Glycerol's Contribution to Improved Drug Delivery Systems
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycerol in Drug Delivery: Background and Objectives
Glycerol, a simple polyol compound, has emerged as a significant player in the field of drug delivery systems over the past few decades. Its unique physicochemical properties, including high viscosity, hygroscopicity, and excellent solubility, have made it an attractive candidate for enhancing drug formulations and delivery mechanisms. The evolution of glycerol's role in pharmaceutical applications can be traced back to its initial use as a simple excipient, progressing to its current status as a multifunctional component in advanced drug delivery systems.
The primary objective of incorporating glycerol into drug delivery systems is to overcome various challenges associated with traditional drug formulations. These challenges include poor solubility, limited bioavailability, and inadequate stability of active pharmaceutical ingredients. By leveraging glycerol's properties, researchers aim to develop more efficient and targeted drug delivery methods, ultimately improving therapeutic outcomes and patient compliance.
In recent years, the pharmaceutical industry has witnessed a paradigm shift towards personalized medicine and nanotechnology-based drug delivery systems. This shift has further amplified the importance of glycerol in drug formulations. Its ability to act as a stabilizer, solubilizer, and permeation enhancer has opened up new avenues for developing novel drug delivery platforms, such as nanocarriers, liposomes, and hydrogels.
The technological progression in this field has been marked by several key milestones. These include the development of glycerol-based self-emulsifying drug delivery systems (SEDDS), which have significantly improved the oral bioavailability of poorly water-soluble drugs. Additionally, the integration of glycerol in transdermal drug delivery systems has enhanced skin permeation and drug absorption rates.
As we look towards the future, the potential of glycerol in drug delivery systems continues to expand. Emerging trends indicate a growing interest in utilizing glycerol for the development of stimuli-responsive drug delivery systems and in combination with other advanced materials to create hybrid delivery platforms. These advancements aim to achieve greater control over drug release kinetics and targeting specificity.
The ongoing research in this domain is driven by the need for more effective treatments for chronic diseases, the demand for non-invasive drug administration routes, and the push towards sustainable and biocompatible pharmaceutical excipients. As such, understanding the fundamental principles and exploring innovative applications of glycerol in drug delivery systems remains a critical focus for pharmaceutical research and development.
The primary objective of incorporating glycerol into drug delivery systems is to overcome various challenges associated with traditional drug formulations. These challenges include poor solubility, limited bioavailability, and inadequate stability of active pharmaceutical ingredients. By leveraging glycerol's properties, researchers aim to develop more efficient and targeted drug delivery methods, ultimately improving therapeutic outcomes and patient compliance.
In recent years, the pharmaceutical industry has witnessed a paradigm shift towards personalized medicine and nanotechnology-based drug delivery systems. This shift has further amplified the importance of glycerol in drug formulations. Its ability to act as a stabilizer, solubilizer, and permeation enhancer has opened up new avenues for developing novel drug delivery platforms, such as nanocarriers, liposomes, and hydrogels.
The technological progression in this field has been marked by several key milestones. These include the development of glycerol-based self-emulsifying drug delivery systems (SEDDS), which have significantly improved the oral bioavailability of poorly water-soluble drugs. Additionally, the integration of glycerol in transdermal drug delivery systems has enhanced skin permeation and drug absorption rates.
As we look towards the future, the potential of glycerol in drug delivery systems continues to expand. Emerging trends indicate a growing interest in utilizing glycerol for the development of stimuli-responsive drug delivery systems and in combination with other advanced materials to create hybrid delivery platforms. These advancements aim to achieve greater control over drug release kinetics and targeting specificity.
The ongoing research in this domain is driven by the need for more effective treatments for chronic diseases, the demand for non-invasive drug administration routes, and the push towards sustainable and biocompatible pharmaceutical excipients. As such, understanding the fundamental principles and exploring innovative applications of glycerol in drug delivery systems remains a critical focus for pharmaceutical research and development.
Market Analysis for Glycerol-Enhanced Drug Delivery
The market for glycerol-enhanced drug delivery systems has shown significant growth potential in recent years, driven by the increasing demand for more effective and targeted drug delivery methods. Glycerol, a versatile compound with excellent biocompatibility and solubility properties, has emerged as a key component in improving drug delivery systems across various pharmaceutical applications.
The global drug delivery market is expected to reach substantial value in the coming years, with glycerol-based systems playing an increasingly important role. This growth is primarily attributed to the rising prevalence of chronic diseases, the need for more efficient drug administration, and the continuous advancements in pharmaceutical research and development.
One of the key factors driving the market for glycerol-enhanced drug delivery is the growing emphasis on personalized medicine. As healthcare providers and patients seek more tailored treatment options, drug delivery systems that can offer improved bioavailability and controlled release are gaining traction. Glycerol's ability to enhance drug solubility and stability makes it an attractive option for developing such advanced delivery systems.
The pharmaceutical industry's focus on reducing side effects and improving patient compliance has also contributed to the increased adoption of glycerol-based drug delivery systems. These systems can help in minimizing dosage frequency and enhancing the therapeutic efficacy of various drugs, leading to better patient outcomes and reduced healthcare costs.
Geographically, North America and Europe currently dominate the market for glycerol-enhanced drug delivery systems, owing to their well-established pharmaceutical industries and high healthcare expenditure. However, emerging economies in Asia-Pacific and Latin America are expected to witness rapid growth in this sector, driven by improving healthcare infrastructure and increasing investments in pharmaceutical research.
The market landscape is characterized by intense competition among key players, including major pharmaceutical companies and specialized drug delivery technology firms. These companies are investing heavily in research and development to create innovative glycerol-based formulations and delivery platforms. Collaborations between pharmaceutical companies and academic institutions are also becoming more common, fostering the development of novel drug delivery solutions.
Despite the positive outlook, the market faces certain challenges. Regulatory hurdles and the high costs associated with developing and commercializing new drug delivery systems can potentially slow down market growth. Additionally, the need for extensive clinical trials to prove the safety and efficacy of glycerol-enhanced delivery systems may prolong the time-to-market for new products.
The global drug delivery market is expected to reach substantial value in the coming years, with glycerol-based systems playing an increasingly important role. This growth is primarily attributed to the rising prevalence of chronic diseases, the need for more efficient drug administration, and the continuous advancements in pharmaceutical research and development.
One of the key factors driving the market for glycerol-enhanced drug delivery is the growing emphasis on personalized medicine. As healthcare providers and patients seek more tailored treatment options, drug delivery systems that can offer improved bioavailability and controlled release are gaining traction. Glycerol's ability to enhance drug solubility and stability makes it an attractive option for developing such advanced delivery systems.
The pharmaceutical industry's focus on reducing side effects and improving patient compliance has also contributed to the increased adoption of glycerol-based drug delivery systems. These systems can help in minimizing dosage frequency and enhancing the therapeutic efficacy of various drugs, leading to better patient outcomes and reduced healthcare costs.
Geographically, North America and Europe currently dominate the market for glycerol-enhanced drug delivery systems, owing to their well-established pharmaceutical industries and high healthcare expenditure. However, emerging economies in Asia-Pacific and Latin America are expected to witness rapid growth in this sector, driven by improving healthcare infrastructure and increasing investments in pharmaceutical research.
The market landscape is characterized by intense competition among key players, including major pharmaceutical companies and specialized drug delivery technology firms. These companies are investing heavily in research and development to create innovative glycerol-based formulations and delivery platforms. Collaborations between pharmaceutical companies and academic institutions are also becoming more common, fostering the development of novel drug delivery solutions.
Despite the positive outlook, the market faces certain challenges. Regulatory hurdles and the high costs associated with developing and commercializing new drug delivery systems can potentially slow down market growth. Additionally, the need for extensive clinical trials to prove the safety and efficacy of glycerol-enhanced delivery systems may prolong the time-to-market for new products.
Current Challenges in Glycerol-Based Drug Delivery
Despite the promising potential of glycerol in drug delivery systems, several challenges persist in its effective implementation. One of the primary obstacles is the high viscosity of glycerol, which can impede the flow and dispersion of drug formulations. This property often necessitates the use of additional excipients or processing techniques to achieve desired rheological characteristics, potentially complicating formulation processes and increasing production costs.
Another significant challenge lies in the hygroscopic nature of glycerol. Its ability to absorb moisture from the environment can lead to stability issues in drug formulations, particularly for moisture-sensitive active pharmaceutical ingredients. This hygroscopicity may result in changes in drug concentration, compromised product integrity, and reduced shelf life, necessitating careful consideration in packaging and storage conditions.
The high osmolality of glycerol-based formulations presents yet another hurdle. When used in high concentrations, glycerol can cause local tissue irritation and discomfort upon administration, especially in sensitive areas such as mucous membranes. This limitation restricts the use of glycerol in certain drug delivery routes and requires careful balancing of glycerol content to maintain efficacy while minimizing adverse effects.
Furthermore, the non-specific nature of glycerol's interactions with drugs poses challenges in controlled release applications. While glycerol can enhance the solubility of many compounds, achieving precise control over drug release kinetics remains difficult. This lack of specificity can result in burst release effects or inconsistent drug delivery profiles, potentially compromising therapeutic outcomes.
The metabolic fate of glycerol in the body also presents concerns. Although generally recognized as safe, high doses of glycerol can affect osmotic balance and potentially lead to adverse effects such as headaches, nausea, and in extreme cases, renal impairment. Consequently, dosing limitations and careful monitoring may be necessary, particularly in long-term or high-dose applications.
Lastly, the regulatory landscape surrounding glycerol-based drug delivery systems poses challenges. While glycerol is widely accepted as a safe excipient, its use in novel drug delivery platforms may require extensive safety and efficacy studies to gain regulatory approval. This process can be time-consuming and costly, potentially deterring pharmaceutical companies from exploring innovative glycerol-based formulations.
Another significant challenge lies in the hygroscopic nature of glycerol. Its ability to absorb moisture from the environment can lead to stability issues in drug formulations, particularly for moisture-sensitive active pharmaceutical ingredients. This hygroscopicity may result in changes in drug concentration, compromised product integrity, and reduced shelf life, necessitating careful consideration in packaging and storage conditions.
The high osmolality of glycerol-based formulations presents yet another hurdle. When used in high concentrations, glycerol can cause local tissue irritation and discomfort upon administration, especially in sensitive areas such as mucous membranes. This limitation restricts the use of glycerol in certain drug delivery routes and requires careful balancing of glycerol content to maintain efficacy while minimizing adverse effects.
Furthermore, the non-specific nature of glycerol's interactions with drugs poses challenges in controlled release applications. While glycerol can enhance the solubility of many compounds, achieving precise control over drug release kinetics remains difficult. This lack of specificity can result in burst release effects or inconsistent drug delivery profiles, potentially compromising therapeutic outcomes.
The metabolic fate of glycerol in the body also presents concerns. Although generally recognized as safe, high doses of glycerol can affect osmotic balance and potentially lead to adverse effects such as headaches, nausea, and in extreme cases, renal impairment. Consequently, dosing limitations and careful monitoring may be necessary, particularly in long-term or high-dose applications.
Lastly, the regulatory landscape surrounding glycerol-based drug delivery systems poses challenges. While glycerol is widely accepted as a safe excipient, its use in novel drug delivery platforms may require extensive safety and efficacy studies to gain regulatory approval. This process can be time-consuming and costly, potentially deterring pharmaceutical companies from exploring innovative glycerol-based formulations.
Existing Glycerol-Based Drug Delivery Systems
01 Glycerol-based drug delivery systems
Glycerol is utilized as a key component in various drug delivery systems due to its biocompatibility and versatility. These systems can enhance drug solubility, improve absorption, and provide controlled release of active ingredients. Glycerol-based formulations can be designed for different routes of administration, including oral, topical, and parenteral delivery.- Glycerol-based drug delivery systems: Glycerol is utilized as a key component in various drug delivery systems due to its biocompatibility and versatility. These systems can enhance drug solubility, improve absorption, and provide controlled release of active ingredients. Glycerol-based formulations can be designed for different administration routes, including oral, topical, and parenteral delivery.
- Glycerol in transdermal drug delivery: Glycerol plays a significant role in transdermal drug delivery systems. It acts as a penetration enhancer, improving the permeation of active ingredients through the skin. Additionally, glycerol's moisturizing properties can help maintain skin hydration, potentially enhancing drug absorption and patient comfort during application.
- Glycerol-based nanocarriers for drug delivery: Nanocarriers incorporating glycerol have shown promise in targeted drug delivery. These nanostructures can encapsulate drugs, protect them from degradation, and facilitate their transport to specific sites in the body. Glycerol-based nanocarriers may improve drug bioavailability and reduce side effects associated with systemic administration.
- Glycerol in pulmonary drug delivery: Glycerol is employed in formulations for pulmonary drug delivery, particularly in inhalers and nebulizers. It can act as a humectant, preventing the drying of drug particles and improving their dispersion in the lungs. This application of glycerol enhances the efficacy of inhaled medications for respiratory conditions.
- Glycerol in sustained-release formulations: Glycerol is utilized in the development of sustained-release drug formulations. It can be incorporated into matrices or coatings to modulate drug release over extended periods. This approach helps maintain therapeutic drug levels, reduce dosing frequency, and improve patient compliance in various treatment regimens.
02 Glycerol in transdermal drug delivery
Glycerol plays a significant role in transdermal drug delivery systems. It acts as a penetration enhancer, improving the permeation of active ingredients through the skin. Additionally, glycerol's moisturizing properties can help maintain skin hydration, potentially increasing drug absorption and efficacy in topical formulations.Expand Specific Solutions03 Glycerol in nanoparticle-based drug delivery
Glycerol is incorporated into nanoparticle-based drug delivery systems to enhance stability and improve drug encapsulation efficiency. These nanoparticles can be designed for targeted delivery, controlled release, and improved bioavailability of various therapeutic agents. Glycerol-containing nanoformulations show promise in areas such as cancer therapy and gene delivery.Expand Specific Solutions04 Glycerol in pulmonary drug delivery
Glycerol is utilized in pulmonary drug delivery systems to improve the dispersion and stability of inhaled formulations. It can act as a humectant in dry powder inhalers, preventing moisture-induced aggregation of particles. In nebulizer formulations, glycerol can help adjust viscosity and enhance drug solubility, leading to more efficient delivery to the lungs.Expand Specific Solutions05 Glycerol in sustained-release formulations
Glycerol is employed in the development of sustained-release drug delivery systems. It can be used to create matrix systems or as a component in polymer-based formulations to modulate drug release kinetics. These glycerol-containing sustained-release systems can provide prolonged therapeutic effects and reduce dosing frequency, potentially improving patient compliance.Expand Specific Solutions
Key Players in Glycerol-Enhanced Drug Delivery
The glycerol-based drug delivery systems market is in a growth phase, driven by increasing demand for improved pharmaceutical formulations. The global market size is expanding, with projections indicating significant growth in the coming years. Technologically, the field is advancing rapidly, with various players contributing to its maturation. Key companies like Novartis AG and Santen Pharmaceutical Co., Ltd. are investing in research and development, while academic institutions such as Monash University and California Institute of Technology are pushing the boundaries of glycerol-based drug delivery technologies. The involvement of both industry leaders and research institutions suggests a collaborative ecosystem that is fostering innovation and driving the technology towards commercial viability.
Monash University
Technical Solution: Monash University has developed innovative glycerol-based drug delivery systems, focusing on enhancing the bioavailability and efficacy of various therapeutic compounds. Their approach involves creating glycerol-based nanocarriers that can encapsulate both hydrophilic and hydrophobic drugs, improving their solubility and stability[1]. These nanocarriers are designed with surface modifications to target specific tissues or cells, enhancing drug delivery precision[2]. The university has also explored the use of glycerol as a cryoprotectant in liposomal formulations, which has shown promise in preserving the integrity of drug-loaded liposomes during freeze-drying processes, thereby extending the shelf-life of pharmaceutical products[3].
Strengths: Enhanced drug solubility and stability, targeted delivery capabilities, and improved preservation of drug formulations. Weaknesses: Potential scalability issues and the need for extensive clinical trials to validate the safety and efficacy of these novel delivery systems.
Novartis AG
Technical Solution: Novartis AG has leveraged glycerol in developing advanced drug delivery platforms, particularly for challenging therapeutic areas such as ophthalmology and dermatology. Their approach incorporates glycerol as a key excipient in sustained-release formulations, exploiting its hygroscopic properties to maintain drug stability and control release kinetics[4]. Novartis has also pioneered the use of glycerol-based hydrogels for topical drug delivery, which offer improved skin penetration and prolonged drug residence time[5]. In ophthalmology, they have developed glycerol-enhanced eye drop formulations that increase corneal permeability and reduce dosing frequency, significantly improving patient compliance[6].
Strengths: Improved drug stability, controlled release capabilities, and enhanced patient compliance. Weaknesses: Potential limitations in systemic drug delivery applications and the need for specialized manufacturing processes.
Innovative Glycerol Formulations for Drug Delivery
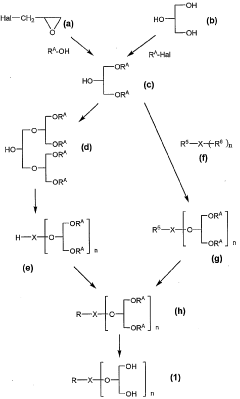
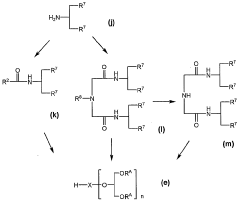
Compound modified with glycerol derivative
PatentWO2005023844A1
Innovation
- Development of compounds modified with glycerol derivatives as surface modifiers for drug carriers, which enhance blood retention and improve drug delivery by modifying amphipathic or hydrophobic substances with glycerol derivatives, creating microparticles that can effectively target and retain drugs in the bloodstream.
Polymer-drug conjugates
PatentPendingUS20250205356A1
Innovation
- A polymeric scaffold delivery system with a polyglycerol backbone and functional groups for targeted drug conjugation, enabling high drug load and specific binding to antigen sites for efficient drug delivery and release.
Regulatory Considerations for Glycerol in Pharmaceuticals
The regulatory landscape for glycerol in pharmaceuticals is complex and multifaceted, requiring careful consideration by drug developers and manufacturers. Glycerol, also known as glycerin, is widely used in pharmaceutical formulations as a solvent, sweetener, and preservative. Its status as Generally Recognized as Safe (GRAS) by the U.S. Food and Drug Administration (FDA) has facilitated its incorporation into various drug delivery systems.
However, the use of glycerol in pharmaceuticals is subject to specific regulatory guidelines and requirements. The FDA's Code of Federal Regulations (CFR) Title 21 outlines the acceptable uses and limitations of glycerol in drug products. For instance, when used as an active ingredient in over-the-counter drug products, glycerol must comply with the monograph requirements specified in 21 CFR Part 310 and 21 CFR Part 344.
In the European Union, the European Medicines Agency (EMA) provides guidance on the use of glycerol in medicinal products. The EMA's guidelines on excipients in the labeling and package leaflet of medicinal products for human use specifically address glycerol, requiring appropriate labeling when certain concentration thresholds are exceeded.
Manufacturers must also consider the quality standards for glycerol used in pharmaceuticals. The United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) provide detailed specifications for pharmaceutical-grade glycerol, including purity requirements and testing methods. Adherence to these pharmacopoeial standards is crucial for regulatory compliance.
When developing novel drug delivery systems incorporating glycerol, pharmaceutical companies must navigate additional regulatory considerations. These may include demonstrating the safety and efficacy of the delivery system through appropriate preclinical and clinical studies. The regulatory pathway may vary depending on whether the glycerol-based delivery system is considered a new drug, a drug-device combination, or a significant change to an existing approved product.
Furthermore, the use of glycerol in parenteral formulations requires special attention to ensure sterility and absence of pyrogens. Manufacturers must comply with Good Manufacturing Practice (GMP) guidelines and implement appropriate quality control measures to mitigate risks associated with microbial contamination and endotoxin levels.
As the pharmaceutical industry continues to innovate in drug delivery technologies, regulatory agencies are adapting their approaches to evaluate novel formulations. This dynamic regulatory environment necessitates ongoing vigilance and adaptability from pharmaceutical companies utilizing glycerol in their drug delivery systems. Staying abreast of regulatory updates, engaging in early dialogue with regulatory authorities, and maintaining robust quality management systems are essential strategies for navigating the regulatory landscape surrounding glycerol in pharmaceuticals.
However, the use of glycerol in pharmaceuticals is subject to specific regulatory guidelines and requirements. The FDA's Code of Federal Regulations (CFR) Title 21 outlines the acceptable uses and limitations of glycerol in drug products. For instance, when used as an active ingredient in over-the-counter drug products, glycerol must comply with the monograph requirements specified in 21 CFR Part 310 and 21 CFR Part 344.
In the European Union, the European Medicines Agency (EMA) provides guidance on the use of glycerol in medicinal products. The EMA's guidelines on excipients in the labeling and package leaflet of medicinal products for human use specifically address glycerol, requiring appropriate labeling when certain concentration thresholds are exceeded.
Manufacturers must also consider the quality standards for glycerol used in pharmaceuticals. The United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) provide detailed specifications for pharmaceutical-grade glycerol, including purity requirements and testing methods. Adherence to these pharmacopoeial standards is crucial for regulatory compliance.
When developing novel drug delivery systems incorporating glycerol, pharmaceutical companies must navigate additional regulatory considerations. These may include demonstrating the safety and efficacy of the delivery system through appropriate preclinical and clinical studies. The regulatory pathway may vary depending on whether the glycerol-based delivery system is considered a new drug, a drug-device combination, or a significant change to an existing approved product.
Furthermore, the use of glycerol in parenteral formulations requires special attention to ensure sterility and absence of pyrogens. Manufacturers must comply with Good Manufacturing Practice (GMP) guidelines and implement appropriate quality control measures to mitigate risks associated with microbial contamination and endotoxin levels.
As the pharmaceutical industry continues to innovate in drug delivery technologies, regulatory agencies are adapting their approaches to evaluate novel formulations. This dynamic regulatory environment necessitates ongoing vigilance and adaptability from pharmaceutical companies utilizing glycerol in their drug delivery systems. Staying abreast of regulatory updates, engaging in early dialogue with regulatory authorities, and maintaining robust quality management systems are essential strategies for navigating the regulatory landscape surrounding glycerol in pharmaceuticals.
Environmental Impact of Glycerol in Drug Production
The environmental impact of glycerol in drug production is a crucial aspect to consider when evaluating the sustainability of improved drug delivery systems. Glycerol, a byproduct of biodiesel production, has gained significant attention in the pharmaceutical industry due to its versatile applications and potential to enhance drug formulations.
One of the primary environmental benefits of using glycerol in drug production is its renewable nature. As a byproduct of biodiesel manufacturing, glycerol offers a sustainable alternative to petroleum-based excipients commonly used in pharmaceutical formulations. This shift towards bio-based materials aligns with global efforts to reduce dependence on fossil fuels and minimize carbon footprints in industrial processes.
Furthermore, the utilization of glycerol in drug production contributes to waste reduction and resource efficiency. By repurposing a byproduct that would otherwise be discarded, the pharmaceutical industry can minimize waste generation and optimize resource utilization. This circular economy approach not only reduces environmental burden but also enhances the overall sustainability of drug manufacturing processes.
Glycerol's biodegradability is another significant environmental advantage. Unlike some synthetic excipients that persist in the environment, glycerol can be readily broken down by microorganisms, reducing the potential for long-term ecological impacts. This characteristic is particularly important when considering the fate of pharmaceutical residues in aquatic ecosystems and soil environments.
However, it is essential to consider the potential environmental challenges associated with glycerol production and purification. The increasing demand for glycerol in various industries, including pharmaceuticals, may lead to intensified biodiesel production, which could have implications for land use and agricultural practices. Careful management of these upstream processes is necessary to ensure that the environmental benefits of glycerol use in drug delivery systems are not offset by negative impacts in other areas.
Additionally, the purification of crude glycerol to pharmaceutical-grade quality requires energy-intensive processes. While these processes are necessary to meet stringent quality standards for drug formulations, they contribute to the overall environmental footprint of glycerol-based drug delivery systems. Ongoing research and development efforts are focused on optimizing these purification methods to enhance energy efficiency and reduce associated emissions.
In conclusion, the incorporation of glycerol in drug production offers several environmental advantages, including renewable sourcing, waste reduction, and biodegradability. However, a comprehensive life cycle assessment is necessary to fully understand and quantify the net environmental impact of glycerol-based drug delivery systems. As the pharmaceutical industry continues to prioritize sustainability, the role of glycerol in environmentally conscious drug production is likely to expand, driving further innovations in green chemistry and sustainable manufacturing practices.
One of the primary environmental benefits of using glycerol in drug production is its renewable nature. As a byproduct of biodiesel manufacturing, glycerol offers a sustainable alternative to petroleum-based excipients commonly used in pharmaceutical formulations. This shift towards bio-based materials aligns with global efforts to reduce dependence on fossil fuels and minimize carbon footprints in industrial processes.
Furthermore, the utilization of glycerol in drug production contributes to waste reduction and resource efficiency. By repurposing a byproduct that would otherwise be discarded, the pharmaceutical industry can minimize waste generation and optimize resource utilization. This circular economy approach not only reduces environmental burden but also enhances the overall sustainability of drug manufacturing processes.
Glycerol's biodegradability is another significant environmental advantage. Unlike some synthetic excipients that persist in the environment, glycerol can be readily broken down by microorganisms, reducing the potential for long-term ecological impacts. This characteristic is particularly important when considering the fate of pharmaceutical residues in aquatic ecosystems and soil environments.
However, it is essential to consider the potential environmental challenges associated with glycerol production and purification. The increasing demand for glycerol in various industries, including pharmaceuticals, may lead to intensified biodiesel production, which could have implications for land use and agricultural practices. Careful management of these upstream processes is necessary to ensure that the environmental benefits of glycerol use in drug delivery systems are not offset by negative impacts in other areas.
Additionally, the purification of crude glycerol to pharmaceutical-grade quality requires energy-intensive processes. While these processes are necessary to meet stringent quality standards for drug formulations, they contribute to the overall environmental footprint of glycerol-based drug delivery systems. Ongoing research and development efforts are focused on optimizing these purification methods to enhance energy efficiency and reduce associated emissions.
In conclusion, the incorporation of glycerol in drug production offers several environmental advantages, including renewable sourcing, waste reduction, and biodegradability. However, a comprehensive life cycle assessment is necessary to fully understand and quantify the net environmental impact of glycerol-based drug delivery systems. As the pharmaceutical industry continues to prioritize sustainability, the role of glycerol in environmentally conscious drug production is likely to expand, driving further innovations in green chemistry and sustainable manufacturing practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!