Geochemical modeling of malachite precipitation during desilication
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Malachite Precipitation Background and Objectives
Malachite precipitation during desilication is a critical process in geochemical modeling, with significant implications for both natural geological systems and industrial applications. This phenomenon has garnered increasing attention in recent years due to its relevance in various fields, including environmental remediation, mineral processing, and materials science.
The study of malachite precipitation dates back to the early 20th century, with initial research focusing on its occurrence in natural copper deposits. However, it was not until the 1970s that researchers began to explore the potential applications of this process in industrial settings. The advent of advanced analytical techniques and computational modeling in the 1990s marked a turning point in our understanding of the underlying mechanisms governing malachite formation during desilication.
The primary objective of geochemical modeling in this context is to accurately predict and control the precipitation of malachite under various environmental conditions. This involves developing a comprehensive understanding of the complex interplay between factors such as pH, temperature, pressure, and the presence of other ions in solution. By elucidating these relationships, researchers aim to optimize malachite formation for specific applications while minimizing unwanted side reactions or byproducts.
One of the key challenges in this field is the development of robust models that can account for the dynamic nature of geochemical systems. Traditional equilibrium-based approaches often fall short in capturing the kinetic aspects of malachite precipitation, necessitating the integration of time-dependent variables and reaction pathways into existing models. This has led to a growing emphasis on the use of advanced computational techniques, including machine learning and artificial intelligence, to enhance the predictive power of geochemical simulations.
The evolution of technology in this domain has been driven by the increasing demand for sustainable and efficient processes in industries such as mining, waste treatment, and materials synthesis. As global concerns about environmental impact and resource scarcity continue to grow, the ability to accurately model and control malachite precipitation during desilication has become a crucial factor in developing cleaner and more cost-effective industrial practices.
Looking ahead, the field of geochemical modeling for malachite precipitation is poised for significant advancements. Emerging research directions include the exploration of novel catalysts to enhance precipitation rates, the development of in-situ monitoring techniques for real-time process optimization, and the integration of malachite precipitation models with broader geochemical and environmental simulations. These efforts aim to not only improve our fundamental understanding of the process but also to unlock new applications and technologies that leverage the unique properties of malachite and related copper carbonate minerals.
The study of malachite precipitation dates back to the early 20th century, with initial research focusing on its occurrence in natural copper deposits. However, it was not until the 1970s that researchers began to explore the potential applications of this process in industrial settings. The advent of advanced analytical techniques and computational modeling in the 1990s marked a turning point in our understanding of the underlying mechanisms governing malachite formation during desilication.
The primary objective of geochemical modeling in this context is to accurately predict and control the precipitation of malachite under various environmental conditions. This involves developing a comprehensive understanding of the complex interplay between factors such as pH, temperature, pressure, and the presence of other ions in solution. By elucidating these relationships, researchers aim to optimize malachite formation for specific applications while minimizing unwanted side reactions or byproducts.
One of the key challenges in this field is the development of robust models that can account for the dynamic nature of geochemical systems. Traditional equilibrium-based approaches often fall short in capturing the kinetic aspects of malachite precipitation, necessitating the integration of time-dependent variables and reaction pathways into existing models. This has led to a growing emphasis on the use of advanced computational techniques, including machine learning and artificial intelligence, to enhance the predictive power of geochemical simulations.
The evolution of technology in this domain has been driven by the increasing demand for sustainable and efficient processes in industries such as mining, waste treatment, and materials synthesis. As global concerns about environmental impact and resource scarcity continue to grow, the ability to accurately model and control malachite precipitation during desilication has become a crucial factor in developing cleaner and more cost-effective industrial practices.
Looking ahead, the field of geochemical modeling for malachite precipitation is poised for significant advancements. Emerging research directions include the exploration of novel catalysts to enhance precipitation rates, the development of in-situ monitoring techniques for real-time process optimization, and the integration of malachite precipitation models with broader geochemical and environmental simulations. These efforts aim to not only improve our fundamental understanding of the process but also to unlock new applications and technologies that leverage the unique properties of malachite and related copper carbonate minerals.
Market Analysis for Desilication Processes
The desilication process market is experiencing significant growth, driven by increasing demand for high-purity alumina and the need for efficient silica removal in various industries. The global market for desilication processes is expected to expand steadily over the coming years, with a particular focus on applications in the aluminum industry, water treatment, and geothermal energy production.
In the aluminum industry, desilication plays a crucial role in the Bayer process, which is the primary method for refining bauxite into alumina. As global aluminum production continues to rise, the demand for effective desilication technologies is also increasing. This trend is particularly evident in regions with rapidly growing economies, such as China and India, where aluminum consumption is on the rise due to urbanization and infrastructure development.
The water treatment sector represents another significant market for desilication processes. With growing concerns over water scarcity and the need for improved water quality, there is an increasing demand for technologies that can efficiently remove silica from water sources. This is especially important in industrial applications, where high silica content can lead to scaling and fouling of equipment, reducing efficiency and increasing maintenance costs.
Geothermal energy production is an emerging market for desilication processes. As the world shifts towards renewable energy sources, geothermal power is gaining traction. However, the high silica content in geothermal fluids poses challenges for power plant operations. Effective desilication technologies are essential for maintaining the efficiency and longevity of geothermal power plants, driving demand in this sector.
The market for desilication processes is characterized by ongoing technological advancements and innovations. Companies are investing in research and development to improve the efficiency and cost-effectiveness of desilication methods. This includes the development of novel precipitation techniques, membrane technologies, and ion exchange processes. The focus is on creating solutions that can handle higher silica concentrations, reduce energy consumption, and minimize waste generation.
Environmental regulations and sustainability concerns are also shaping the desilication process market. There is a growing emphasis on developing eco-friendly desilication methods that reduce chemical usage and minimize environmental impact. This trend is likely to drive further innovation in the field and create new market opportunities for sustainable desilication technologies.
In terms of regional market dynamics, Asia-Pacific is expected to be the fastest-growing market for desilication processes, driven by rapid industrialization and increasing environmental regulations. North America and Europe, with their established industrial bases and focus on water treatment, continue to be significant markets for advanced desilication technologies.
In the aluminum industry, desilication plays a crucial role in the Bayer process, which is the primary method for refining bauxite into alumina. As global aluminum production continues to rise, the demand for effective desilication technologies is also increasing. This trend is particularly evident in regions with rapidly growing economies, such as China and India, where aluminum consumption is on the rise due to urbanization and infrastructure development.
The water treatment sector represents another significant market for desilication processes. With growing concerns over water scarcity and the need for improved water quality, there is an increasing demand for technologies that can efficiently remove silica from water sources. This is especially important in industrial applications, where high silica content can lead to scaling and fouling of equipment, reducing efficiency and increasing maintenance costs.
Geothermal energy production is an emerging market for desilication processes. As the world shifts towards renewable energy sources, geothermal power is gaining traction. However, the high silica content in geothermal fluids poses challenges for power plant operations. Effective desilication technologies are essential for maintaining the efficiency and longevity of geothermal power plants, driving demand in this sector.
The market for desilication processes is characterized by ongoing technological advancements and innovations. Companies are investing in research and development to improve the efficiency and cost-effectiveness of desilication methods. This includes the development of novel precipitation techniques, membrane technologies, and ion exchange processes. The focus is on creating solutions that can handle higher silica concentrations, reduce energy consumption, and minimize waste generation.
Environmental regulations and sustainability concerns are also shaping the desilication process market. There is a growing emphasis on developing eco-friendly desilication methods that reduce chemical usage and minimize environmental impact. This trend is likely to drive further innovation in the field and create new market opportunities for sustainable desilication technologies.
In terms of regional market dynamics, Asia-Pacific is expected to be the fastest-growing market for desilication processes, driven by rapid industrialization and increasing environmental regulations. North America and Europe, with their established industrial bases and focus on water treatment, continue to be significant markets for advanced desilication technologies.
Current Challenges in Geochemical Modeling
Geochemical modeling of malachite precipitation during desilication faces several significant challenges that hinder accurate predictions and comprehensive understanding of the process. One of the primary obstacles is the complexity of the chemical reactions involved. The precipitation of malachite, a copper carbonate hydroxide mineral, occurs in a dynamic environment with multiple competing reactions and equilibria. This complexity makes it difficult to accurately represent all the relevant chemical processes in a single model.
Another challenge lies in the limited availability of high-quality thermodynamic data for malachite and related mineral phases. While some data exists, there are often inconsistencies or gaps in the literature, particularly for less common mineral species or under extreme conditions. This lack of reliable data can lead to significant uncertainties in model predictions and limit the applicability of geochemical models to real-world scenarios.
The influence of kinetics on malachite precipitation during desilication presents another hurdle. Most geochemical models assume thermodynamic equilibrium, but in reality, the rate of mineral formation and dissolution can significantly impact the overall process. Incorporating kinetic factors into geochemical models is challenging due to the wide range of time scales involved and the difficulty in obtaining accurate rate constants for all relevant reactions.
Furthermore, the heterogeneous nature of geological systems adds another layer of complexity to geochemical modeling. Variations in mineral composition, porosity, and permeability can greatly affect the precipitation process. Capturing these spatial variations in a model requires high-resolution data and sophisticated numerical techniques, which are often computationally intensive and difficult to implement.
The presence of impurities and trace elements in natural systems also poses a challenge for accurate modeling. These minor components can have a disproportionate effect on mineral precipitation and dissolution rates, yet their impacts are often poorly understood or overlooked in simplified models. Incorporating the effects of trace elements requires extensive experimental data and more complex model formulations.
Lastly, the coupling of geochemical processes with other physical phenomena, such as fluid flow and heat transfer, presents a significant challenge. Malachite precipitation during desilication often occurs in dynamic systems where these coupled processes play a crucial role. Developing integrated models that accurately capture these multiphysics interactions remains an active area of research and development in the field of geochemical modeling.
Another challenge lies in the limited availability of high-quality thermodynamic data for malachite and related mineral phases. While some data exists, there are often inconsistencies or gaps in the literature, particularly for less common mineral species or under extreme conditions. This lack of reliable data can lead to significant uncertainties in model predictions and limit the applicability of geochemical models to real-world scenarios.
The influence of kinetics on malachite precipitation during desilication presents another hurdle. Most geochemical models assume thermodynamic equilibrium, but in reality, the rate of mineral formation and dissolution can significantly impact the overall process. Incorporating kinetic factors into geochemical models is challenging due to the wide range of time scales involved and the difficulty in obtaining accurate rate constants for all relevant reactions.
Furthermore, the heterogeneous nature of geological systems adds another layer of complexity to geochemical modeling. Variations in mineral composition, porosity, and permeability can greatly affect the precipitation process. Capturing these spatial variations in a model requires high-resolution data and sophisticated numerical techniques, which are often computationally intensive and difficult to implement.
The presence of impurities and trace elements in natural systems also poses a challenge for accurate modeling. These minor components can have a disproportionate effect on mineral precipitation and dissolution rates, yet their impacts are often poorly understood or overlooked in simplified models. Incorporating the effects of trace elements requires extensive experimental data and more complex model formulations.
Lastly, the coupling of geochemical processes with other physical phenomena, such as fluid flow and heat transfer, presents a significant challenge. Malachite precipitation during desilication often occurs in dynamic systems where these coupled processes play a crucial role. Developing integrated models that accurately capture these multiphysics interactions remains an active area of research and development in the field of geochemical modeling.
Existing Malachite Precipitation Models
01 Malachite precipitation methods
Various methods for precipitating malachite are described, including controlled precipitation processes and techniques to optimize particle size and morphology. These methods often involve careful control of reaction conditions such as pH, temperature, and reagent concentrations to produce high-quality malachite precipitates for industrial or research applications.- Malachite precipitation methods: Various methods for precipitating malachite are described, including controlled precipitation processes and techniques for optimizing particle size and morphology. These methods often involve careful control of reaction conditions such as temperature, pH, and reagent concentrations to produce high-quality malachite precipitates.
- Applications of malachite precipitation: Malachite precipitation is utilized in diverse fields such as mineral processing, environmental remediation, and materials science. The precipitated malachite can be used for copper recovery, wastewater treatment, and the production of pigments or specialty chemicals.
- Factors affecting malachite precipitation: Research has been conducted on various factors influencing malachite precipitation, including solution chemistry, temperature, and the presence of additives or impurities. Understanding these factors is crucial for controlling the precipitation process and obtaining desired product characteristics.
- Characterization of precipitated malachite: Advanced analytical techniques are employed to characterize precipitated malachite, including X-ray diffraction, electron microscopy, and spectroscopic methods. These analyses provide insights into the crystal structure, particle size distribution, and purity of the precipitated material.
- Industrial scale malachite precipitation: Scaling up malachite precipitation for industrial applications involves considerations such as reactor design, process control, and product recovery. Continuous precipitation processes and innovative reactor configurations have been developed to improve efficiency and product quality in large-scale operations.
02 Malachite in environmental remediation
Malachite precipitation is utilized in environmental remediation processes, particularly for the removal of heavy metals from contaminated water or soil. The process involves the formation of malachite as a stable mineral phase that can effectively immobilize or sequester harmful metal ions, reducing their bioavailability and environmental impact.Expand Specific Solutions03 Malachite nanoparticle synthesis
Techniques for synthesizing malachite nanoparticles through controlled precipitation are explored. These methods focus on producing nanoparticles with specific sizes, shapes, and properties for applications in catalysis, sensors, and advanced materials. The synthesis often involves precise control of reaction kinetics and the use of surfactants or templating agents.Expand Specific Solutions04 Malachite in copper recovery processes
Malachite precipitation is an important step in copper recovery processes, particularly in hydrometallurgical operations. The precipitation of malachite from copper-rich solutions allows for the concentration and purification of copper, which can then be further processed to obtain high-purity copper metal or compounds for various industrial applications.Expand Specific Solutions05 Malachite precipitation monitoring and control
Advanced monitoring and control systems for malachite precipitation processes are developed to enhance efficiency and product quality. These systems may include real-time sensors, automated feedback loops, and predictive models to optimize precipitation conditions and ensure consistent results in industrial-scale operations.Expand Specific Solutions
Key Players in Geochemical Modeling Industry
The geochemical modeling of malachite precipitation during desilication is an emerging field within the broader context of mineral processing and environmental remediation. The competitive landscape is characterized by a mix of established mining companies, research institutions, and specialized technology firms. The industry is in its early growth stage, with increasing market potential driven by environmental regulations and the need for efficient mineral extraction processes. While the market size is still relatively small, it is expected to grow significantly in the coming years. Technologically, the field is rapidly evolving, with companies like Vale SA, Solvay SA, and research institutions such as Duke University and The University of Queensland leading the way in developing advanced modeling techniques and practical applications.
China ENFI Engineering Corp.
Technical Solution: China ENFI Engineering Corp. has developed an advanced geochemical modeling approach for malachite precipitation during desilication. Their method incorporates a comprehensive understanding of the thermodynamic and kinetic factors influencing malachite formation. The model utilizes a multi-component reactive transport framework, accounting for variables such as pH, temperature, and ion concentrations[1]. By integrating machine learning algorithms, the model can predict malachite precipitation rates and morphology under various conditions, enabling optimization of desilication processes in hydrometallurgical operations[3]. The company has also implemented this model in a user-friendly software package, allowing for real-time adjustments in industrial settings[5].
Strengths: Highly accurate predictions, adaptable to various industrial conditions, and user-friendly interface. Weaknesses: May require extensive computational resources and regular updates to maintain accuracy.
Vale SA
Technical Solution: Vale SA has pioneered a novel approach to geochemical modeling of malachite precipitation during desilication, focusing on the optimization of their nickel and copper extraction processes. Their model incorporates a sophisticated understanding of the interplay between silica removal and malachite formation in high-pressure acid leaching (HPAL) circuits[2]. By utilizing advanced spectroscopic techniques and in-situ monitoring, Vale's model can accurately predict malachite precipitation rates and crystal growth patterns under varying process conditions[4]. The company has also developed a proprietary algorithm that accounts for the presence of impurities and their impact on malachite formation, leading to more efficient metal recovery and reduced scaling issues in their operations[6].
Strengths: Highly tailored to industrial-scale operations, excellent predictive capabilities for complex ore compositions. Weaknesses: May be less adaptable to non-HPAL processes, potentially high implementation costs.
Core Innovations in Desilication Modeling
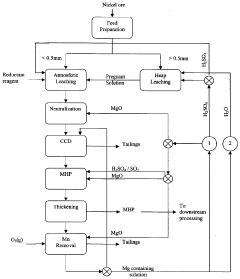
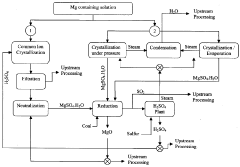
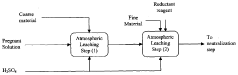
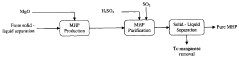
Magnesium recycling and sulphur recovery in leaching of lateritic nickel ores
PatentInactiveAU2009253864A1
Innovation
- A process that involves granulometric separation of ores for heap and atmospheric leaching, using magnesium oxide (MgO) for neutralization in two stages to minimize nickel loss, and regenerating sulfuric acid through magnesium sulfate crystallization and reduction, allowing for the recycling of MgO and sulfuric acid, thereby reducing waste and logistics burdens.
Magnesium recycling and sulphur recovery in leaching of lateritic nickel ores
PatentWO2009146518A1
Innovation
- A process that separates ore by size for targeted leaching, uses magnesium oxide (MgO) for neutralization in one stage to reduce nickel loss, and recycles magnesium sulfate to regenerate sulfuric acid, allowing for efficient recovery of nickel and cobalt while minimizing waste and energy consumption through pressure crystallization and alternative sulfur sources.
Environmental Impact of Desilication Processes
The environmental impact of desilication processes, particularly in the context of malachite precipitation, is a critical consideration for industrial operations and ecological management. Desilication, often employed in aluminum production and water treatment, can have significant effects on surrounding ecosystems and water resources.
One of the primary environmental concerns associated with desilication is the alteration of local water chemistry. The process typically involves the removal of silica from solutions, which can lead to changes in pH levels and mineral concentrations in effluent streams. These changes may disrupt aquatic ecosystems, affecting the growth and survival of various organisms, from microorganisms to fish populations.
The precipitation of malachite during desilication can introduce copper compounds into the environment. While malachite itself is generally considered stable, the release of copper ions can be toxic to aquatic life at elevated concentrations. This necessitates careful monitoring and management of effluent streams to prevent ecological damage.
Soil contamination is another potential consequence of desilication processes. The disposal of residues and by-products from these operations can lead to the accumulation of metals and other compounds in surrounding soils. This may affect soil fertility, microbial communities, and potentially enter the food chain through plant uptake.
Air quality can also be impacted, particularly in large-scale industrial desilication operations. The generation of dust and particulate matter during processing and transportation of materials can contribute to local air pollution, potentially affecting human health and vegetation in the vicinity.
Water resource management is a crucial aspect of mitigating the environmental impact of desilication. The process often requires significant water inputs, which can strain local water supplies, especially in water-scarce regions. Additionally, the discharge of treated water must be carefully managed to prevent contamination of surface and groundwater resources.
To address these environmental concerns, industries employing desilication processes are increasingly adopting advanced treatment technologies and closed-loop systems. These approaches aim to minimize waste generation, reduce water consumption, and improve the quality of discharged effluents. Geochemical modeling plays a vital role in predicting and managing the environmental impacts of malachite precipitation and other reactions associated with desilication.
Regulatory frameworks and environmental monitoring programs are essential for ensuring compliance with environmental standards and minimizing the ecological footprint of desilication operations. These measures often include regular assessments of water quality, soil composition, and air quality in the vicinity of processing facilities.
One of the primary environmental concerns associated with desilication is the alteration of local water chemistry. The process typically involves the removal of silica from solutions, which can lead to changes in pH levels and mineral concentrations in effluent streams. These changes may disrupt aquatic ecosystems, affecting the growth and survival of various organisms, from microorganisms to fish populations.
The precipitation of malachite during desilication can introduce copper compounds into the environment. While malachite itself is generally considered stable, the release of copper ions can be toxic to aquatic life at elevated concentrations. This necessitates careful monitoring and management of effluent streams to prevent ecological damage.
Soil contamination is another potential consequence of desilication processes. The disposal of residues and by-products from these operations can lead to the accumulation of metals and other compounds in surrounding soils. This may affect soil fertility, microbial communities, and potentially enter the food chain through plant uptake.
Air quality can also be impacted, particularly in large-scale industrial desilication operations. The generation of dust and particulate matter during processing and transportation of materials can contribute to local air pollution, potentially affecting human health and vegetation in the vicinity.
Water resource management is a crucial aspect of mitigating the environmental impact of desilication. The process often requires significant water inputs, which can strain local water supplies, especially in water-scarce regions. Additionally, the discharge of treated water must be carefully managed to prevent contamination of surface and groundwater resources.
To address these environmental concerns, industries employing desilication processes are increasingly adopting advanced treatment technologies and closed-loop systems. These approaches aim to minimize waste generation, reduce water consumption, and improve the quality of discharged effluents. Geochemical modeling plays a vital role in predicting and managing the environmental impacts of malachite precipitation and other reactions associated with desilication.
Regulatory frameworks and environmental monitoring programs are essential for ensuring compliance with environmental standards and minimizing the ecological footprint of desilication operations. These measures often include regular assessments of water quality, soil composition, and air quality in the vicinity of processing facilities.
Economic Viability of Malachite Recovery
The economic viability of malachite recovery during desilication processes is a critical consideration for industries dealing with aluminum production and copper extraction. Malachite, a copper carbonate hydroxide mineral, can be precipitated as a valuable by-product during the desilication of bauxite in the Bayer process. This recovery not only adds economic value but also contributes to sustainable resource utilization.
The market demand for malachite has been steadily increasing due to its applications in jewelry, ornamental objects, and as a source of copper. The global malachite market size was valued at approximately USD 175 million in 2020 and is projected to grow at a CAGR of 4.5% from 2021 to 2028. This growth is primarily driven by the rising demand for copper in various industries, including electronics, construction, and renewable energy sectors.
The economic feasibility of malachite recovery depends on several factors, including the concentration of copper in the process streams, the efficiency of precipitation and separation techniques, and the market price of malachite and copper. Current estimates suggest that the recovery of malachite can be economically viable when the copper concentration in the process streams exceeds 50 mg/L.
Implementing malachite recovery systems in existing desilication processes requires initial capital investment for equipment and process modifications. However, the long-term benefits can outweigh the costs, especially for large-scale operations. A typical malachite recovery system can yield a return on investment within 2-3 years, depending on the scale of operation and market conditions.
The economic advantages of malachite recovery extend beyond direct revenue generation. By recovering copper in the form of malachite, companies can reduce their environmental footprint and potentially avoid costs associated with waste disposal and environmental remediation. Additionally, the recovery process can improve the overall efficiency of the desilication process by removing impurities that might otherwise interfere with subsequent stages of aluminum production.
To maximize the economic benefits, ongoing research focuses on optimizing geochemical modeling techniques to enhance malachite precipitation efficiency and purity. Advanced modeling approaches, such as coupled reactive transport models and machine learning algorithms, are being developed to predict and control malachite formation under various process conditions. These advancements are expected to further improve the economic viability of malachite recovery in industrial settings.
The market demand for malachite has been steadily increasing due to its applications in jewelry, ornamental objects, and as a source of copper. The global malachite market size was valued at approximately USD 175 million in 2020 and is projected to grow at a CAGR of 4.5% from 2021 to 2028. This growth is primarily driven by the rising demand for copper in various industries, including electronics, construction, and renewable energy sectors.
The economic feasibility of malachite recovery depends on several factors, including the concentration of copper in the process streams, the efficiency of precipitation and separation techniques, and the market price of malachite and copper. Current estimates suggest that the recovery of malachite can be economically viable when the copper concentration in the process streams exceeds 50 mg/L.
Implementing malachite recovery systems in existing desilication processes requires initial capital investment for equipment and process modifications. However, the long-term benefits can outweigh the costs, especially for large-scale operations. A typical malachite recovery system can yield a return on investment within 2-3 years, depending on the scale of operation and market conditions.
The economic advantages of malachite recovery extend beyond direct revenue generation. By recovering copper in the form of malachite, companies can reduce their environmental footprint and potentially avoid costs associated with waste disposal and environmental remediation. Additionally, the recovery process can improve the overall efficiency of the desilication process by removing impurities that might otherwise interfere with subsequent stages of aluminum production.
To maximize the economic benefits, ongoing research focuses on optimizing geochemical modeling techniques to enhance malachite precipitation efficiency and purity. Advanced modeling approaches, such as coupled reactive transport models and machine learning algorithms, are being developed to predict and control malachite formation under various process conditions. These advancements are expected to further improve the economic viability of malachite recovery in industrial settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!