Gel Electrophoresis in Clinical Trials: Impact Analysis
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gel Electrophoresis Evolution and Objectives
Gel electrophoresis has evolved significantly since its inception in the 1930s, becoming a cornerstone technique in molecular biology and clinical diagnostics. Initially developed for protein separation, the method has undergone substantial refinements to address the growing demands of genetic and proteomic research. The evolution of gel electrophoresis has been marked by key milestones, including the introduction of polyacrylamide gels in the 1950s, the development of two-dimensional electrophoresis in the 1970s, and the advent of pulsed-field gel electrophoresis in the 1980s.
In the context of clinical trials, gel electrophoresis has emerged as a critical tool for analyzing biomolecules, particularly nucleic acids and proteins. Its ability to separate molecules based on size and charge has made it indispensable for a wide range of applications, from genetic profiling to protein characterization. The technique's evolution has been driven by the need for higher resolution, increased sensitivity, and improved reproducibility in clinical research settings.
The primary objectives of gel electrophoresis in clinical trials are multifaceted. Firstly, it aims to provide accurate and reliable separation of biomolecules, enabling researchers to identify and quantify specific markers or genetic variations associated with diseases or drug responses. Secondly, it seeks to enhance the detection of low-abundance molecules, which is crucial for early disease diagnosis and monitoring treatment efficacy.
Another key objective is to improve the throughput and automation of the technique, allowing for the processing of large sample numbers typical in clinical trials. This has led to the development of high-throughput electrophoresis systems and microfluidic devices that can handle multiple samples simultaneously. Additionally, there is a growing focus on integrating gel electrophoresis with other analytical techniques, such as mass spectrometry, to provide more comprehensive molecular profiling.
The ongoing evolution of gel electrophoresis also aims to address challenges in reproducibility and standardization across different laboratories and clinical trial sites. This includes the development of standardized protocols, quality control measures, and reference materials to ensure consistent and comparable results. Furthermore, there is a push towards more environmentally friendly and cost-effective electrophoresis methods, including the use of alternative gel materials and buffer systems.
As clinical trials become increasingly complex and personalized, the objectives of gel electrophoresis continue to expand. Current research focuses on developing novel applications, such as the analysis of circulating tumor DNA, exosomes, and other biomarkers that require ultra-sensitive detection methods. The integration of gel electrophoresis with advanced imaging and data analysis techniques is also a key objective, aiming to extract more information from electrophoretic separations and facilitate the interpretation of complex molecular profiles in clinical trial data.
In the context of clinical trials, gel electrophoresis has emerged as a critical tool for analyzing biomolecules, particularly nucleic acids and proteins. Its ability to separate molecules based on size and charge has made it indispensable for a wide range of applications, from genetic profiling to protein characterization. The technique's evolution has been driven by the need for higher resolution, increased sensitivity, and improved reproducibility in clinical research settings.
The primary objectives of gel electrophoresis in clinical trials are multifaceted. Firstly, it aims to provide accurate and reliable separation of biomolecules, enabling researchers to identify and quantify specific markers or genetic variations associated with diseases or drug responses. Secondly, it seeks to enhance the detection of low-abundance molecules, which is crucial for early disease diagnosis and monitoring treatment efficacy.
Another key objective is to improve the throughput and automation of the technique, allowing for the processing of large sample numbers typical in clinical trials. This has led to the development of high-throughput electrophoresis systems and microfluidic devices that can handle multiple samples simultaneously. Additionally, there is a growing focus on integrating gel electrophoresis with other analytical techniques, such as mass spectrometry, to provide more comprehensive molecular profiling.
The ongoing evolution of gel electrophoresis also aims to address challenges in reproducibility and standardization across different laboratories and clinical trial sites. This includes the development of standardized protocols, quality control measures, and reference materials to ensure consistent and comparable results. Furthermore, there is a push towards more environmentally friendly and cost-effective electrophoresis methods, including the use of alternative gel materials and buffer systems.
As clinical trials become increasingly complex and personalized, the objectives of gel electrophoresis continue to expand. Current research focuses on developing novel applications, such as the analysis of circulating tumor DNA, exosomes, and other biomarkers that require ultra-sensitive detection methods. The integration of gel electrophoresis with advanced imaging and data analysis techniques is also a key objective, aiming to extract more information from electrophoretic separations and facilitate the interpretation of complex molecular profiles in clinical trial data.
Clinical Trial Market Demand Analysis
The clinical trial market has witnessed significant growth in recent years, driven by the increasing demand for new and innovative therapies across various therapeutic areas. Gel electrophoresis, a fundamental technique in molecular biology, plays a crucial role in this expanding market. The demand for gel electrophoresis in clinical trials stems from its ability to separate and analyze DNA, RNA, and proteins, making it an essential tool for drug development and biomarker discovery.
The global clinical trial market is projected to continue its upward trajectory, with a compound annual growth rate (CAGR) expected to remain strong over the next five years. This growth is fueled by factors such as the rising prevalence of chronic diseases, increased R&D investments by pharmaceutical and biotechnology companies, and the growing emphasis on personalized medicine. As a result, the demand for gel electrophoresis techniques in clinical trials is also expected to surge.
One of the key drivers of gel electrophoresis demand in clinical trials is the increasing focus on genomics and proteomics research. As more clinical trials incorporate genetic and protein-based biomarkers, the need for reliable and efficient separation techniques becomes paramount. Gel electrophoresis offers a cost-effective and well-established method for analyzing these biomolecules, making it an attractive option for researchers and pharmaceutical companies alike.
The oncology segment represents a significant portion of the clinical trial market, and it is expected to be a major contributor to the demand for gel electrophoresis. Cancer research often involves the analysis of genetic mutations and protein expression patterns, areas where gel electrophoresis excels. Additionally, the growing interest in rare diseases and orphan drugs is likely to drive further demand for gel electrophoresis techniques in clinical trials targeting these conditions.
Another factor influencing the market demand for gel electrophoresis in clinical trials is the increasing adoption of personalized medicine approaches. As clinical trials move towards more targeted therapies based on individual genetic profiles, the need for precise molecular analysis techniques like gel electrophoresis becomes more pronounced. This trend is particularly evident in fields such as pharmacogenomics, where gel electrophoresis can help identify genetic variations that influence drug response.
The COVID-19 pandemic has also had a significant impact on the clinical trial landscape, with a surge in vaccine and therapeutic development efforts. This has led to an increased demand for gel electrophoresis techniques in the analysis of viral proteins and nucleic acids, as well as in the evaluation of immune responses in vaccine trials. The ongoing need for COVID-19 research and the potential for future pandemic preparedness are likely to sustain this demand in the coming years.
The global clinical trial market is projected to continue its upward trajectory, with a compound annual growth rate (CAGR) expected to remain strong over the next five years. This growth is fueled by factors such as the rising prevalence of chronic diseases, increased R&D investments by pharmaceutical and biotechnology companies, and the growing emphasis on personalized medicine. As a result, the demand for gel electrophoresis techniques in clinical trials is also expected to surge.
One of the key drivers of gel electrophoresis demand in clinical trials is the increasing focus on genomics and proteomics research. As more clinical trials incorporate genetic and protein-based biomarkers, the need for reliable and efficient separation techniques becomes paramount. Gel electrophoresis offers a cost-effective and well-established method for analyzing these biomolecules, making it an attractive option for researchers and pharmaceutical companies alike.
The oncology segment represents a significant portion of the clinical trial market, and it is expected to be a major contributor to the demand for gel electrophoresis. Cancer research often involves the analysis of genetic mutations and protein expression patterns, areas where gel electrophoresis excels. Additionally, the growing interest in rare diseases and orphan drugs is likely to drive further demand for gel electrophoresis techniques in clinical trials targeting these conditions.
Another factor influencing the market demand for gel electrophoresis in clinical trials is the increasing adoption of personalized medicine approaches. As clinical trials move towards more targeted therapies based on individual genetic profiles, the need for precise molecular analysis techniques like gel electrophoresis becomes more pronounced. This trend is particularly evident in fields such as pharmacogenomics, where gel electrophoresis can help identify genetic variations that influence drug response.
The COVID-19 pandemic has also had a significant impact on the clinical trial landscape, with a surge in vaccine and therapeutic development efforts. This has led to an increased demand for gel electrophoresis techniques in the analysis of viral proteins and nucleic acids, as well as in the evaluation of immune responses in vaccine trials. The ongoing need for COVID-19 research and the potential for future pandemic preparedness are likely to sustain this demand in the coming years.
Current Challenges in Gel Electrophoresis
Gel electrophoresis, a cornerstone technique in molecular biology and clinical diagnostics, faces several significant challenges in its application to clinical trials. These challenges stem from both technical limitations and the evolving demands of modern clinical research.
One of the primary challenges is the limited resolution and sensitivity of traditional gel electrophoresis methods. As clinical trials increasingly focus on detecting minute changes in biomarkers or rare genetic variants, the ability to resolve and visualize small differences in DNA or protein fragments becomes crucial. Standard agarose and polyacrylamide gels often struggle to provide the necessary level of detail, particularly for complex mixtures or low-abundance targets.
Reproducibility and standardization pose another significant hurdle. The manual nature of gel preparation and running conditions can lead to variability between experiments and laboratories. This inconsistency can complicate multi-center clinical trials, where uniformity in results across different sites is essential for data integrity and regulatory compliance.
Time and labor intensity represent additional challenges. Gel electrophoresis, especially when dealing with large sample numbers typical in clinical trials, can be time-consuming and labor-intensive. This not only impacts the efficiency of trials but also increases the potential for human error, which can compromise data quality and reliability.
The quantitative analysis of gel electrophoresis results presents another obstacle. While densitometry techniques have improved, achieving accurate and reproducible quantification remains challenging, particularly for complex protein mixtures or when dealing with post-translational modifications. This limitation can hinder the precise measurement of biomarkers or drug effects in clinical trials.
Environmental and safety concerns also pose challenges. The use of potentially hazardous chemicals, such as ethidium bromide for DNA staining, raises safety issues and necessitates special handling and disposal procedures. This can complicate the implementation of gel electrophoresis in clinical settings where such facilities may be limited.
Automation and integration with other analytical techniques represent ongoing challenges. While some aspects of gel electrophoresis have been automated, fully integrated systems that seamlessly fit into the workflow of modern clinical trials are still lacking. This gap hinders the technique's incorporation into high-throughput screening processes often required in large-scale clinical studies.
Lastly, the emergence of alternative technologies, such as capillary electrophoresis and next-generation sequencing, presents a challenge to the continued relevance of traditional gel electrophoresis in clinical trials. These newer methods often offer advantages in terms of speed, sensitivity, and automation, pushing gel electrophoresis to evolve and find its niche in the changing landscape of clinical research methodologies.
One of the primary challenges is the limited resolution and sensitivity of traditional gel electrophoresis methods. As clinical trials increasingly focus on detecting minute changes in biomarkers or rare genetic variants, the ability to resolve and visualize small differences in DNA or protein fragments becomes crucial. Standard agarose and polyacrylamide gels often struggle to provide the necessary level of detail, particularly for complex mixtures or low-abundance targets.
Reproducibility and standardization pose another significant hurdle. The manual nature of gel preparation and running conditions can lead to variability between experiments and laboratories. This inconsistency can complicate multi-center clinical trials, where uniformity in results across different sites is essential for data integrity and regulatory compliance.
Time and labor intensity represent additional challenges. Gel electrophoresis, especially when dealing with large sample numbers typical in clinical trials, can be time-consuming and labor-intensive. This not only impacts the efficiency of trials but also increases the potential for human error, which can compromise data quality and reliability.
The quantitative analysis of gel electrophoresis results presents another obstacle. While densitometry techniques have improved, achieving accurate and reproducible quantification remains challenging, particularly for complex protein mixtures or when dealing with post-translational modifications. This limitation can hinder the precise measurement of biomarkers or drug effects in clinical trials.
Environmental and safety concerns also pose challenges. The use of potentially hazardous chemicals, such as ethidium bromide for DNA staining, raises safety issues and necessitates special handling and disposal procedures. This can complicate the implementation of gel electrophoresis in clinical settings where such facilities may be limited.
Automation and integration with other analytical techniques represent ongoing challenges. While some aspects of gel electrophoresis have been automated, fully integrated systems that seamlessly fit into the workflow of modern clinical trials are still lacking. This gap hinders the technique's incorporation into high-throughput screening processes often required in large-scale clinical studies.
Lastly, the emergence of alternative technologies, such as capillary electrophoresis and next-generation sequencing, presents a challenge to the continued relevance of traditional gel electrophoresis in clinical trials. These newer methods often offer advantages in terms of speed, sensitivity, and automation, pushing gel electrophoresis to evolve and find its niche in the changing landscape of clinical research methodologies.
Current Gel Electrophoresis Techniques
01 Improved separation and resolution techniques
Advanced gel electrophoresis methods have been developed to enhance separation and resolution of biomolecules. These techniques include pulsed-field gel electrophoresis, two-dimensional gel electrophoresis, and capillary gel electrophoresis. These improvements allow for better analysis of complex mixtures and larger molecules, increasing the accuracy and efficiency of molecular biology research.- Improved separation and resolution techniques: Advanced gel electrophoresis methods have been developed to enhance separation and resolution of biomolecules. These techniques include pulsed-field gel electrophoresis, two-dimensional gel electrophoresis, and capillary gel electrophoresis. These improvements allow for better analysis of complex mixtures and larger molecules, increasing the accuracy and efficiency of molecular biology research.
- Novel gel compositions and materials: Researchers have developed new gel compositions and materials to optimize electrophoresis performance. These include temperature-responsive hydrogels, composite gels with nanoparticles, and specialized polymer matrices. These novel materials can improve separation efficiency, reduce run times, and enhance the detection of specific biomolecules.
- Integration with other analytical techniques: Gel electrophoresis has been integrated with other analytical techniques to create powerful hybrid methods. These combinations include gel electrophoresis-mass spectrometry, electrophoresis-immunoassays, and microfluidic electrophoresis systems. Such integrations expand the capabilities of gel electrophoresis, allowing for more comprehensive analysis of complex biological samples.
- Automation and high-throughput systems: Advancements in automation and high-throughput systems have significantly increased the efficiency and reproducibility of gel electrophoresis. These developments include robotic sample loading, automated gel casting, and integrated imaging systems. Such innovations reduce human error, increase sample throughput, and allow for more standardized and comparable results across experiments.
- Environmental and safety improvements: Efforts have been made to develop more environmentally friendly and safer gel electrophoresis methods. These include the use of non-toxic gel stains, biodegradable gel materials, and low-voltage systems. Such improvements reduce the environmental impact of electrophoresis experiments and enhance laboratory safety for researchers.
02 Novel gel compositions and materials
Researchers have developed new gel compositions and materials to improve the performance of gel electrophoresis. These include temperature-responsive hydrogels, composite gels with nanoparticles, and specialized polymer matrices. These novel materials can enhance separation efficiency, reduce run times, and improve the overall quality of results in various applications.Expand Specific Solutions03 Integration with other analytical techniques
Gel electrophoresis has been integrated with other analytical techniques to create powerful hybrid methods. These combinations include gel electrophoresis-mass spectrometry, electrophoresis-chromatography systems, and microfluidic devices incorporating gel electrophoresis. These integrated approaches expand the capabilities of gel electrophoresis and enable more comprehensive analysis of complex biological samples.Expand Specific Solutions04 Automation and high-throughput systems
Advancements in automation and high-throughput systems have significantly impacted gel electrophoresis. These developments include robotic sample loading, automated gel imaging and analysis, and parallel electrophoresis systems. Such innovations increase sample throughput, reduce human error, and improve reproducibility in research and diagnostic applications.Expand Specific Solutions05 Environmental and safety improvements
Efforts have been made to develop more environmentally friendly and safer gel electrophoresis methods. These include the use of non-toxic gel alternatives, reduced chemical waste production, and improved safety features in electrophoresis equipment. These advancements contribute to a more sustainable and safer laboratory environment while maintaining the effectiveness of gel electrophoresis techniques.Expand Specific Solutions
Key Industry Players and Competition
The gel electrophoresis market in clinical trials is experiencing significant growth, driven by increasing demand for personalized medicine and advancements in genomic research. The industry is in a mature stage, with established players like Life Technologies Corp., Agilent Technologies, and Beckman Coulter dominating the market. However, emerging technologies and innovative startups are continuously challenging the status quo. The global market size for gel electrophoresis is projected to reach several billion dollars by 2025, with a steady CAGR. Technologically, the field is evolving rapidly, with companies like Thermo Fisher Scientific (through Pierce Biotechnology) and Helena Laboratories Corp. investing heavily in R&D to improve resolution, speed, and automation of gel electrophoresis systems for clinical applications.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed advanced gel electrophoresis systems for clinical trials, focusing on high-resolution protein separation. Their 2100 Bioanalyzer system combines microfluidics and gel electrophoresis for rapid, automated analysis of DNA, RNA, and proteins[1]. This technology allows for precise quantification and sizing of nucleic acids and proteins, crucial for clinical trial sample analysis. Agilent has also introduced the TapeStation systems, which offer automated electrophoresis with minimal sample input, ideal for precious clinical samples[2]. These systems provide digital data output, enabling easy integration into clinical trial workflows and data management systems.
Strengths: High automation, minimal sample requirements, and digital data output. Weaknesses: Higher cost compared to traditional gel electrophoresis methods, may require specialized training for operation.
Beckman Coulter, Inc.
Technical Solution: Beckman Coulter has innovated in the field of gel electrophoresis with their ProteomeLab PA 800 system, which combines capillary electrophoresis with various detection methods for high-resolution protein analysis in clinical trials[3]. This system allows for the separation and characterization of complex protein mixtures, post-translational modifications, and biomarkers. Beckman Coulter has also developed the P/ACE MDQ Plus Capillary Electrophoresis System, which offers multiple detection options and can be used for both protein and nucleic acid analysis in clinical trial samples[4]. These systems provide high sensitivity and reproducibility, critical for the rigorous standards of clinical trials.
Strengths: High resolution, versatility in detection methods, and applicability to various biomolecules. Weaknesses: Complexity of operation may require specialized training, higher initial investment compared to traditional gel electrophoresis.
Innovative Gel Electrophoresis Methods
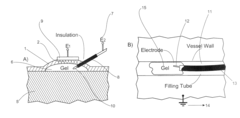
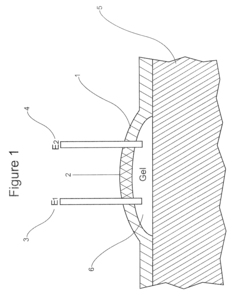
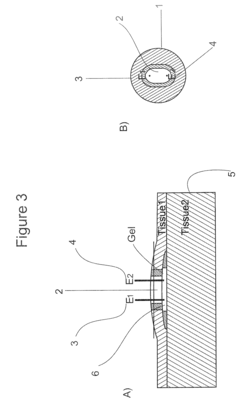
Gels with predetermined conductivity used in electroporation of tissue
PatentActiveUS7674249B2
Innovation
- The use of gels with adjustable conductivity, either lower or matched to surrounding tissue, to direct current flow to specific areas during electroporation, combined with finite element computer simulations and injectable electrodes to optimize electric field distribution and protect sensitive tissues.
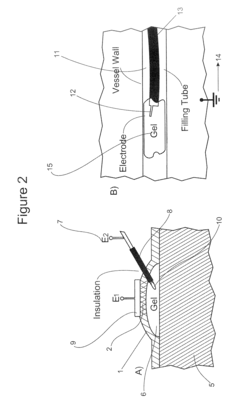
Electrode for controlling and monitoring gel strips individually
PatentInactiveEP1686370A1
Innovation
- An electrode arrangement that allows for individual electrical contact and control of each gel strip, enabling separate monitoring and control of electrical properties, such as voltage and current, to optimize the electrophoresis process and detect errors like dehydration or short circuits.
Regulatory Compliance in Clinical Trials
Regulatory compliance is a critical aspect of clinical trials involving gel electrophoresis. The use of this technique in clinical studies must adhere to strict guidelines set forth by regulatory bodies such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe. These regulations ensure the safety of participants, the integrity of data, and the validity of trial results.
One of the primary regulatory considerations for gel electrophoresis in clinical trials is the validation of the method. Researchers must demonstrate that the gel electrophoresis technique used is accurate, precise, and reproducible. This involves developing and documenting standard operating procedures (SOPs) that detail every step of the process, from sample preparation to data analysis.
Quality control measures are essential to maintain regulatory compliance. This includes regular calibration of equipment, use of appropriate controls, and implementation of a robust quality management system. Laboratories conducting gel electrophoresis for clinical trials must also be certified and accredited, often requiring compliance with Good Laboratory Practice (GLP) standards.
Data integrity is another crucial aspect of regulatory compliance. All raw data, including gel images and densitometry results, must be securely stored and easily retrievable. Electronic data systems used in gel electrophoresis analysis must comply with 21 CFR Part 11, which outlines requirements for electronic records and electronic signatures in FDA-regulated industries.
Informed consent is a fundamental requirement in clinical trials, and this extends to the use of gel electrophoresis. Participants must be informed about the purpose of the technique, the type of samples that will be analyzed, and how their genetic information will be used and protected. This is particularly important when gel electrophoresis is used for genetic analysis or biomarker studies.
Ethical considerations also play a significant role in regulatory compliance. The use of gel electrophoresis in clinical trials must be approved by institutional review boards (IRBs) or ethics committees. These bodies ensure that the research protocol is scientifically sound and that the rights and welfare of participants are protected.
Lastly, reporting and documentation are critical components of regulatory compliance. Researchers must maintain detailed records of all gel electrophoresis procedures, results, and any deviations from the protocol. These records may be subject to regulatory inspections and audits, and they form an essential part of the final clinical trial report submitted to regulatory agencies.
One of the primary regulatory considerations for gel electrophoresis in clinical trials is the validation of the method. Researchers must demonstrate that the gel electrophoresis technique used is accurate, precise, and reproducible. This involves developing and documenting standard operating procedures (SOPs) that detail every step of the process, from sample preparation to data analysis.
Quality control measures are essential to maintain regulatory compliance. This includes regular calibration of equipment, use of appropriate controls, and implementation of a robust quality management system. Laboratories conducting gel electrophoresis for clinical trials must also be certified and accredited, often requiring compliance with Good Laboratory Practice (GLP) standards.
Data integrity is another crucial aspect of regulatory compliance. All raw data, including gel images and densitometry results, must be securely stored and easily retrievable. Electronic data systems used in gel electrophoresis analysis must comply with 21 CFR Part 11, which outlines requirements for electronic records and electronic signatures in FDA-regulated industries.
Informed consent is a fundamental requirement in clinical trials, and this extends to the use of gel electrophoresis. Participants must be informed about the purpose of the technique, the type of samples that will be analyzed, and how their genetic information will be used and protected. This is particularly important when gel electrophoresis is used for genetic analysis or biomarker studies.
Ethical considerations also play a significant role in regulatory compliance. The use of gel electrophoresis in clinical trials must be approved by institutional review boards (IRBs) or ethics committees. These bodies ensure that the research protocol is scientifically sound and that the rights and welfare of participants are protected.
Lastly, reporting and documentation are critical components of regulatory compliance. Researchers must maintain detailed records of all gel electrophoresis procedures, results, and any deviations from the protocol. These records may be subject to regulatory inspections and audits, and they form an essential part of the final clinical trial report submitted to regulatory agencies.
Cost-Benefit Analysis of Gel Electrophoresis
The cost-benefit analysis of gel electrophoresis in clinical trials reveals a complex interplay of financial considerations and scientific value. Initial equipment costs for gel electrophoresis systems can be substantial, ranging from $5,000 to $20,000 for basic setups to over $100,000 for advanced automated systems. However, these upfront investments are often offset by the technique's versatility and long-term utility in various clinical trial applications.
Operational costs, including reagents, buffers, and consumables, typically amount to $10-$50 per sample. While this may seem modest, the cumulative expense can be significant in large-scale clinical trials involving thousands of samples. Labor costs also contribute substantially, as skilled technicians are required to prepare and run gels, as well as interpret results. On average, a single gel electrophoresis experiment may require 2-4 hours of hands-on time.
Despite these costs, gel electrophoresis offers numerous benefits that justify its use in clinical trials. Its high resolution and sensitivity allow for precise separation and identification of biomolecules, crucial for accurate patient stratification and biomarker analysis. The technique's reliability and reproducibility contribute to data integrity, a critical factor in regulatory submissions and peer-reviewed publications.
Time-efficiency is another key advantage. Gel electrophoresis can process multiple samples simultaneously, reducing overall analysis time compared to some alternative methods. This capability is particularly valuable in large-scale clinical trials where rapid data generation is essential for timely decision-making and interim analyses.
The technique's adaptability to various biomolecules (DNA, RNA, proteins) provides a cost-effective solution for multi-omics studies, potentially reducing the need for multiple specialized instruments. This versatility can lead to significant cost savings in comprehensive clinical trial designs that investigate multiple molecular endpoints.
When considering alternatives, such as capillary electrophoresis or next-generation sequencing, gel electrophoresis often emerges as a cost-effective option for specific applications. While these newer technologies may offer higher throughput or more detailed molecular information, they typically come with substantially higher costs per sample and require more specialized expertise.
In conclusion, while gel electrophoresis does incur notable costs in clinical trials, its scientific value, versatility, and reliability often outweigh these expenses. The technique's ability to provide crucial molecular insights at a relatively moderate cost makes it an indispensable tool in many clinical trial settings, particularly in early-phase studies or when budget constraints are significant considerations.
Operational costs, including reagents, buffers, and consumables, typically amount to $10-$50 per sample. While this may seem modest, the cumulative expense can be significant in large-scale clinical trials involving thousands of samples. Labor costs also contribute substantially, as skilled technicians are required to prepare and run gels, as well as interpret results. On average, a single gel electrophoresis experiment may require 2-4 hours of hands-on time.
Despite these costs, gel electrophoresis offers numerous benefits that justify its use in clinical trials. Its high resolution and sensitivity allow for precise separation and identification of biomolecules, crucial for accurate patient stratification and biomarker analysis. The technique's reliability and reproducibility contribute to data integrity, a critical factor in regulatory submissions and peer-reviewed publications.
Time-efficiency is another key advantage. Gel electrophoresis can process multiple samples simultaneously, reducing overall analysis time compared to some alternative methods. This capability is particularly valuable in large-scale clinical trials where rapid data generation is essential for timely decision-making and interim analyses.
The technique's adaptability to various biomolecules (DNA, RNA, proteins) provides a cost-effective solution for multi-omics studies, potentially reducing the need for multiple specialized instruments. This versatility can lead to significant cost savings in comprehensive clinical trial designs that investigate multiple molecular endpoints.
When considering alternatives, such as capillary electrophoresis or next-generation sequencing, gel electrophoresis often emerges as a cost-effective option for specific applications. While these newer technologies may offer higher throughput or more detailed molecular information, they typically come with substantially higher costs per sample and require more specialized expertise.
In conclusion, while gel electrophoresis does incur notable costs in clinical trials, its scientific value, versatility, and reliability often outweigh these expenses. The technique's ability to provide crucial molecular insights at a relatively moderate cost makes it an indispensable tool in many clinical trial settings, particularly in early-phase studies or when budget constraints are significant considerations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!