How Decane Affects Chain Scission in Polymer Decomposition Studies
JUL 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Decane-Polymer Interaction Background and Objectives
The study of decane's influence on chain scission in polymer decomposition has gained significant attention in recent years due to its implications for various industrial applications and environmental concerns. This research area bridges the fields of polymer science, organic chemistry, and materials engineering, focusing on the intricate interactions between decane, a common hydrocarbon, and complex polymer structures during decomposition processes.
Historically, polymer decomposition studies have primarily concentrated on thermal and oxidative degradation mechanisms. However, the introduction of decane as a variable in these studies has opened new avenues for understanding the nuanced effects of solvent-polymer interactions on decomposition pathways. The presence of decane, a straight-chain alkane with ten carbon atoms, can potentially alter the kinetics and mechanisms of polymer chain scission, leading to unique degradation patterns and product distributions.
The primary objective of this research is to elucidate the specific role of decane in polymer chain scission during decomposition. This involves investigating how decane influences the breaking of chemical bonds within polymer chains, potentially acting as a plasticizer, a reactant, or a medium that affects the local environment of the polymer molecules. Understanding these interactions is crucial for predicting and controlling polymer behavior in various applications, from fuel systems to waste management.
Another key goal is to quantify the impact of decane on the rate and extent of polymer decomposition. This includes examining how different concentrations of decane affect the initiation, propagation, and termination stages of chain scission reactions. Such knowledge is essential for developing more accurate models of polymer degradation in complex environments, which can inform the design of more durable materials or more efficient recycling processes.
Furthermore, this research aims to explore the potential for decane to selectively influence certain types of polymers or specific chemical bonds within polymer structures. This selectivity, if present, could have profound implications for polymer recycling technologies, allowing for more targeted and efficient breakdown of complex polymer mixtures.
The technological evolution in this field has been driven by advancements in analytical techniques, such as high-resolution spectroscopy and chromatography, which enable researchers to observe and quantify the subtle effects of decane on polymer decomposition at a molecular level. These tools, combined with computational modeling, are pushing the boundaries of our understanding of polymer-solvent interactions and their consequences for material properties and lifecycle.
Historically, polymer decomposition studies have primarily concentrated on thermal and oxidative degradation mechanisms. However, the introduction of decane as a variable in these studies has opened new avenues for understanding the nuanced effects of solvent-polymer interactions on decomposition pathways. The presence of decane, a straight-chain alkane with ten carbon atoms, can potentially alter the kinetics and mechanisms of polymer chain scission, leading to unique degradation patterns and product distributions.
The primary objective of this research is to elucidate the specific role of decane in polymer chain scission during decomposition. This involves investigating how decane influences the breaking of chemical bonds within polymer chains, potentially acting as a plasticizer, a reactant, or a medium that affects the local environment of the polymer molecules. Understanding these interactions is crucial for predicting and controlling polymer behavior in various applications, from fuel systems to waste management.
Another key goal is to quantify the impact of decane on the rate and extent of polymer decomposition. This includes examining how different concentrations of decane affect the initiation, propagation, and termination stages of chain scission reactions. Such knowledge is essential for developing more accurate models of polymer degradation in complex environments, which can inform the design of more durable materials or more efficient recycling processes.
Furthermore, this research aims to explore the potential for decane to selectively influence certain types of polymers or specific chemical bonds within polymer structures. This selectivity, if present, could have profound implications for polymer recycling technologies, allowing for more targeted and efficient breakdown of complex polymer mixtures.
The technological evolution in this field has been driven by advancements in analytical techniques, such as high-resolution spectroscopy and chromatography, which enable researchers to observe and quantify the subtle effects of decane on polymer decomposition at a molecular level. These tools, combined with computational modeling, are pushing the boundaries of our understanding of polymer-solvent interactions and their consequences for material properties and lifecycle.
Market Analysis for Polymer Decomposition Research
The polymer decomposition research market has been experiencing significant growth in recent years, driven by the increasing demand for sustainable and recyclable materials across various industries. This market segment is closely tied to the broader polymer industry, which was valued at approximately $600 billion globally in 2020 and is expected to continue its upward trajectory.
The focus on polymer decomposition studies, particularly those involving chain scission mechanisms, has gained traction due to the growing emphasis on circular economy principles and environmental regulations. Industries such as packaging, automotive, and electronics are actively seeking ways to improve the recyclability and end-of-life management of polymer-based products.
Within this context, research on how decane affects chain scission in polymer decomposition has emerged as a niche but crucial area of study. Decane, a hydrocarbon solvent, plays a significant role in understanding the degradation processes of certain polymers, especially those used in high-temperature applications or exposed to hydrocarbon-rich environments.
The market for this specific research area is primarily driven by the petrochemical and polymer manufacturing sectors. These industries are investing in research and development to enhance their understanding of polymer behavior under various conditions, ultimately aiming to develop more resilient and easily recyclable materials.
Furthermore, the automotive industry has shown increased interest in polymer decomposition studies involving decane. As vehicles continue to use a higher percentage of polymer-based components, understanding their degradation mechanisms becomes crucial for improving durability and end-of-life recycling processes.
The packaging industry, another major consumer of polymers, is also contributing to the growth of this research market. With stringent regulations on single-use plastics and growing consumer awareness about environmental issues, companies are investing in research to develop packaging materials that can be more easily decomposed or recycled.
Academic institutions and research laboratories are key players in advancing the understanding of decane's role in polymer chain scission. Their findings often translate into practical applications for industry, creating a symbiotic relationship between academic research and industrial development.
Government initiatives and funding for sustainable materials research have further bolstered the market for polymer decomposition studies. Many countries have implemented policies to reduce plastic waste and promote the development of biodegradable or easily recyclable polymers, indirectly supporting research in this field.
As the global push for sustainability continues, the market for polymer decomposition research, including studies on decane's effects on chain scission, is expected to expand. This growth is likely to be accompanied by increased collaboration between academic institutions, industry players, and government bodies, fostering innovation and practical applications in polymer science and technology.
The focus on polymer decomposition studies, particularly those involving chain scission mechanisms, has gained traction due to the growing emphasis on circular economy principles and environmental regulations. Industries such as packaging, automotive, and electronics are actively seeking ways to improve the recyclability and end-of-life management of polymer-based products.
Within this context, research on how decane affects chain scission in polymer decomposition has emerged as a niche but crucial area of study. Decane, a hydrocarbon solvent, plays a significant role in understanding the degradation processes of certain polymers, especially those used in high-temperature applications or exposed to hydrocarbon-rich environments.
The market for this specific research area is primarily driven by the petrochemical and polymer manufacturing sectors. These industries are investing in research and development to enhance their understanding of polymer behavior under various conditions, ultimately aiming to develop more resilient and easily recyclable materials.
Furthermore, the automotive industry has shown increased interest in polymer decomposition studies involving decane. As vehicles continue to use a higher percentage of polymer-based components, understanding their degradation mechanisms becomes crucial for improving durability and end-of-life recycling processes.
The packaging industry, another major consumer of polymers, is also contributing to the growth of this research market. With stringent regulations on single-use plastics and growing consumer awareness about environmental issues, companies are investing in research to develop packaging materials that can be more easily decomposed or recycled.
Academic institutions and research laboratories are key players in advancing the understanding of decane's role in polymer chain scission. Their findings often translate into practical applications for industry, creating a symbiotic relationship between academic research and industrial development.
Government initiatives and funding for sustainable materials research have further bolstered the market for polymer decomposition studies. Many countries have implemented policies to reduce plastic waste and promote the development of biodegradable or easily recyclable polymers, indirectly supporting research in this field.
As the global push for sustainability continues, the market for polymer decomposition research, including studies on decane's effects on chain scission, is expected to expand. This growth is likely to be accompanied by increased collaboration between academic institutions, industry players, and government bodies, fostering innovation and practical applications in polymer science and technology.
Current Challenges in Polymer Chain Scission Studies
Polymer chain scission studies face several significant challenges that hinder progress in understanding and controlling polymer decomposition processes. One of the primary obstacles is the complexity of the chemical reactions involved in chain scission. The presence of decane, a common solvent in these studies, introduces additional variables that complicate the analysis of polymer degradation mechanisms.
The heterogeneity of polymer samples presents another major challenge. Polymers often contain a distribution of chain lengths and molecular weights, making it difficult to isolate and study specific chain scission events. This variability can lead to inconsistent results and complicate the interpretation of experimental data, especially when decane is present as a solvent or reaction medium.
Furthermore, the detection and quantification of chain scission products remain problematic. Current analytical techniques may not be sensitive enough to capture all the intermediate species formed during polymer decomposition, particularly when decane interacts with these products. This limitation hampers the development of comprehensive reaction models and mechanistic understanding.
The influence of environmental factors on chain scission processes adds another layer of complexity. Temperature, pressure, and the presence of impurities or additives can significantly affect the rate and mechanism of polymer degradation. The interaction between these factors and decane further complicates the experimental design and data analysis.
Time-resolved studies of chain scission events pose technical challenges as well. Many polymer decomposition reactions occur rapidly, making it difficult to capture the kinetics of individual scission events in real-time. The presence of decane may alter reaction rates or introduce competing processes, further complicating kinetic analyses.
Additionally, the development of predictive models for polymer chain scission in the presence of decane remains a significant challenge. Current models often struggle to account for the complex interplay between polymer structure, decane interactions, and environmental conditions. This limitation hinders the ability to design more stable polymers or optimize decomposition processes for specific applications.
Lastly, the scalability of laboratory findings to industrial processes presents ongoing difficulties. Translating insights gained from small-scale experiments with decane to large-scale polymer processing and recycling operations requires overcoming numerous engineering and economic hurdles. Bridging this gap is crucial for advancing sustainable polymer technologies and improving waste management strategies.
The heterogeneity of polymer samples presents another major challenge. Polymers often contain a distribution of chain lengths and molecular weights, making it difficult to isolate and study specific chain scission events. This variability can lead to inconsistent results and complicate the interpretation of experimental data, especially when decane is present as a solvent or reaction medium.
Furthermore, the detection and quantification of chain scission products remain problematic. Current analytical techniques may not be sensitive enough to capture all the intermediate species formed during polymer decomposition, particularly when decane interacts with these products. This limitation hampers the development of comprehensive reaction models and mechanistic understanding.
The influence of environmental factors on chain scission processes adds another layer of complexity. Temperature, pressure, and the presence of impurities or additives can significantly affect the rate and mechanism of polymer degradation. The interaction between these factors and decane further complicates the experimental design and data analysis.
Time-resolved studies of chain scission events pose technical challenges as well. Many polymer decomposition reactions occur rapidly, making it difficult to capture the kinetics of individual scission events in real-time. The presence of decane may alter reaction rates or introduce competing processes, further complicating kinetic analyses.
Additionally, the development of predictive models for polymer chain scission in the presence of decane remains a significant challenge. Current models often struggle to account for the complex interplay between polymer structure, decane interactions, and environmental conditions. This limitation hinders the ability to design more stable polymers or optimize decomposition processes for specific applications.
Lastly, the scalability of laboratory findings to industrial processes presents ongoing difficulties. Translating insights gained from small-scale experiments with decane to large-scale polymer processing and recycling operations requires overcoming numerous engineering and economic hurdles. Bridging this gap is crucial for advancing sustainable polymer technologies and improving waste management strategies.
Existing Methodologies for Studying Decane Effects
01 Catalytic cracking of decane
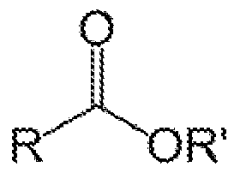
Catalytic cracking is a method used for decane chain scission. This process involves the use of catalysts to break down the long hydrocarbon chains of decane into smaller, more valuable products. The catalysts used can include zeolites, metal oxides, or other materials that facilitate the breaking of carbon-carbon bonds.- Catalytic cracking of decane: Catalytic cracking is a process used to break down long-chain hydrocarbons like decane into smaller, more valuable products. This process typically involves the use of specific catalysts and controlled reaction conditions to achieve selective chain scission. The method can be optimized for various desired product distributions and can be applied in petroleum refining and petrochemical industries.
- Thermal decomposition of decane: Thermal decomposition is a method used to break down decane molecules without the use of catalysts. This process relies on high temperatures to overcome the activation energy required for bond cleavage. The thermal cracking of decane can lead to a variety of smaller hydrocarbon products, including olefins and shorter-chain alkanes. The process conditions can be adjusted to influence the product distribution.
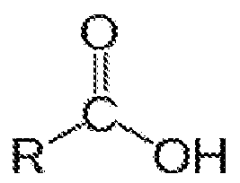
- Oxidative decane chain scission: Oxidative chain scission of decane involves the use of oxidizing agents to break the carbon-carbon bonds. This process can be carried out under milder conditions compared to thermal cracking and can lead to the formation of oxygenated products such as aldehydes, ketones, and carboxylic acids. The selectivity of the oxidation can be controlled by the choice of oxidizing agent and reaction conditions.
- Photochemical decane chain scission: Photochemical methods can be employed to induce chain scission in decane molecules. This approach typically involves the use of specific wavelengths of light to excite the molecules or to generate reactive species that can initiate the chain-breaking process. Photochemical chain scission can offer advantages in terms of selectivity and energy efficiency compared to thermal methods.
- Enzymatic decane chain scission: Enzymatic methods for decane chain scission involve the use of specific enzymes capable of breaking down long-chain hydrocarbons. This bio-based approach can offer high selectivity and the ability to operate under mild conditions. Enzymatic chain scission of decane is of interest in bioremediation applications and in the development of bio-based processes for hydrocarbon conversion.
02 Thermal decomposition of decane
Thermal decomposition is another method for decane chain scission. This process involves subjecting decane to high temperatures, which causes the molecules to break apart into smaller hydrocarbons. The temperature and pressure conditions can be controlled to optimize the yield of desired products.Expand Specific Solutions03 Oxidative cleavage of decane
Oxidative cleavage is a technique used for decane chain scission that involves the use of oxidizing agents. These agents can selectively break carbon-carbon bonds in the decane molecule, resulting in the formation of shorter chain hydrocarbons and oxygenated compounds. This method can be useful for producing specific chemical intermediates.Expand Specific Solutions04 Photochemical degradation of decane
Photochemical degradation is a method that uses light energy to initiate the chain scission of decane. This process can involve the use of photocatalysts or direct photolysis. The absorption of light energy causes the breaking of chemical bonds in the decane molecule, leading to the formation of smaller hydrocarbon fragments.Expand Specific Solutions05 Enzymatic degradation of decane
Enzymatic degradation is a biological approach to decane chain scission. This method utilizes specific enzymes, often from microorganisms, that are capable of breaking down long-chain hydrocarbons like decane. The enzymes catalyze the cleavage of carbon-carbon bonds, resulting in the formation of shorter chain molecules that can be further metabolized.Expand Specific Solutions
Key Players in Polymer Science and Decane Research
The competitive landscape for studying decane's effects on chain scission in polymer decomposition is characterized by a mature market with established players and ongoing research. The field is in a consolidation phase, with major chemical companies like ExxonMobil Chemical, Dow Global Technologies, and BASF leading industrial applications. Academic institutions such as Soochow University and Ghent University contribute to fundamental research. The market size is moderate, driven by industrial demand for polymer stability and recycling solutions. Technologically, the field is well-developed but continues to evolve, with companies like Corning and Honeywell International Technologies pursuing innovations in material science and analytical techniques to enhance understanding of polymer degradation mechanisms and improve product performance.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed advanced techniques for studying polymer decomposition in the presence of decane. Their approach involves using high-resolution nuclear magnetic resonance (NMR) spectroscopy to analyze the chemical changes occurring during chain scission[1]. They have also implemented molecular dynamics simulations to model the interaction between decane molecules and polymer chains at the atomic level[3]. This combination of experimental and computational methods allows for a detailed understanding of how decane affects the breaking of chemical bonds in various polymer structures[5].
Strengths: Comprehensive approach combining experimental and computational methods. Weaknesses: May be limited to specific polymer types studied by ExxonMobil.
Dow Global Technologies LLC
Technical Solution: Dow has pioneered a multi-faceted approach to studying decane's impact on polymer decomposition. They utilize thermogravimetric analysis (TGA) coupled with mass spectrometry to precisely measure weight loss and identify decomposition products in real-time[2]. Additionally, Dow has developed proprietary software that can predict the effect of decane on various polymer structures, allowing for rapid screening of potential polymer formulations[4]. Their research also includes the use of in-situ FTIR spectroscopy to monitor chemical changes during the decomposition process[6].
Strengths: Comprehensive analytical techniques and predictive modeling capabilities. Weaknesses: Proprietary nature of some tools may limit broader scientific collaboration.
Core Innovations in Polymer Chain Scission Analysis
Process for making linear long-chain alkanes from renewable feedstocks using catalysts comprising heteropolyacids
PatentWO2014159484A1
Innovation
- A hydrodeoxygenation process using a catalyst comprising Group IB, VIB, or VIII metals and heteropolyacids or their salts at temperatures between 150 °C to 250 °C and hydrogen gas pressures of at least 300 psig, which converts C10-18 oxygenates to linear alkanes without significant carbon chain shortening.
Degradable elastic polyurethane composition
PatentWO2024129790A2
Innovation
- Development of a polyurethaneurea fiber with a glycol component comprising greater than 50% by weight of a polyester glycol with a number average molecular weight between 450 to 3300, combined with a diisocyanate and a chain extender, and optionally including a biodegradability enhancing additive, to achieve high retractive force and controlled biodegradation.
Environmental Impact of Decane in Polymer Decomposition
The environmental impact of decane in polymer decomposition is a critical consideration in the broader context of plastic waste management and environmental sustainability. Decane, a hydrocarbon commonly used as a solvent in polymer decomposition studies, plays a significant role in the chain scission process and subsequent environmental consequences.
When polymers decompose in the presence of decane, the solvent can facilitate the breakdown of long polymer chains into smaller fragments. This process, while beneficial for studying polymer degradation mechanisms, can lead to the release of microplastics and other potentially harmful byproducts into the environment. The interaction between decane and polymer chains during decomposition may accelerate the formation of these microplastics, which are known to persist in ecosystems for extended periods.
The release of decane itself during polymer decomposition studies also raises environmental concerns. As a volatile organic compound (VOC), decane can contribute to air pollution and the formation of ground-level ozone when released into the atmosphere. Furthermore, if not properly contained or disposed of, decane can contaminate soil and water systems, potentially affecting aquatic and terrestrial ecosystems.
The persistence of decane in the environment is another factor to consider. While it is biodegradable, the rate of degradation can vary depending on environmental conditions. In anaerobic environments, such as landfills or deep water bodies, the breakdown of decane may be significantly slower, prolonging its environmental impact.
The use of decane in polymer decomposition studies also raises questions about the scalability and real-world applicability of such research. While controlled laboratory conditions may yield valuable insights, the environmental impact of using decane-based decomposition methods on an industrial scale could be substantial. This necessitates the exploration of more environmentally friendly alternatives or the development of closed-loop systems that minimize the release of decane and other potentially harmful substances.
Researchers and industry professionals must consider the life cycle assessment of decane use in polymer decomposition studies. This includes evaluating the environmental footprint of decane production, transportation, use in experiments, and ultimate disposal. Such comprehensive analysis can help in developing more sustainable practices and identifying areas for improvement in the polymer decomposition research process.
In conclusion, while decane plays a crucial role in understanding polymer decomposition mechanisms, its environmental impact cannot be overlooked. Balancing the scientific benefits with ecological considerations is essential for developing sustainable solutions in polymer science and waste management.
When polymers decompose in the presence of decane, the solvent can facilitate the breakdown of long polymer chains into smaller fragments. This process, while beneficial for studying polymer degradation mechanisms, can lead to the release of microplastics and other potentially harmful byproducts into the environment. The interaction between decane and polymer chains during decomposition may accelerate the formation of these microplastics, which are known to persist in ecosystems for extended periods.
The release of decane itself during polymer decomposition studies also raises environmental concerns. As a volatile organic compound (VOC), decane can contribute to air pollution and the formation of ground-level ozone when released into the atmosphere. Furthermore, if not properly contained or disposed of, decane can contaminate soil and water systems, potentially affecting aquatic and terrestrial ecosystems.
The persistence of decane in the environment is another factor to consider. While it is biodegradable, the rate of degradation can vary depending on environmental conditions. In anaerobic environments, such as landfills or deep water bodies, the breakdown of decane may be significantly slower, prolonging its environmental impact.
The use of decane in polymer decomposition studies also raises questions about the scalability and real-world applicability of such research. While controlled laboratory conditions may yield valuable insights, the environmental impact of using decane-based decomposition methods on an industrial scale could be substantial. This necessitates the exploration of more environmentally friendly alternatives or the development of closed-loop systems that minimize the release of decane and other potentially harmful substances.
Researchers and industry professionals must consider the life cycle assessment of decane use in polymer decomposition studies. This includes evaluating the environmental footprint of decane production, transportation, use in experiments, and ultimate disposal. Such comprehensive analysis can help in developing more sustainable practices and identifying areas for improvement in the polymer decomposition research process.
In conclusion, while decane plays a crucial role in understanding polymer decomposition mechanisms, its environmental impact cannot be overlooked. Balancing the scientific benefits with ecological considerations is essential for developing sustainable solutions in polymer science and waste management.
Regulatory Framework for Chemical Decomposition Research
The regulatory framework for chemical decomposition research, particularly in the context of how decane affects chain scission in polymer decomposition studies, is a complex and evolving landscape. Governments and international bodies have established various regulations and guidelines to ensure the safety, environmental protection, and ethical conduct of such research.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating chemical research and its potential environmental impacts. The Toxic Substances Control Act (TSCA) provides the EPA with authority to require reporting, record-keeping, and testing requirements for chemical substances and mixtures. This act has been amended to address specific concerns related to polymer decomposition and the use of hydrocarbons like decane in research settings.
The Occupational Safety and Health Administration (OSHA) also contributes to the regulatory framework by setting and enforcing standards for workplace safety in laboratories and research facilities. Their guidelines cover the handling, storage, and disposal of chemicals used in polymer decomposition studies, including decane and other hydrocarbons.
Internationally, the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation provides a comprehensive framework for chemical safety. This regulation impacts research involving decane and polymer decomposition by requiring thorough documentation of chemical properties, potential risks, and safe handling procedures.
The United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) has been widely adopted, providing a standardized approach to communicating chemical hazards. This system is particularly relevant for research involving decane and its effects on polymer chain scission, as it ensures consistent safety information across international borders.
Research institutions and universities often have their own internal regulatory frameworks that complement national and international regulations. These may include specific protocols for conducting polymer decomposition studies, waste management procedures, and safety training requirements for researchers working with hydrocarbons like decane.
Funding agencies also play a role in shaping the regulatory landscape by imposing their own requirements on funded research projects. These may include adherence to specific ethical guidelines, data sharing policies, and environmental impact assessments, particularly for studies involving potentially hazardous chemicals or processes.
As the field of polymer decomposition research advances, regulatory frameworks continue to evolve. There is an increasing focus on sustainable chemistry practices, which may influence future regulations regarding the use of hydrocarbons like decane in chain scission studies. Researchers must stay informed about these changing regulations to ensure compliance and responsible conduct in their work.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating chemical research and its potential environmental impacts. The Toxic Substances Control Act (TSCA) provides the EPA with authority to require reporting, record-keeping, and testing requirements for chemical substances and mixtures. This act has been amended to address specific concerns related to polymer decomposition and the use of hydrocarbons like decane in research settings.
The Occupational Safety and Health Administration (OSHA) also contributes to the regulatory framework by setting and enforcing standards for workplace safety in laboratories and research facilities. Their guidelines cover the handling, storage, and disposal of chemicals used in polymer decomposition studies, including decane and other hydrocarbons.
Internationally, the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation provides a comprehensive framework for chemical safety. This regulation impacts research involving decane and polymer decomposition by requiring thorough documentation of chemical properties, potential risks, and safe handling procedures.
The United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) has been widely adopted, providing a standardized approach to communicating chemical hazards. This system is particularly relevant for research involving decane and its effects on polymer chain scission, as it ensures consistent safety information across international borders.
Research institutions and universities often have their own internal regulatory frameworks that complement national and international regulations. These may include specific protocols for conducting polymer decomposition studies, waste management procedures, and safety training requirements for researchers working with hydrocarbons like decane.
Funding agencies also play a role in shaping the regulatory landscape by imposing their own requirements on funded research projects. These may include adherence to specific ethical guidelines, data sharing policies, and environmental impact assessments, particularly for studies involving potentially hazardous chemicals or processes.
As the field of polymer decomposition research advances, regulatory frameworks continue to evolve. There is an increasing focus on sustainable chemistry practices, which may influence future regulations regarding the use of hydrocarbons like decane in chain scission studies. Researchers must stay informed about these changing regulations to ensure compliance and responsible conduct in their work.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!