How malachite forms in sediment-hosted copper systems?
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Malachite Formation Background and Objectives
Malachite, a copper carbonate hydroxide mineral, plays a significant role in sediment-hosted copper systems. These systems are crucial sources of copper worldwide, accounting for approximately 20-25% of global copper production. The formation of malachite in these environments is a complex process influenced by various geological, chemical, and environmental factors.
Sediment-hosted copper deposits typically form in sedimentary basins where oxidized, copper-bearing fluids interact with reduced sediments or fluids. The primary copper minerals in these systems are often sulfides, such as chalcocite, bornite, and chalcopyrite. Malachite, however, forms as a secondary mineral during the weathering and oxidation of these primary copper sulfides.
The formation of malachite in sediment-hosted copper systems is closely tied to the near-surface weathering processes. As groundwater rich in oxygen and carbon dioxide percolates through the copper-bearing rocks, it oxidizes the primary copper sulfides. This oxidation process releases copper ions into solution, which then combine with carbonate ions derived from the dissolution of carbonate minerals or atmospheric CO2 to form malachite.
The specific conditions required for malachite formation include a pH range of 6.5-8.5, oxidizing conditions, and the presence of sufficient copper and carbonate ions. These conditions are often met in the upper portions of sediment-hosted copper deposits, particularly in arid to semi-arid climates where evaporation can concentrate the necessary ions.
Understanding the formation of malachite in these systems is crucial for several reasons. Firstly, it serves as an important indicator mineral for copper mineralization, guiding exploration efforts in sedimentary basins. Secondly, the presence and distribution of malachite can provide valuable insights into the weathering history and near-surface processes affecting copper deposits.
The objectives of studying malachite formation in sediment-hosted copper systems are multifaceted. They include: (1) elucidating the geochemical and environmental conditions that favor malachite precipitation, (2) understanding the relationship between malachite formation and the evolution of copper deposits over time, (3) developing more accurate models for predicting the distribution and abundance of copper resources in sedimentary basins, and (4) improving exploration techniques for identifying and characterizing sediment-hosted copper deposits.
By comprehensively investigating these aspects, researchers and industry professionals can enhance their understanding of copper mineralization processes, refine exploration strategies, and potentially uncover new copper resources in sedimentary environments. This knowledge is particularly valuable as the global demand for copper continues to rise, driven by its essential role in renewable energy technologies and infrastructure development.
Sediment-hosted copper deposits typically form in sedimentary basins where oxidized, copper-bearing fluids interact with reduced sediments or fluids. The primary copper minerals in these systems are often sulfides, such as chalcocite, bornite, and chalcopyrite. Malachite, however, forms as a secondary mineral during the weathering and oxidation of these primary copper sulfides.
The formation of malachite in sediment-hosted copper systems is closely tied to the near-surface weathering processes. As groundwater rich in oxygen and carbon dioxide percolates through the copper-bearing rocks, it oxidizes the primary copper sulfides. This oxidation process releases copper ions into solution, which then combine with carbonate ions derived from the dissolution of carbonate minerals or atmospheric CO2 to form malachite.
The specific conditions required for malachite formation include a pH range of 6.5-8.5, oxidizing conditions, and the presence of sufficient copper and carbonate ions. These conditions are often met in the upper portions of sediment-hosted copper deposits, particularly in arid to semi-arid climates where evaporation can concentrate the necessary ions.
Understanding the formation of malachite in these systems is crucial for several reasons. Firstly, it serves as an important indicator mineral for copper mineralization, guiding exploration efforts in sedimentary basins. Secondly, the presence and distribution of malachite can provide valuable insights into the weathering history and near-surface processes affecting copper deposits.
The objectives of studying malachite formation in sediment-hosted copper systems are multifaceted. They include: (1) elucidating the geochemical and environmental conditions that favor malachite precipitation, (2) understanding the relationship between malachite formation and the evolution of copper deposits over time, (3) developing more accurate models for predicting the distribution and abundance of copper resources in sedimentary basins, and (4) improving exploration techniques for identifying and characterizing sediment-hosted copper deposits.
By comprehensively investigating these aspects, researchers and industry professionals can enhance their understanding of copper mineralization processes, refine exploration strategies, and potentially uncover new copper resources in sedimentary environments. This knowledge is particularly valuable as the global demand for copper continues to rise, driven by its essential role in renewable energy technologies and infrastructure development.
Market Demand for Malachite
Malachite, a copper carbonate hydroxide mineral, has been experiencing a steady increase in market demand across various industries. The global malachite market is primarily driven by its applications in jewelry, ornamental objects, and as a source of copper ore. In recent years, there has been a growing interest in malachite's potential use in advanced technologies and sustainable applications, further expanding its market prospects.
The jewelry industry remains a significant consumer of high-quality malachite, with demand for unique, natural gemstones on the rise. Luxury brands and artisanal jewelers alike are incorporating malachite into their designs, capitalizing on its distinctive green color and banding patterns. This trend is particularly strong in emerging markets, where consumers are increasingly seeking out alternative gemstones beyond traditional options.
In the field of decorative arts and interior design, malachite continues to be sought after for its aesthetic appeal. High-end furniture makers, architectural firms, and interior designers are utilizing malachite in statement pieces, wall claddings, and bespoke installations. The mineral's rich green hues and natural patterns make it a popular choice for creating luxurious and unique spaces in both residential and commercial settings.
The industrial sector represents another significant market for malachite, primarily as a source of copper. As global demand for copper continues to grow, driven by infrastructure development, renewable energy technologies, and electric vehicle production, the importance of copper-bearing minerals like malachite is expected to increase. This trend is particularly relevant in regions with sediment-hosted copper deposits, where malachite often occurs as a secondary mineral.
Emerging applications in sustainable technologies are opening new avenues for malachite utilization. Research into malachite's potential as a catalyst for water purification and carbon dioxide conversion has shown promising results. These developments could lead to increased demand for malachite in environmental remediation and green chemistry applications, aligning with global efforts to address climate change and water scarcity.
The market for malachite-based pigments and dyes is also experiencing growth, particularly in the fine arts and specialty coatings sectors. The unique color properties of malachite-derived pigments are valued for their stability and vibrancy, finding applications in high-quality paints, inks, and protective coatings.
As awareness of malachite's properties and potential applications grows, there is an increasing interest in its use in alternative medicine and wellness products. While scientific evidence for its efficacy is limited, the mineral is being incorporated into various holistic health practices and products, contributing to a niche but growing market segment.
The jewelry industry remains a significant consumer of high-quality malachite, with demand for unique, natural gemstones on the rise. Luxury brands and artisanal jewelers alike are incorporating malachite into their designs, capitalizing on its distinctive green color and banding patterns. This trend is particularly strong in emerging markets, where consumers are increasingly seeking out alternative gemstones beyond traditional options.
In the field of decorative arts and interior design, malachite continues to be sought after for its aesthetic appeal. High-end furniture makers, architectural firms, and interior designers are utilizing malachite in statement pieces, wall claddings, and bespoke installations. The mineral's rich green hues and natural patterns make it a popular choice for creating luxurious and unique spaces in both residential and commercial settings.
The industrial sector represents another significant market for malachite, primarily as a source of copper. As global demand for copper continues to grow, driven by infrastructure development, renewable energy technologies, and electric vehicle production, the importance of copper-bearing minerals like malachite is expected to increase. This trend is particularly relevant in regions with sediment-hosted copper deposits, where malachite often occurs as a secondary mineral.
Emerging applications in sustainable technologies are opening new avenues for malachite utilization. Research into malachite's potential as a catalyst for water purification and carbon dioxide conversion has shown promising results. These developments could lead to increased demand for malachite in environmental remediation and green chemistry applications, aligning with global efforts to address climate change and water scarcity.
The market for malachite-based pigments and dyes is also experiencing growth, particularly in the fine arts and specialty coatings sectors. The unique color properties of malachite-derived pigments are valued for their stability and vibrancy, finding applications in high-quality paints, inks, and protective coatings.
As awareness of malachite's properties and potential applications grows, there is an increasing interest in its use in alternative medicine and wellness products. While scientific evidence for its efficacy is limited, the mineral is being incorporated into various holistic health practices and products, contributing to a niche but growing market segment.
Current State of Malachite Formation Research
The current state of malachite formation research in sediment-hosted copper systems has seen significant advancements in recent years. Researchers have made substantial progress in understanding the complex processes involved in the genesis of malachite within these geological settings. The formation of malachite, a copper carbonate hydroxide mineral, is now recognized as a multifaceted process influenced by various factors within sediment-hosted copper deposits.
Recent studies have focused on the geochemical conditions necessary for malachite precipitation. It has been established that malachite formation typically occurs in oxidizing environments with a pH range of 6.5 to 8.5. The presence of dissolved copper ions and carbonate-rich fluids is crucial for the crystallization process. Researchers have identified that the interaction between copper-bearing solutions and carbonate-rich host rocks or fluids plays a pivotal role in malachite formation.
Advances in analytical techniques have allowed for more detailed investigations of the mineralogical and textural characteristics of malachite in sediment-hosted copper systems. High-resolution imaging and spectroscopic methods have revealed intricate growth patterns and zonation within malachite crystals, providing insights into the dynamic nature of their formation. These observations have led to improved models of malachite growth mechanisms and the factors controlling crystal morphology.
The role of microbial activity in malachite formation has gained increased attention. Some studies suggest that certain bacteria can facilitate the precipitation of malachite by altering local geochemical conditions or serving as nucleation sites. This biogenic influence adds another layer of complexity to the understanding of malachite genesis in sediment-hosted environments.
Researchers have also made progress in elucidating the temporal aspects of malachite formation. It is now understood that malachite can form at various stages during the evolution of sediment-hosted copper deposits, from early diagenesis to late-stage weathering processes. This temporal variability has implications for the interpretation of malachite occurrences in exploration and the reconstruction of deposit histories.
The relationship between malachite and other copper minerals in sediment-hosted systems has been a focus of recent investigations. Studies have shown that malachite often forms as a secondary mineral, resulting from the alteration of primary copper sulfides such as chalcopyrite or bornite. The transformation pathways and kinetics of these reactions are being actively researched to better understand the overall copper mineralization process in these deposits.
Recent studies have focused on the geochemical conditions necessary for malachite precipitation. It has been established that malachite formation typically occurs in oxidizing environments with a pH range of 6.5 to 8.5. The presence of dissolved copper ions and carbonate-rich fluids is crucial for the crystallization process. Researchers have identified that the interaction between copper-bearing solutions and carbonate-rich host rocks or fluids plays a pivotal role in malachite formation.
Advances in analytical techniques have allowed for more detailed investigations of the mineralogical and textural characteristics of malachite in sediment-hosted copper systems. High-resolution imaging and spectroscopic methods have revealed intricate growth patterns and zonation within malachite crystals, providing insights into the dynamic nature of their formation. These observations have led to improved models of malachite growth mechanisms and the factors controlling crystal morphology.
The role of microbial activity in malachite formation has gained increased attention. Some studies suggest that certain bacteria can facilitate the precipitation of malachite by altering local geochemical conditions or serving as nucleation sites. This biogenic influence adds another layer of complexity to the understanding of malachite genesis in sediment-hosted environments.
Researchers have also made progress in elucidating the temporal aspects of malachite formation. It is now understood that malachite can form at various stages during the evolution of sediment-hosted copper deposits, from early diagenesis to late-stage weathering processes. This temporal variability has implications for the interpretation of malachite occurrences in exploration and the reconstruction of deposit histories.
The relationship between malachite and other copper minerals in sediment-hosted systems has been a focus of recent investigations. Studies have shown that malachite often forms as a secondary mineral, resulting from the alteration of primary copper sulfides such as chalcopyrite or bornite. The transformation pathways and kinetics of these reactions are being actively researched to better understand the overall copper mineralization process in these deposits.
Existing Models of Malachite Formation
01 Synthesis and preparation of malachite
Various methods for synthesizing and preparing malachite, including chemical reactions, hydrothermal processes, and precipitation techniques. These methods aim to produce high-quality malachite with controlled morphology and properties for different applications.- Malachite-based catalysts for chemical reactions: Malachite is used as a precursor or component in catalysts for various chemical reactions. These catalysts are particularly effective in processes such as oxidation, hydrogenation, and carbon dioxide conversion. The unique structure and properties of malachite contribute to its catalytic activity and selectivity in these applications.
- Malachite in environmental remediation: Malachite is utilized in environmental remediation processes, particularly for the removal of heavy metals and other pollutants from water and soil. Its adsorption properties and ability to form complexes with metal ions make it an effective material for water treatment and soil decontamination applications.
- Malachite-based pigments and dyes: Malachite is used as a source for green pigments and dyes in various industries, including paints, inks, and textiles. The unique color and stability of malachite-based pigments make them valuable in artistic and industrial applications. Processes for extracting, purifying, and formulating malachite-based colorants have been developed to enhance their performance and versatility.
- Malachite in nanotechnology and materials science: Malachite is employed in the synthesis of nanostructures and advanced materials. Its unique crystal structure and chemical properties are exploited to create novel nanomaterials with applications in electronics, sensors, and energy storage devices. Malachite-based nanoparticles and composites are being developed for their potential in various technological applications.
- Malachite in biomedical applications: Malachite and its derivatives are being investigated for potential biomedical applications. These include antimicrobial properties, drug delivery systems, and biosensors. The biocompatibility and unique chemical characteristics of malachite-based materials are being explored for their potential in medical diagnostics and therapeutic applications.
02 Applications of malachite in catalysis
Malachite and its derivatives are used as catalysts or catalyst supports in various chemical reactions. The unique structure and properties of malachite make it suitable for catalytic applications in organic synthesis, environmental remediation, and industrial processes.Expand Specific Solutions03 Malachite-based materials for environmental applications
Development of malachite-based materials for environmental applications, such as water treatment, pollutant removal, and adsorption of heavy metals. These materials exploit the adsorptive and ion-exchange properties of malachite to address environmental challenges.Expand Specific Solutions04 Malachite in pigments and colorants
Utilization of malachite as a pigment or colorant in various industries, including paints, inks, and cosmetics. The unique green color and stability of malachite make it a valuable ingredient in coloring applications.Expand Specific Solutions05 Malachite-based nanostructures and composites
Fabrication and characterization of malachite-based nanostructures and composites for advanced applications. These materials combine the properties of malachite with other components to create novel functional materials with enhanced performance in various fields.Expand Specific Solutions
Key Players in Copper Mineralization Research
The sediment-hosted copper system market is in a mature stage, with a global market size estimated in the billions of dollars. The technology for malachite formation in these systems is well-established, with key players like Freeport-McMoRan, China Nonferrous Metal Mining, and Sumitomo Metal Mining leading the field. Research institutions such as Kunming University of Science & Technology and Central South University contribute to advancing the understanding of these processes. The competitive landscape is characterized by a mix of large mining corporations and specialized research entities, with ongoing efforts to optimize extraction techniques and improve environmental sustainability in copper production from sedimentary deposits.
Freeport-McMoRan, Inc.
Technical Solution: Freeport-McMoRan, as a major copper producer, has developed proprietary techniques for identifying and extracting malachite in sediment-hosted copper systems. Their approach combines advanced geological mapping with innovative geochemical analysis methods. They utilize high-resolution spectral imaging to detect malachite signatures in sedimentary formations[2]. Freeport-McMoRan has also implemented machine learning algorithms to analyze vast datasets of geological and geochemical information, enhancing their ability to predict malachite occurrences. Their research has shown that malachite formation is often associated with specific sedimentary facies and structural features, which they use to guide their exploration efforts[4].
Strengths: Extensive field experience, advanced technological applications. Weaknesses: Proprietary nature of techniques may limit broader scientific contributions.
China Nonferrous Metal Mining (Group) Co., Ltd.
Technical Solution: China Nonferrous Metal Mining has developed a multi-faceted approach to understanding malachite formation in sediment-hosted copper systems. Their research combines field observations with laboratory experiments to elucidate the mechanisms of malachite precipitation. They have identified specific microbial communities that play a crucial role in facilitating the oxidation of copper sulfides and the subsequent formation of malachite[5]. The company has also developed innovative in-situ leaching techniques that take advantage of the natural processes of malachite formation to extract copper more efficiently from low-grade ores[6].
Strengths: Integration of microbial processes in understanding malachite formation, innovative extraction techniques. Weaknesses: May face challenges in scaling up laboratory findings to industrial applications.
Core Innovations in Copper Mineralization Studies
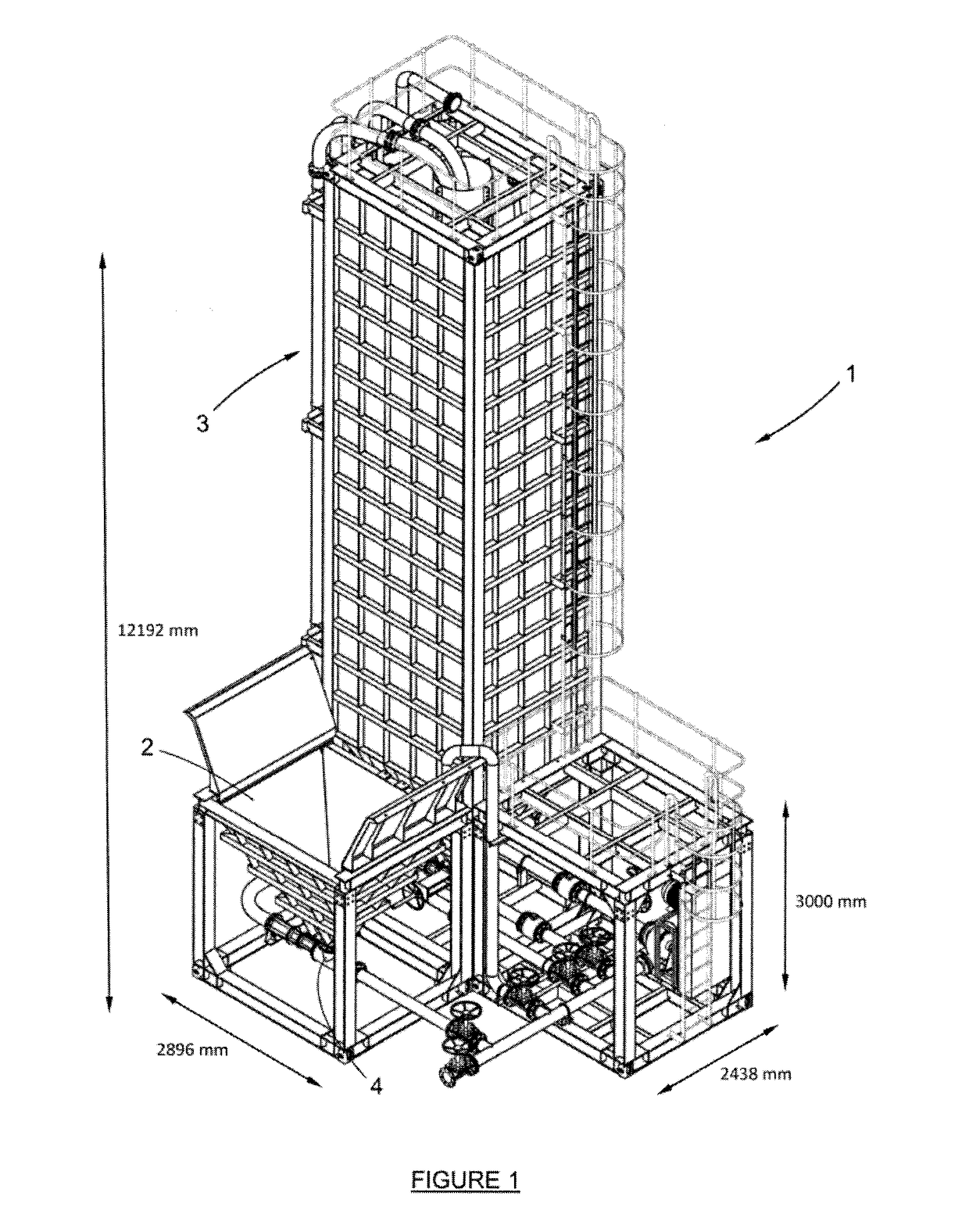
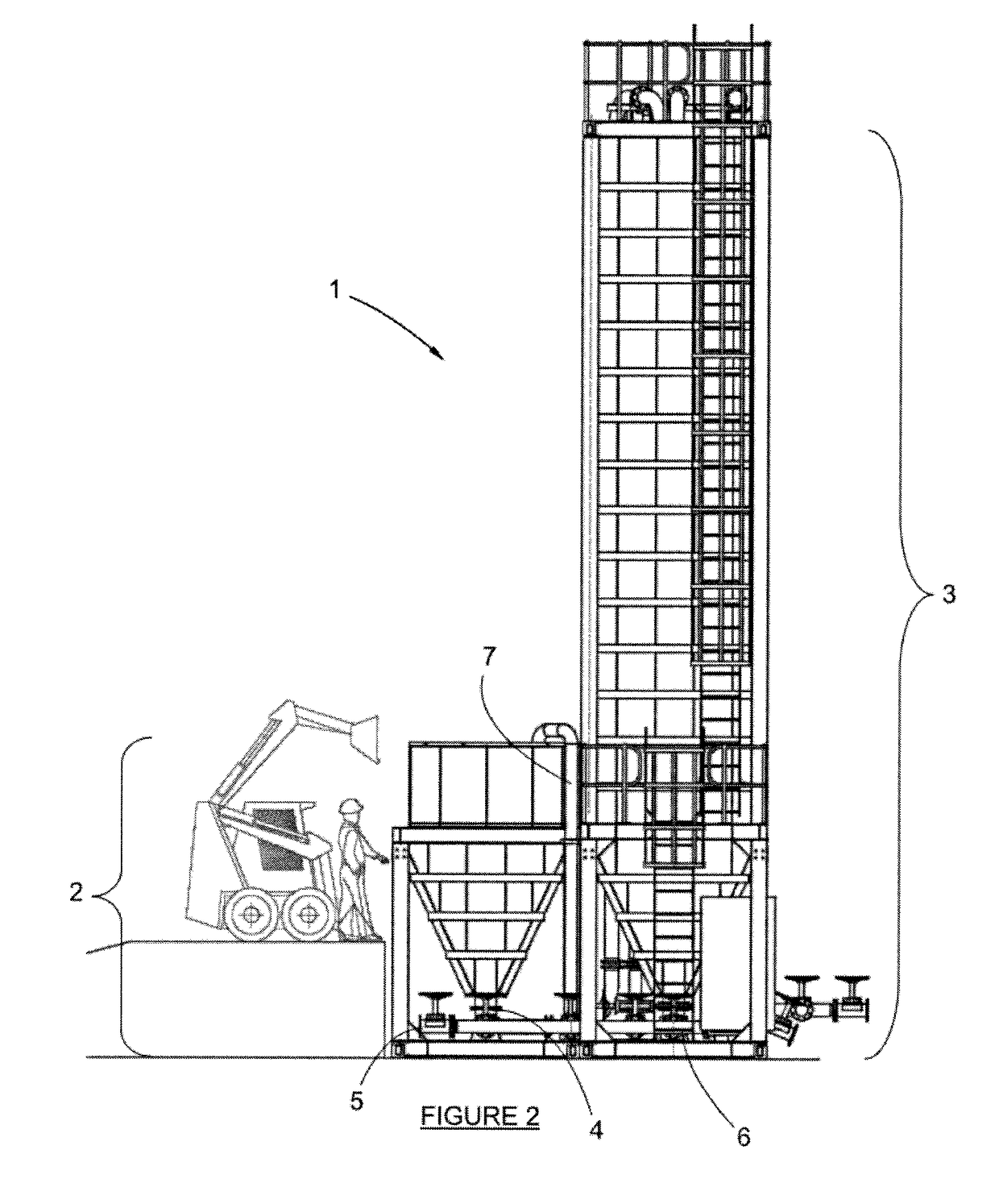
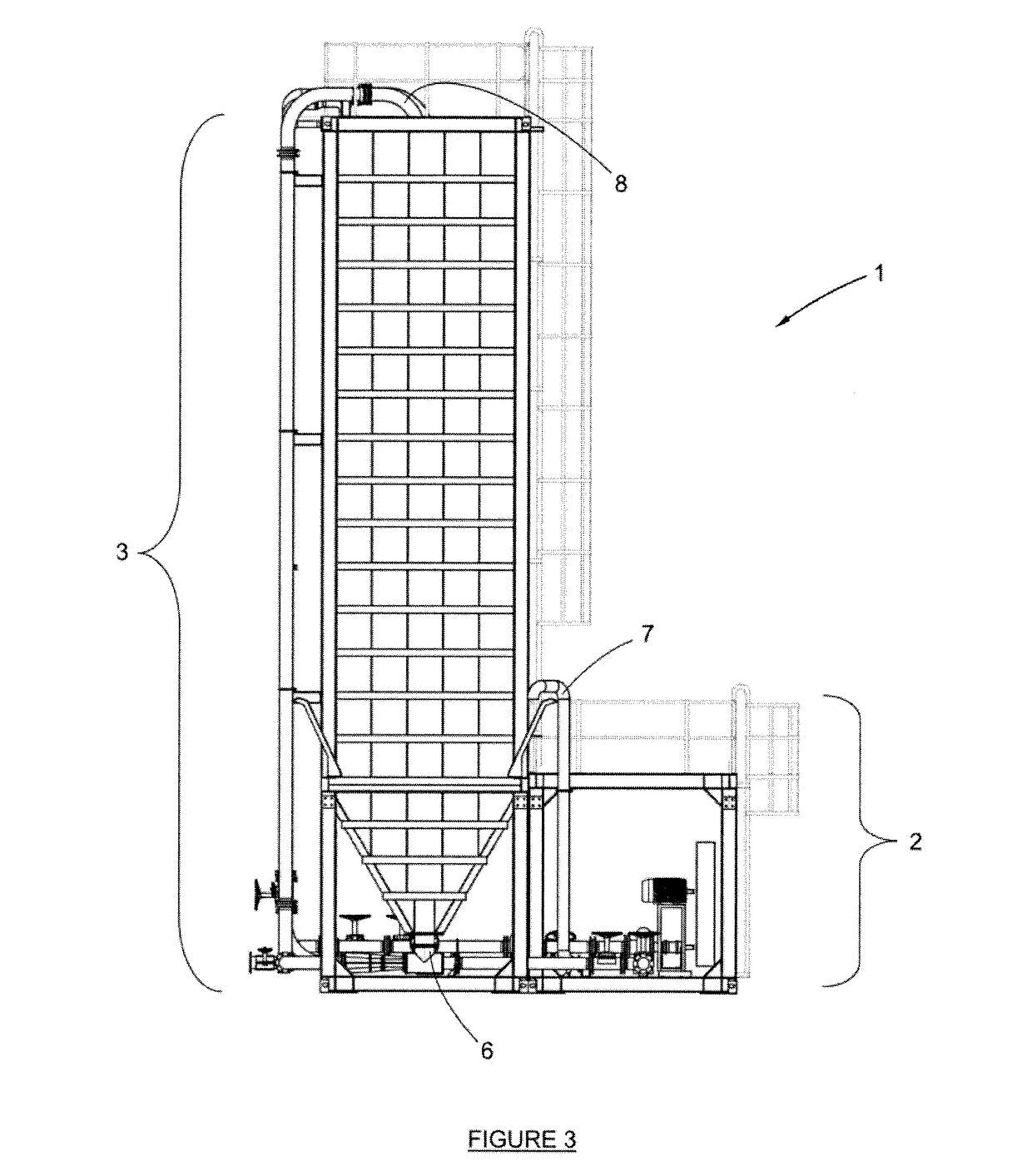
Apparatus and Process for the Improved Economic Extraction of Metal from a Metal-Bearing Material
PatentInactiveUS20170175226A1
Innovation
- A modular, re-locatable apparatus that combines leaching and liquid/solid separation processes in a single unit, allowing for easy transportation and assembly on-site, reducing capital costs and time to first production, and enabling the extraction of metals from both oxide and sulphide ores.
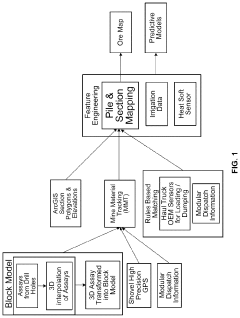
Chemical impacts on a leach stockpile
PatentActiveUS20240037462A1
Innovation
- A system that utilizes predictive models and data analytics to optimize ore routing and leaching operations by integrating mineralogy, irrigation, and temperature data, adjusting process parameters in real-time to enhance copper recovery and reduce costs.
Environmental Impact of Malachite Mining
The environmental impact of malachite mining is a significant concern in sediment-hosted copper systems. The extraction process often involves open-pit mining or underground mining techniques, which can lead to substantial landscape alterations and habitat destruction. These activities may result in the removal of vegetation, soil erosion, and changes in local topography, affecting both terrestrial and aquatic ecosystems.
Water pollution is a major issue associated with malachite mining. The extraction and processing of malachite can release heavy metals, such as copper, lead, and arsenic, into nearby water bodies. These contaminants can persist in the environment for extended periods, potentially harming aquatic life and posing risks to human health through contaminated drinking water sources. Acid mine drainage is another water-related concern, as the exposure of sulfide minerals to air and water can generate acidic runoff, further exacerbating water pollution and ecosystem degradation.
Air quality is also affected by malachite mining operations. Dust generated during excavation, transportation, and processing activities can contain fine particulate matter and potentially harmful substances. This airborne pollution can have adverse effects on human respiratory health and impact surrounding vegetation through the deposition of dust on leaves, potentially inhibiting photosynthesis and plant growth.
The mining process often requires significant energy consumption and may contribute to greenhouse gas emissions, particularly if fossil fuels are used as the primary energy source. This can have broader implications for climate change and global environmental concerns. Additionally, the disposal of mining waste, including tailings and overburden, can create long-term environmental challenges if not managed properly.
Biodiversity loss is another critical environmental impact of malachite mining. The destruction of natural habitats and the introduction of pollutants can lead to the displacement or decline of local flora and fauna. This can disrupt ecosystem balance and potentially result in the loss of rare or endemic species in the affected areas.
Efforts to mitigate these environmental impacts include implementing more sustainable mining practices, such as improved waste management, water treatment technologies, and land reclamation strategies. Regulatory frameworks and environmental impact assessments play crucial roles in minimizing the negative effects of malachite mining on the environment. However, the long-term consequences of mining activities often require ongoing monitoring and remediation efforts to ensure the protection of ecosystems and human health.
Water pollution is a major issue associated with malachite mining. The extraction and processing of malachite can release heavy metals, such as copper, lead, and arsenic, into nearby water bodies. These contaminants can persist in the environment for extended periods, potentially harming aquatic life and posing risks to human health through contaminated drinking water sources. Acid mine drainage is another water-related concern, as the exposure of sulfide minerals to air and water can generate acidic runoff, further exacerbating water pollution and ecosystem degradation.
Air quality is also affected by malachite mining operations. Dust generated during excavation, transportation, and processing activities can contain fine particulate matter and potentially harmful substances. This airborne pollution can have adverse effects on human respiratory health and impact surrounding vegetation through the deposition of dust on leaves, potentially inhibiting photosynthesis and plant growth.
The mining process often requires significant energy consumption and may contribute to greenhouse gas emissions, particularly if fossil fuels are used as the primary energy source. This can have broader implications for climate change and global environmental concerns. Additionally, the disposal of mining waste, including tailings and overburden, can create long-term environmental challenges if not managed properly.
Biodiversity loss is another critical environmental impact of malachite mining. The destruction of natural habitats and the introduction of pollutants can lead to the displacement or decline of local flora and fauna. This can disrupt ecosystem balance and potentially result in the loss of rare or endemic species in the affected areas.
Efforts to mitigate these environmental impacts include implementing more sustainable mining practices, such as improved waste management, water treatment technologies, and land reclamation strategies. Regulatory frameworks and environmental impact assessments play crucial roles in minimizing the negative effects of malachite mining on the environment. However, the long-term consequences of mining activities often require ongoing monitoring and remediation efforts to ensure the protection of ecosystems and human health.
Geochemical Processes in Sediment-Hosted Copper Systems
Sediment-hosted copper systems are complex geological environments where various geochemical processes interact to form mineral deposits, including malachite. These processes involve the transport, concentration, and precipitation of copper and other elements within sedimentary basins.
The formation of malachite in these systems is primarily driven by the oxidation of copper sulfides in the presence of carbonate-rich fluids. This process typically occurs in the near-surface environment, where oxygenated groundwater interacts with copper-bearing minerals. The oxidation of primary copper sulfides, such as chalcopyrite or bornite, releases copper ions into solution.
Simultaneously, the dissolution of carbonate minerals in the host rocks provides a source of carbonate ions. As these copper-rich, carbonate-bearing fluids migrate through the sedimentary sequence, changes in pH, temperature, and pressure can trigger the precipitation of malachite. This process is often facilitated by the presence of organic matter, which can act as a reducing agent and promote the formation of copper carbonate minerals.
The geochemical environment plays a crucial role in determining the stability and distribution of malachite within sediment-hosted copper systems. Factors such as the availability of copper, carbonate saturation, and redox conditions influence the formation and preservation of malachite. In some cases, malachite may form as a secondary mineral during weathering processes, replacing primary copper sulfides near the surface.
The presence of other elements, such as iron and sulfur, can also impact malachite formation. For instance, iron-rich environments may favor the formation of other copper minerals, such as chrysocolla or azurite, over malachite. The interplay between these various geochemical factors creates a complex system where malachite formation is highly dependent on local conditions within the sedimentary basin.
Understanding these geochemical processes is essential for exploring and evaluating sediment-hosted copper deposits. By studying the distribution and characteristics of malachite and associated minerals, geologists can gain insights into the depositional environment, fluid chemistry, and post-depositional alterations that have occurred within these systems.
The formation of malachite in these systems is primarily driven by the oxidation of copper sulfides in the presence of carbonate-rich fluids. This process typically occurs in the near-surface environment, where oxygenated groundwater interacts with copper-bearing minerals. The oxidation of primary copper sulfides, such as chalcopyrite or bornite, releases copper ions into solution.
Simultaneously, the dissolution of carbonate minerals in the host rocks provides a source of carbonate ions. As these copper-rich, carbonate-bearing fluids migrate through the sedimentary sequence, changes in pH, temperature, and pressure can trigger the precipitation of malachite. This process is often facilitated by the presence of organic matter, which can act as a reducing agent and promote the formation of copper carbonate minerals.
The geochemical environment plays a crucial role in determining the stability and distribution of malachite within sediment-hosted copper systems. Factors such as the availability of copper, carbonate saturation, and redox conditions influence the formation and preservation of malachite. In some cases, malachite may form as a secondary mineral during weathering processes, replacing primary copper sulfides near the surface.
The presence of other elements, such as iron and sulfur, can also impact malachite formation. For instance, iron-rich environments may favor the formation of other copper minerals, such as chrysocolla or azurite, over malachite. The interplay between these various geochemical factors creates a complex system where malachite formation is highly dependent on local conditions within the sedimentary basin.
Understanding these geochemical processes is essential for exploring and evaluating sediment-hosted copper deposits. By studying the distribution and characteristics of malachite and associated minerals, geologists can gain insights into the depositional environment, fluid chemistry, and post-depositional alterations that have occurred within these systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!