more efficient and cost-effective ways to develop and produce therapeutic proteins and vaccines.
In the academic research sector, there is a growing interest in using cell-free systems for studying complex biological processes, protein interactions, and metabolic pathways. This has led to an increased demand for analytical tools like gel electrophoresis that can provide detailed insights into the composition and quality of cell-free culture media.
The biopharmaceutical industry, in particular, has shown a strong demand for cell-free culture media analysis. As companies strive to optimize their production processes and ensure product quality, the need for robust analytical methods has become paramount. Gel electrophoresis offers a reliable and well-established technique for monitoring protein expression, purity, and degradation in cell-free systems.
Furthermore, the emergence of synthetic biology and the development of novel cell-free biosensors have created new market opportunities for gel electrophoresis in cell-free culture media analysis. These applications require precise characterization of protein components and their interactions, driving the demand for advanced analytical tools.
The market for gel electrophoresis equipment and consumables specifically tailored for cell-free culture media analysis is also expanding. Manufacturers are developing specialized kits and reagents optimized for this application, catering to the growing needs of researchers and industry professionals working with cell-free systems.
As the field of cell-free protein synthesis continues to evolve, there is an increasing demand for high-throughput and automated gel electrophoresis systems. This trend is driven by the need for faster and more efficient analysis of large numbers of samples, particularly in industrial settings where rapid product development and quality control are essential.
In conclusion, the market demand for gel electrophoresis in cell-free culture media analysis is robust and expected to grow further. This growth is fueled by advancements in biotechnology, the expansion of the biopharmaceutical industry, and the increasing adoption of cell-free protein synthesis systems across various research and industrial applications.
Current Challenges in Gel Electrophoresis Techniques
Gel electrophoresis, a cornerstone technique in molecular biology, faces several challenges when applied to cell-free culture media analysis. One of the primary issues is the complexity of the sample matrix. Cell-free culture media often contain a diverse array of proteins, metabolites, and other biomolecules, which can interfere with the separation and resolution of target analytes. This complexity can lead to smearing, background noise, and reduced band clarity, making it difficult to accurately interpret results.
Another significant challenge is the low concentration of certain analytes of interest in cell-free culture media. Many important biomarkers or proteins may be present in trace amounts, pushing the limits of detection for traditional gel electrophoresis techniques. This necessitates the development of more sensitive methods or pre-concentration steps, which can introduce additional variability and complexity to the analysis process.
The dynamic range of protein concentrations in cell-free culture media also poses a challenge. High-abundance proteins can mask the presence of low-abundance proteins, making it difficult to detect and quantify less prevalent but potentially crucial components. This issue is particularly problematic when trying to identify subtle changes in protein expression or when searching for novel biomarkers.
Reproducibility is another key concern in gel electrophoresis of cell-free culture media. The inherent variability in sample preparation, gel casting, and running conditions can lead to inconsistencies between experiments. This variability is exacerbated by the complex nature of the media, making it challenging to achieve consistent and comparable results across different batches or laboratories.
The time-consuming nature of traditional gel electrophoresis techniques is also a significant drawback, especially in high-throughput screening applications. The lengthy process of gel preparation, sample loading, electrophoresis, and subsequent staining or imaging can limit the number of samples that can be analyzed in a given timeframe, potentially slowing down research and development efforts.
Furthermore, the limited resolution of conventional gel electrophoresis techniques can be problematic when analyzing closely related proteins or small peptides in cell-free culture media. This limitation becomes particularly evident when trying to distinguish between post-translational modifications or subtle structural differences that may be critical in understanding cellular processes or product quality.
Lastly, the quantitative analysis of gel electrophoresis results remains challenging. While densitometry can provide semi-quantitative data, achieving accurate and precise quantification of specific components in complex cell-free culture media is often difficult. This limitation hampers efforts to perform detailed comparative analyses or to establish reliable quality control metrics for cell-free expression systems.
Another significant challenge is the low concentration of certain analytes of interest in cell-free culture media. Many important biomarkers or proteins may be present in trace amounts, pushing the limits of detection for traditional gel electrophoresis techniques. This necessitates the development of more sensitive methods or pre-concentration steps, which can introduce additional variability and complexity to the analysis process.
The dynamic range of protein concentrations in cell-free culture media also poses a challenge. High-abundance proteins can mask the presence of low-abundance proteins, making it difficult to detect and quantify less prevalent but potentially crucial components. This issue is particularly problematic when trying to identify subtle changes in protein expression or when searching for novel biomarkers.
Reproducibility is another key concern in gel electrophoresis of cell-free culture media. The inherent variability in sample preparation, gel casting, and running conditions can lead to inconsistencies between experiments. This variability is exacerbated by the complex nature of the media, making it challenging to achieve consistent and comparable results across different batches or laboratories.
The time-consuming nature of traditional gel electrophoresis techniques is also a significant drawback, especially in high-throughput screening applications. The lengthy process of gel preparation, sample loading, electrophoresis, and subsequent staining or imaging can limit the number of samples that can be analyzed in a given timeframe, potentially slowing down research and development efforts.
Furthermore, the limited resolution of conventional gel electrophoresis techniques can be problematic when analyzing closely related proteins or small peptides in cell-free culture media. This limitation becomes particularly evident when trying to distinguish between post-translational modifications or subtle structural differences that may be critical in understanding cellular processes or product quality.
Lastly, the quantitative analysis of gel electrophoresis results remains challenging. While densitometry can provide semi-quantitative data, achieving accurate and precise quantification of specific components in complex cell-free culture media is often difficult. This limitation hampers efforts to perform detailed comparative analyses or to establish reliable quality control metrics for cell-free expression systems.
Existing Gel Electrophoresis Protocols for Cell-Free Media
01 Gel composition and preparation
Gel electrophoresis involves the use of specialized gel compositions. These gels are typically made from materials such as agarose or polyacrylamide. The composition and preparation of these gels are crucial for achieving optimal separation of molecules. Factors such as gel concentration, crosslinking, and additives can be adjusted to enhance resolution and separation efficiency.- Gel composition and preparation: Various gel compositions and preparation methods are used in gel electrophoresis. These include specific formulations of agarose, polyacrylamide, and other polymers to create gels with desired properties for different applications. The composition and preparation of the gel can significantly affect the separation and resolution of molecules during electrophoresis.
- Electrophoresis apparatus design: Innovations in electrophoresis apparatus design focus on improving efficiency, resolution, and ease of use. These designs may include novel electrode configurations, buffer systems, or integrated cooling mechanisms. Some apparatus designs also incorporate features for automated sample loading or real-time monitoring of the electrophoresis process.
- Detection and analysis methods: Advanced detection and analysis methods are developed to enhance the sensitivity and accuracy of gel electrophoresis results. These may include fluorescence-based detection, image analysis software, or integration with mass spectrometry. Some methods focus on real-time monitoring of the separation process or automated data interpretation.
- Specialized electrophoresis techniques: Specialized electrophoresis techniques are developed for specific applications or to overcome limitations of traditional methods. These may include pulsed-field gel electrophoresis, two-dimensional electrophoresis, or capillary electrophoresis. Such techniques can offer improved resolution, separation of larger molecules, or analysis of complex mixtures.
- Sample preparation and loading: Innovations in sample preparation and loading aim to improve the efficiency and reproducibility of gel electrophoresis. These may include novel buffer formulations, sample concentration techniques, or automated loading systems. Some methods focus on minimizing sample degradation or improving the uniformity of sample application.
02 Electrophoresis apparatus design
The design of gel electrophoresis apparatus plays a significant role in the effectiveness of the technique. This includes the development of innovative electrode configurations, buffer chambers, and cooling systems. Advanced designs aim to improve separation speed, resolution, and reproducibility while minimizing sample volume requirements and enhancing ease of use.Expand Specific Solutions03 Detection and imaging methods
Various detection and imaging methods are employed in gel electrophoresis to visualize and analyze separated molecules. These include fluorescence-based detection, UV absorption, and staining techniques. Advanced imaging systems and software are developed to enhance sensitivity, quantification accuracy, and data analysis capabilities.Expand Specific Solutions04 Sample preparation and loading techniques
Efficient sample preparation and loading are critical for successful gel electrophoresis. This includes methods for concentrating samples, removing interfering substances, and ensuring uniform sample application. Innovative loading techniques aim to improve sample resolution, prevent sample diffusion, and increase the number of samples that can be analyzed simultaneously.Expand Specific Solutions05 Specialized electrophoresis techniques
Various specialized electrophoresis techniques have been developed to address specific analytical needs. These include pulsed-field gel electrophoresis, two-dimensional gel electrophoresis, and capillary gel electrophoresis. These techniques offer improved resolution, separation of complex mixtures, and analysis of larger molecules or specific types of biomolecules.Expand Specific Solutions
Key Players in Gel Electrophoresis Industry
The gel electrophoresis market for cell-free culture media analysis is in a growth phase, driven by increasing demand for biopharmaceutical research and development. The global market size is estimated to be in the hundreds of millions of dollars, with steady expansion projected. Technologically, the field is moderately mature, with established players like Agilent Technologies and 3M Innovative Properties offering advanced solutions. However, there's room for innovation, particularly in automation and high-throughput systems. Emerging companies such as Expedeon are introducing novel reagents and services, while academic institutions like Zhejiang University and Cornell University contribute to ongoing research and development, indicating a dynamic competitive landscape with potential for further advancements.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed advanced gel electrophoresis systems for cell-free culture media analysis. Their 2100 Bioanalyzer system utilizes microfluidic technology to perform automated electrophoresis, providing high-resolution separation of proteins and nucleic acids[1]. This system integrates sample loading, separation, staining, destaining, detection, and data analysis into a single platform. For cell-free culture media analysis, Agilent's technology allows for rapid quantification and sizing of proteins, enabling researchers to monitor product quality and process consistency[2]. The company has also introduced the Fragment Analyzer system, which offers higher throughput and sensitivity for analyzing larger DNA fragments and RNA samples from cell-free culture media[3].
Strengths: High automation, rapid analysis, and integration of multiple steps. Weaknesses: Higher initial cost compared to traditional gel electrophoresis systems and potential limitations in analyzing very large molecules.
Helena Laboratories Corp.
Technical Solution: Helena Laboratories specializes in clinical electrophoresis systems and has adapted their technology for cell-free culture media analysis. Their SPIFE 3000 system offers high-resolution protein separation using agarose gel electrophoresis, which is particularly useful for analyzing complex protein mixtures in cell-free culture media[4]. The company has developed specific protocols for the analysis of monoclonal antibodies and other biopharmaceutical products in culture media. Helena's QuickGel system provides rapid electrophoresis for time-sensitive applications, allowing for quick assessment of media components and product quality[5]. Additionally, their V8 Nexus Capillary Electrophoresis System combines the principles of gel electrophoresis with capillary technology, offering enhanced sensitivity and resolution for analyzing low-abundance proteins in cell-free culture media[6].
Strengths: Specialized protocols for biopharmaceutical applications and high-resolution protein separation. Weaknesses: May require more manual intervention compared to fully automated systems.
Innovations in Gel Electrophoresis for Complex Samples
Force-promoted sample recovery in GEL electrophoresis
PatentInactiveEP1946094A1
Innovation
- A gel strip device with a permeable substrate that allows fluid samples to pass through while preventing gel material from doing so, utilizing mechanical force to facilitate sample recovery, either through centrifugation or pressure differences, in conjunction with a compartment frame for off-gel or in-gel electrophoresis modes.
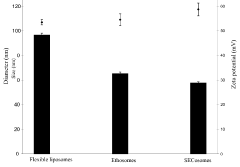
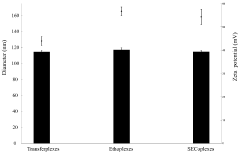
Cationic liposomes for the delivery of high molecular weight compounds
PatentWO2010149785A1
Innovation
- Development of SECosomes, a new type of cationic liposome composed of 1,2-dioleoyl-3-trimethylammonium propane (DOTAP), sodium cholate, cholesterol, and a high percentage of ethanol or propanol, which enhances skin penetration and delivery of high molecular weight compounds by maintaining stability and deformability.
Regulatory Considerations for Analytical Methods
Regulatory considerations play a crucial role in the development and implementation of analytical methods for cell-free culture media analysis using gel electrophoresis. These considerations are essential to ensure the reliability, reproducibility, and compliance of the analytical procedures with regulatory standards.
The primary regulatory bodies overseeing analytical methods in the biopharmaceutical industry include the Food and Drug Administration (FDA) in the United States, the European Medicines Agency (EMA) in Europe, and the International Conference on Harmonisation (ICH). These organizations provide guidelines and regulations that must be adhered to when developing and validating analytical methods.
For gel electrophoresis in cell-free culture media analysis, method validation is a critical regulatory requirement. This process involves demonstrating that the analytical procedure is suitable for its intended purpose and can consistently produce accurate and reliable results. Key validation parameters include specificity, linearity, range, accuracy, precision, detection limit, and quantitation limit.
Regulatory agencies also emphasize the importance of quality control measures throughout the analytical process. This includes the use of appropriate controls, reference standards, and system suitability tests to ensure the consistent performance of the gel electrophoresis method.
Documentation is another crucial aspect of regulatory compliance. Detailed records of method development, validation, and routine use must be maintained. Standard Operating Procedures (SOPs) should be established and followed to ensure consistency in the execution of the analytical method.
The concept of Quality by Design (QbD) is increasingly being applied to analytical method development and validation. This approach involves identifying critical method attributes and process parameters that may impact the quality of the results, and designing the method to consistently meet predefined quality criteria.
Regulatory bodies also require ongoing method performance verification through activities such as periodic revalidation and participation in proficiency testing programs. These measures help ensure the continued reliability and accuracy of the gel electrophoresis method over time.
As analytical technologies evolve, regulatory expectations may also change. It is essential for laboratories to stay informed about updates to regulatory guidelines and adapt their practices accordingly. This may involve implementing new validation approaches or incorporating emerging technologies that enhance the robustness and efficiency of gel electrophoresis methods.
In conclusion, adherence to regulatory considerations is paramount in developing and implementing gel electrophoresis methods for cell-free culture media analysis. By addressing these regulatory aspects, laboratories can ensure the quality and compliance of their analytical procedures, ultimately contributing to the safety and efficacy of biopharmaceutical products.
The primary regulatory bodies overseeing analytical methods in the biopharmaceutical industry include the Food and Drug Administration (FDA) in the United States, the European Medicines Agency (EMA) in Europe, and the International Conference on Harmonisation (ICH). These organizations provide guidelines and regulations that must be adhered to when developing and validating analytical methods.
For gel electrophoresis in cell-free culture media analysis, method validation is a critical regulatory requirement. This process involves demonstrating that the analytical procedure is suitable for its intended purpose and can consistently produce accurate and reliable results. Key validation parameters include specificity, linearity, range, accuracy, precision, detection limit, and quantitation limit.
Regulatory agencies also emphasize the importance of quality control measures throughout the analytical process. This includes the use of appropriate controls, reference standards, and system suitability tests to ensure the consistent performance of the gel electrophoresis method.
Documentation is another crucial aspect of regulatory compliance. Detailed records of method development, validation, and routine use must be maintained. Standard Operating Procedures (SOPs) should be established and followed to ensure consistency in the execution of the analytical method.
The concept of Quality by Design (QbD) is increasingly being applied to analytical method development and validation. This approach involves identifying critical method attributes and process parameters that may impact the quality of the results, and designing the method to consistently meet predefined quality criteria.
Regulatory bodies also require ongoing method performance verification through activities such as periodic revalidation and participation in proficiency testing programs. These measures help ensure the continued reliability and accuracy of the gel electrophoresis method over time.
As analytical technologies evolve, regulatory expectations may also change. It is essential for laboratories to stay informed about updates to regulatory guidelines and adapt their practices accordingly. This may involve implementing new validation approaches or incorporating emerging technologies that enhance the robustness and efficiency of gel electrophoresis methods.
In conclusion, adherence to regulatory considerations is paramount in developing and implementing gel electrophoresis methods for cell-free culture media analysis. By addressing these regulatory aspects, laboratories can ensure the quality and compliance of their analytical procedures, ultimately contributing to the safety and efficacy of biopharmaceutical products.
Sustainability in Gel Electrophoresis Practices
Sustainability in gel electrophoresis practices has become increasingly important as laboratories strive to reduce their environmental impact while maintaining high-quality analytical results. Traditional gel electrophoresis methods often involve the use of toxic chemicals and generate significant waste, prompting researchers to explore more eco-friendly alternatives.
One key area of focus is the development of sustainable gel materials. Biodegradable polymers derived from renewable resources, such as agarose from seaweed or cellulose from plant matter, are being investigated as replacements for synthetic acrylamide gels. These natural polymers not only reduce reliance on petroleum-based products but also offer improved biodegradability and reduced toxicity.
Efforts to minimize the use of harmful chemicals in gel electrophoresis have led to the exploration of safer staining methods. Fluorescent dyes that are less toxic and more sensitive than traditional ethidium bromide are gaining popularity. Additionally, researchers are investigating metal-based stains and even label-free detection methods to further reduce chemical waste.
Water conservation is another critical aspect of sustainable gel electrophoresis. Microfluidic devices and miniaturized gel systems are being developed to significantly reduce buffer volumes and overall water consumption. These systems not only conserve resources but also offer faster analysis times and improved resolution for cell-free culture media components.
Energy efficiency is being addressed through the design of low-power electrophoresis equipment. LED-based illumination systems for gel visualization consume less energy than traditional UV transilluminators. Moreover, ambient temperature electrophoresis techniques are being explored to eliminate the need for energy-intensive cooling systems.
Waste reduction strategies are also being implemented in gel electrophoresis practices. Reusable gel cassettes and combs are replacing single-use plastic components, while digital imaging systems are reducing the need for physical gel documentation. Some laboratories are even investigating gel recycling methods to further minimize waste generation.
The integration of sustainable practices in gel electrophoresis extends to the analysis of cell-free culture media. Researchers are developing optimized protocols that require smaller sample volumes and generate less waste while maintaining the ability to detect and quantify key media components. These advancements not only contribute to environmental sustainability but also improve the efficiency and cost-effectiveness of cell-free culture media analysis.
One key area of focus is the development of sustainable gel materials. Biodegradable polymers derived from renewable resources, such as agarose from seaweed or cellulose from plant matter, are being investigated as replacements for synthetic acrylamide gels. These natural polymers not only reduce reliance on petroleum-based products but also offer improved biodegradability and reduced toxicity.
Efforts to minimize the use of harmful chemicals in gel electrophoresis have led to the exploration of safer staining methods. Fluorescent dyes that are less toxic and more sensitive than traditional ethidium bromide are gaining popularity. Additionally, researchers are investigating metal-based stains and even label-free detection methods to further reduce chemical waste.
Water conservation is another critical aspect of sustainable gel electrophoresis. Microfluidic devices and miniaturized gel systems are being developed to significantly reduce buffer volumes and overall water consumption. These systems not only conserve resources but also offer faster analysis times and improved resolution for cell-free culture media components.
Energy efficiency is being addressed through the design of low-power electrophoresis equipment. LED-based illumination systems for gel visualization consume less energy than traditional UV transilluminators. Moreover, ambient temperature electrophoresis techniques are being explored to eliminate the need for energy-intensive cooling systems.
Waste reduction strategies are also being implemented in gel electrophoresis practices. Reusable gel cassettes and combs are replacing single-use plastic components, while digital imaging systems are reducing the need for physical gel documentation. Some laboratories are even investigating gel recycling methods to further minimize waste generation.
The integration of sustainable practices in gel electrophoresis extends to the analysis of cell-free culture media. Researchers are developing optimized protocols that require smaller sample volumes and generate less waste while maintaining the ability to detect and quantify key media components. These advancements not only contribute to environmental sustainability but also improve the efficiency and cost-effectiveness of cell-free culture media analysis.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!