How to Drive Scalability in Large-Scale Gel Electrophoresis Operations?
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gel Electrophoresis Evolution and Objectives
Gel electrophoresis has been a cornerstone technique in molecular biology since its inception in the 1960s. This method, which separates molecules based on their size and electrical charge, has evolved significantly over the decades. Initially used for protein separation, it quickly found applications in DNA and RNA analysis, becoming an indispensable tool in genomics, proteomics, and forensic science.
The evolution of gel electrophoresis has been marked by several key advancements. The introduction of polyacrylamide gels in the 1970s allowed for higher resolution separation of smaller molecules. The development of pulsed-field gel electrophoresis in the 1980s enabled the separation of much larger DNA fragments, expanding the technique's utility in genomic studies. More recently, the integration of automated systems and digital imaging technologies has greatly enhanced the efficiency and accuracy of gel electrophoresis operations.
As the scale of biological research has grown, so too has the demand for high-throughput gel electrophoresis capabilities. This has led to the development of multi-channel systems and microfluidic devices, allowing for parallel processing of multiple samples. However, scaling up gel electrophoresis operations while maintaining precision and reproducibility remains a significant challenge in many laboratories and industrial settings.
The primary objective in driving scalability in large-scale gel electrophoresis operations is to increase throughput without compromising on resolution or accuracy. This involves addressing several key areas: automation of sample preparation and loading, optimization of gel casting processes, enhancement of electrophoresis run times, and improvement of detection and analysis methods.
Another crucial objective is to develop standardized protocols that can be easily replicated across different laboratories and scales of operation. This standardization is essential for ensuring consistency in results, particularly in clinical diagnostics and quality control applications where reliability is paramount.
Sustainability is also becoming an increasingly important consideration. Objectives in this area include reducing the environmental impact of gel electrophoresis by minimizing waste, developing recyclable or biodegradable gel materials, and improving energy efficiency in large-scale operations.
As we look to the future, the integration of gel electrophoresis with other analytical techniques, such as mass spectrometry or next-generation sequencing, presents exciting possibilities for comprehensive biomolecule analysis. The ultimate goal is to create high-throughput, automated systems that can seamlessly interface with other analytical platforms, providing a more complete picture of complex biological samples.
The evolution of gel electrophoresis has been marked by several key advancements. The introduction of polyacrylamide gels in the 1970s allowed for higher resolution separation of smaller molecules. The development of pulsed-field gel electrophoresis in the 1980s enabled the separation of much larger DNA fragments, expanding the technique's utility in genomic studies. More recently, the integration of automated systems and digital imaging technologies has greatly enhanced the efficiency and accuracy of gel electrophoresis operations.
As the scale of biological research has grown, so too has the demand for high-throughput gel electrophoresis capabilities. This has led to the development of multi-channel systems and microfluidic devices, allowing for parallel processing of multiple samples. However, scaling up gel electrophoresis operations while maintaining precision and reproducibility remains a significant challenge in many laboratories and industrial settings.
The primary objective in driving scalability in large-scale gel electrophoresis operations is to increase throughput without compromising on resolution or accuracy. This involves addressing several key areas: automation of sample preparation and loading, optimization of gel casting processes, enhancement of electrophoresis run times, and improvement of detection and analysis methods.
Another crucial objective is to develop standardized protocols that can be easily replicated across different laboratories and scales of operation. This standardization is essential for ensuring consistency in results, particularly in clinical diagnostics and quality control applications where reliability is paramount.
Sustainability is also becoming an increasingly important consideration. Objectives in this area include reducing the environmental impact of gel electrophoresis by minimizing waste, developing recyclable or biodegradable gel materials, and improving energy efficiency in large-scale operations.
As we look to the future, the integration of gel electrophoresis with other analytical techniques, such as mass spectrometry or next-generation sequencing, presents exciting possibilities for comprehensive biomolecule analysis. The ultimate goal is to create high-throughput, automated systems that can seamlessly interface with other analytical platforms, providing a more complete picture of complex biological samples.
Market Demand Analysis for Large-Scale Gel Electrophoresis
The market demand for large-scale gel electrophoresis operations has been steadily increasing, driven by the growing need for high-throughput DNA and protein analysis in various fields, including genomics, proteomics, and biotechnology. This demand is particularly evident in research institutions, pharmaceutical companies, and diagnostic laboratories where large-scale sample processing is essential for advancing scientific discoveries and developing new therapies.
One of the primary factors fueling this demand is the exponential growth in genomic and proteomic data generation. As sequencing technologies become more affordable and accessible, researchers are conducting larger studies involving thousands of samples. This trend has created a bottleneck in sample preparation and analysis, where traditional gel electrophoresis methods struggle to keep pace with the volume of samples.
The biopharmaceutical industry has emerged as a significant driver of demand for scalable gel electrophoresis solutions. With the rise of personalized medicine and the development of biologics, there is an increasing need for high-throughput protein characterization and quality control. Large-scale gel electrophoresis enables efficient analysis of protein purity, stability, and post-translational modifications, which are critical for ensuring the safety and efficacy of biopharmaceutical products.
In the field of diagnostics, the demand for scalable gel electrophoresis is being driven by the need for rapid and accurate testing of large patient populations. This is particularly relevant in the context of infectious disease outbreaks and genetic screening programs, where high-throughput analysis of nucleic acids and proteins is essential for timely diagnosis and epidemiological studies.
The academic research sector also contributes significantly to the market demand for large-scale gel electrophoresis. As collaborative research projects and multi-center studies become more common, there is a growing need for standardized and scalable analytical methods that can be applied across different laboratories and institutions.
Environmental and food safety testing represent emerging areas of demand for scalable gel electrophoresis. These fields require the analysis of large numbers of samples for the detection of contaminants, pathogens, and genetically modified organisms, driving the need for high-throughput electrophoresis solutions.
The market demand is not limited to hardware solutions alone. There is a growing interest in integrated systems that combine automated sample preparation, electrophoresis, and data analysis. This reflects a broader trend towards laboratory automation and the need for end-to-end solutions that can streamline workflows and reduce manual intervention.
As the demand for large-scale gel electrophoresis continues to grow, there is an increasing focus on developing technologies that can improve throughput, reduce sample volumes, and enhance resolution. This has led to innovations in microfluidic devices, capillary electrophoresis systems, and multiplexed gel formats, all aimed at addressing the scalability challenges in electrophoresis operations.
One of the primary factors fueling this demand is the exponential growth in genomic and proteomic data generation. As sequencing technologies become more affordable and accessible, researchers are conducting larger studies involving thousands of samples. This trend has created a bottleneck in sample preparation and analysis, where traditional gel electrophoresis methods struggle to keep pace with the volume of samples.
The biopharmaceutical industry has emerged as a significant driver of demand for scalable gel electrophoresis solutions. With the rise of personalized medicine and the development of biologics, there is an increasing need for high-throughput protein characterization and quality control. Large-scale gel electrophoresis enables efficient analysis of protein purity, stability, and post-translational modifications, which are critical for ensuring the safety and efficacy of biopharmaceutical products.
In the field of diagnostics, the demand for scalable gel electrophoresis is being driven by the need for rapid and accurate testing of large patient populations. This is particularly relevant in the context of infectious disease outbreaks and genetic screening programs, where high-throughput analysis of nucleic acids and proteins is essential for timely diagnosis and epidemiological studies.
The academic research sector also contributes significantly to the market demand for large-scale gel electrophoresis. As collaborative research projects and multi-center studies become more common, there is a growing need for standardized and scalable analytical methods that can be applied across different laboratories and institutions.
Environmental and food safety testing represent emerging areas of demand for scalable gel electrophoresis. These fields require the analysis of large numbers of samples for the detection of contaminants, pathogens, and genetically modified organisms, driving the need for high-throughput electrophoresis solutions.
The market demand is not limited to hardware solutions alone. There is a growing interest in integrated systems that combine automated sample preparation, electrophoresis, and data analysis. This reflects a broader trend towards laboratory automation and the need for end-to-end solutions that can streamline workflows and reduce manual intervention.
As the demand for large-scale gel electrophoresis continues to grow, there is an increasing focus on developing technologies that can improve throughput, reduce sample volumes, and enhance resolution. This has led to innovations in microfluidic devices, capillary electrophoresis systems, and multiplexed gel formats, all aimed at addressing the scalability challenges in electrophoresis operations.
Current Challenges in Scaling Gel Electrophoresis
Gel electrophoresis is a fundamental technique in molecular biology, widely used for separating and analyzing DNA, RNA, and proteins. As research and diagnostic applications expand, there is an increasing demand for scaling up gel electrophoresis operations. However, this scaling process faces several significant challenges that need to be addressed to ensure efficient and reliable large-scale operations.
One of the primary challenges in scaling gel electrophoresis is maintaining consistent sample separation quality across larger gel formats. As gel size increases, issues such as uneven electric field distribution and heat generation become more pronounced, potentially leading to band distortion and reduced resolution. This problem is exacerbated in high-throughput settings where multiple samples are run simultaneously, requiring careful optimization of buffer systems and gel compositions to ensure uniform separation across the entire gel surface.
Another major hurdle is the increased time and resource consumption associated with larger gels. Scaling up gel size often results in longer run times, higher power requirements, and increased buffer consumption. This not only impacts operational efficiency but also raises concerns about cost-effectiveness and environmental sustainability. Developing strategies to minimize these factors while maintaining separation quality is crucial for successful large-scale implementation.
Sample loading presents another significant challenge in scaled-up operations. Traditional manual loading methods become impractical and error-prone when dealing with a large number of samples. Automated loading systems are necessary but must be designed to handle a variety of sample types and volumes without compromising sample integrity or introducing cross-contamination.
Gel imaging and analysis also face scaling challenges. Larger gels require more sophisticated imaging equipment capable of capturing high-resolution images of the entire gel surface. Additionally, the increased data volume generated from large-scale operations necessitates robust image analysis software and data management systems to efficiently process and interpret results.
Reproducibility and standardization become increasingly critical in large-scale gel electrophoresis. Variations in gel preparation, running conditions, and analysis methods can lead to inconsistent results across different batches or laboratories. Establishing standardized protocols and quality control measures that can be reliably implemented at scale is essential for ensuring comparable and trustworthy results.
Lastly, the integration of gel electrophoresis into high-throughput workflows poses significant challenges. Seamless interfacing with upstream sample preparation and downstream analysis steps requires careful consideration of automation, sample tracking, and data management. Developing modular and flexible systems that can adapt to different experimental requirements while maintaining overall workflow efficiency is a key challenge in scaling gel electrophoresis operations.
One of the primary challenges in scaling gel electrophoresis is maintaining consistent sample separation quality across larger gel formats. As gel size increases, issues such as uneven electric field distribution and heat generation become more pronounced, potentially leading to band distortion and reduced resolution. This problem is exacerbated in high-throughput settings where multiple samples are run simultaneously, requiring careful optimization of buffer systems and gel compositions to ensure uniform separation across the entire gel surface.
Another major hurdle is the increased time and resource consumption associated with larger gels. Scaling up gel size often results in longer run times, higher power requirements, and increased buffer consumption. This not only impacts operational efficiency but also raises concerns about cost-effectiveness and environmental sustainability. Developing strategies to minimize these factors while maintaining separation quality is crucial for successful large-scale implementation.
Sample loading presents another significant challenge in scaled-up operations. Traditional manual loading methods become impractical and error-prone when dealing with a large number of samples. Automated loading systems are necessary but must be designed to handle a variety of sample types and volumes without compromising sample integrity or introducing cross-contamination.
Gel imaging and analysis also face scaling challenges. Larger gels require more sophisticated imaging equipment capable of capturing high-resolution images of the entire gel surface. Additionally, the increased data volume generated from large-scale operations necessitates robust image analysis software and data management systems to efficiently process and interpret results.
Reproducibility and standardization become increasingly critical in large-scale gel electrophoresis. Variations in gel preparation, running conditions, and analysis methods can lead to inconsistent results across different batches or laboratories. Establishing standardized protocols and quality control measures that can be reliably implemented at scale is essential for ensuring comparable and trustworthy results.
Lastly, the integration of gel electrophoresis into high-throughput workflows poses significant challenges. Seamless interfacing with upstream sample preparation and downstream analysis steps requires careful consideration of automation, sample tracking, and data management. Developing modular and flexible systems that can adapt to different experimental requirements while maintaining overall workflow efficiency is a key challenge in scaling gel electrophoresis operations.
Existing Scalability Solutions for Gel Electrophoresis
01 Microfluidic devices for gel electrophoresis
Microfluidic devices are being developed to enhance the scalability of gel electrophoresis. These devices allow for miniaturization of the process, enabling parallel analysis of multiple samples simultaneously. The use of microfluidic channels and integrated electrodes can improve separation efficiency and reduce sample consumption, making the technique more scalable for high-throughput applications.- Microfluidic devices for gel electrophoresis: Microfluidic devices are being developed to improve the scalability of gel electrophoresis. These devices allow for miniaturization of the process, enabling parallel analysis of multiple samples simultaneously. They often incorporate advanced features such as integrated electrodes, sample loading mechanisms, and detection systems, enhancing throughput and efficiency.
- Multi-channel electrophoresis systems: Multi-channel electrophoresis systems have been designed to increase the scalability of gel electrophoresis. These systems allow for the simultaneous analysis of multiple samples in parallel channels, significantly improving throughput. They often include automated sample loading and detection mechanisms to further enhance efficiency.
- Continuous flow electrophoresis: Continuous flow electrophoresis techniques have been developed to address scalability issues in gel electrophoresis. These methods allow for the continuous separation of molecules in a flowing stream, enabling higher sample throughput and potentially larger-scale separations compared to traditional batch processes.
- High-throughput gel electrophoresis systems: High-throughput gel electrophoresis systems have been designed to increase scalability. These systems often incorporate automated sample preparation, loading, and analysis processes. They may also feature advanced imaging and data analysis capabilities to handle large volumes of data generated from multiple samples.
- Novel gel materials and formulations: Research into novel gel materials and formulations aims to improve the scalability of gel electrophoresis. These innovations include the development of gels with enhanced separation properties, increased stability, and improved compatibility with high-throughput systems. Some approaches focus on creating gels that allow for faster separation times or higher resolution, enabling more efficient analysis of large sample sets.
02 Multi-channel electrophoresis systems
Multi-channel electrophoresis systems have been designed to increase the throughput and scalability of gel electrophoresis. These systems allow for the simultaneous analysis of multiple samples in parallel channels, significantly reducing the time required for large-scale studies. Advanced designs incorporate automated sample loading and detection mechanisms to further improve efficiency.Expand Specific Solutions03 Continuous-flow gel electrophoresis
Continuous-flow gel electrophoresis techniques have been developed to address scalability issues. These methods allow for the continuous separation of molecules in a flowing gel medium, enabling the processing of larger sample volumes and potentially increasing throughput. The continuous nature of the process makes it more suitable for industrial-scale applications and large-scale purification tasks.Expand Specific Solutions04 Automated gel electrophoresis systems
Automated gel electrophoresis systems have been introduced to improve scalability and reproducibility. These systems integrate sample preparation, gel casting, electrophoresis, and analysis steps into a single automated platform. By reducing manual intervention and standardizing procedures, these systems can significantly increase throughput and consistency in large-scale studies.Expand Specific Solutions05 Novel gel materials for improved scalability
Research into novel gel materials aims to enhance the scalability of gel electrophoresis. These materials may offer improved separation efficiency, faster run times, or better stability under various conditions. Some developments focus on creating reusable or recyclable gel matrices, which could reduce costs and waste in large-scale applications. Additionally, some novel materials allow for easier post-electrophoresis recovery of separated molecules.Expand Specific Solutions
Key Players in Large-Scale Electrophoresis Industry
The large-scale gel electrophoresis market is in a growth phase, driven by increasing demand in genomics and proteomics research. The global market size is projected to expand significantly in the coming years, fueled by advancements in biotechnology and life sciences. Technologically, the field is evolving rapidly, with companies like Life Technologies Corp., Beckman Coulter, Inc., and Applied Biosystems LLC leading innovation in high-throughput systems and automation. These industry leaders are focusing on improving scalability through enhanced resolution, faster run times, and integration with downstream analysis tools. Emerging players such as Sage Science, Inc. are also contributing to technological advancements, particularly in specialized applications. The competitive landscape is characterized by a mix of established biotechnology firms and niche providers, with ongoing research collaborations driving further innovation in scalable electrophoresis solutions.
Beckman Coulter, Inc.
Technical Solution: Beckman Coulter has developed an automated gel electrophoresis system that significantly enhances scalability in large-scale operations. Their approach integrates robotic sample handling, multi-channel pipetting, and high-throughput gel running capabilities. The system can process up to 96 samples simultaneously, with automated gel loading and imaging[1]. They have also implemented a microfluidic-based gel electrophoresis technique that allows for faster separation and higher resolution, reducing the time required for large-scale analyses[2]. Additionally, Beckman Coulter has introduced a software platform that enables real-time monitoring and control of multiple gel electrophoresis runs, facilitating 24/7 operation and remote management[3].
Strengths: High throughput, automation, and integration capabilities. Weaknesses: High initial investment cost and potential complexity in system setup and maintenance.
Jilin University
Technical Solution: Jilin University has developed innovative approaches to enhance scalability in large-scale gel electrophoresis operations, focusing on microfluidic technologies and novel materials. Their research has led to the development of a high-throughput microfluidic gel electrophoresis chip capable of analyzing hundreds of samples simultaneously[12]. This chip integrates sample loading, separation, and detection into a single platform, significantly reducing processing time and reagent consumption. The university has also pioneered the use of smart hydrogels as separation matrices, which can be dynamically controlled to optimize separation conditions in real-time, adapting to different sample types without the need for gel replacement[13]. Additionally, Jilin University researchers have developed a machine learning algorithm that predicts optimal separation parameters based on sample characteristics, further streamlining large-scale operations[14].
Strengths: Cutting-edge microfluidic technology, smart materials for adaptive separation, and AI-assisted optimization. Weaknesses: Potential challenges in scaling up production of specialized microfluidic chips and the need for specialized expertise to operate advanced systems.
Innovative Approaches to Enhance Throughput
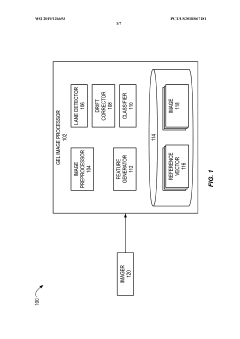
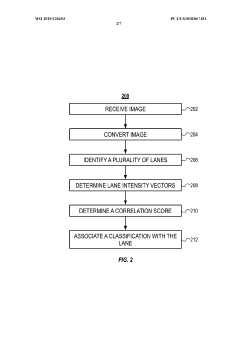
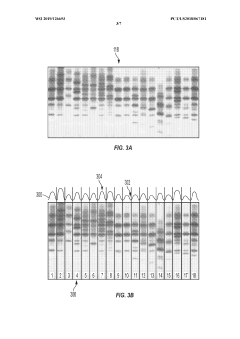
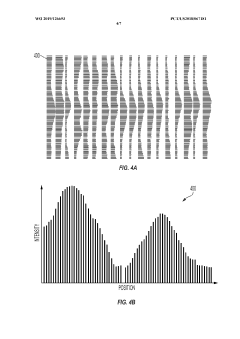
Automated analysis of analytical GELS and blots
PatentWO2019126693A1
Innovation
- An automated system that detects lanes in gels or blots, generates feature vectors, and classifies samples using a classifier based on reference datasets, significantly reducing analysis time and subjectivity through image processing and machine learning techniques.
Method and device for separating biomolecules
PatentInactiveEP1102983A1
Innovation
- A combined electrophoresis chamber that allows for simultaneous or sequential vertical arrangement of gels for both dimensions, with integrated cooling and buffer vessels, enabling full automation and precise temperature control, eliminating the need for manual gel transfer and rehydration, and supporting the use of self-cast or ready-to-use gels.
Automation and Robotics in Gel Electrophoresis
Automation and robotics have revolutionized gel electrophoresis operations, significantly enhancing scalability and efficiency in large-scale applications. The integration of automated systems has addressed many of the challenges associated with manual gel electrophoresis, including inconsistency, time-consumption, and limited throughput.
Robotic systems have been developed to handle various stages of the gel electrophoresis process, from sample preparation to gel loading and imaging. These systems can precisely pipette samples, load gels with minimal cross-contamination, and even perform post-electrophoresis tasks such as gel staining and imaging. The use of robotics ensures reproducibility and reduces human error, critical factors in large-scale operations.
High-throughput automated gel electrophoresis platforms have emerged, capable of processing hundreds of samples simultaneously. These systems often incorporate multiple gel tanks, automated buffer management, and integrated power supplies. Some advanced platforms feature continuous gel casting and running capabilities, allowing for 24/7 operation and maximizing throughput.
Automation has also extended to data analysis and interpretation. Software solutions integrated with automated gel electrophoresis systems can perform lane and band detection, molecular weight calculations, and even generate comprehensive reports. This automation of data processing significantly reduces analysis time and improves result consistency across large sample sets.
Microfluidic technologies have been incorporated into automated gel electrophoresis systems, allowing for miniaturization and increased parallelization. These lab-on-a-chip devices can perform multiple electrophoresis runs simultaneously on a single chip, dramatically increasing sample throughput while reducing reagent consumption.
Artificial intelligence and machine learning algorithms are being applied to optimize gel electrophoresis parameters automatically. These systems can learn from previous runs and adjust variables such as voltage, run time, and buffer composition to achieve optimal separation for different sample types, further enhancing scalability and reproducibility.
The integration of automation and robotics with other analytical techniques, such as mass spectrometry or next-generation sequencing, has created powerful workflows for large-scale genomics and proteomics studies. These integrated platforms allow for seamless sample processing from gel separation to downstream analysis, significantly improving overall laboratory efficiency.
Robotic systems have been developed to handle various stages of the gel electrophoresis process, from sample preparation to gel loading and imaging. These systems can precisely pipette samples, load gels with minimal cross-contamination, and even perform post-electrophoresis tasks such as gel staining and imaging. The use of robotics ensures reproducibility and reduces human error, critical factors in large-scale operations.
High-throughput automated gel electrophoresis platforms have emerged, capable of processing hundreds of samples simultaneously. These systems often incorporate multiple gel tanks, automated buffer management, and integrated power supplies. Some advanced platforms feature continuous gel casting and running capabilities, allowing for 24/7 operation and maximizing throughput.
Automation has also extended to data analysis and interpretation. Software solutions integrated with automated gel electrophoresis systems can perform lane and band detection, molecular weight calculations, and even generate comprehensive reports. This automation of data processing significantly reduces analysis time and improves result consistency across large sample sets.
Microfluidic technologies have been incorporated into automated gel electrophoresis systems, allowing for miniaturization and increased parallelization. These lab-on-a-chip devices can perform multiple electrophoresis runs simultaneously on a single chip, dramatically increasing sample throughput while reducing reagent consumption.
Artificial intelligence and machine learning algorithms are being applied to optimize gel electrophoresis parameters automatically. These systems can learn from previous runs and adjust variables such as voltage, run time, and buffer composition to achieve optimal separation for different sample types, further enhancing scalability and reproducibility.
The integration of automation and robotics with other analytical techniques, such as mass spectrometry or next-generation sequencing, has created powerful workflows for large-scale genomics and proteomics studies. These integrated platforms allow for seamless sample processing from gel separation to downstream analysis, significantly improving overall laboratory efficiency.
Quality Control in Large-Scale Electrophoresis Operations
Quality control is a critical aspect of large-scale gel electrophoresis operations, ensuring consistent and reliable results across numerous samples. Implementing robust quality control measures is essential for maintaining the integrity of data and the efficiency of the overall process.
One key element of quality control in large-scale electrophoresis is the standardization of sample preparation. This involves developing and adhering to strict protocols for sample collection, storage, and processing. Automated liquid handling systems can be employed to minimize human error and ensure precise sample loading.
Gel preparation and casting also require stringent quality control measures. Automated gel casting systems can help maintain consistency in gel thickness and composition. Regular calibration and maintenance of these systems are crucial for optimal performance.
The electrophoresis process itself must be closely monitored. Advanced imaging systems can be integrated to track the progress of sample migration in real-time. This allows for immediate detection of anomalies and enables timely interventions to prevent run failures.
Post-electrophoresis analysis is another critical area for quality control. Automated gel imaging and analysis software can be utilized to ensure objective and consistent interpretation of results. These systems can be programmed to flag any deviations from expected patterns or intensities.
Implementing a comprehensive quality management system (QMS) is essential for large-scale operations. This should include regular equipment calibration, maintenance schedules, and thorough documentation of all procedures and results. The QMS should also incorporate periodic proficiency testing and inter-laboratory comparisons to ensure the reliability of results.
Training and competency assessment of personnel are vital components of quality control. Regular training sessions and assessments can help maintain a high level of expertise among staff and ensure adherence to standard operating procedures.
Environmental control is another crucial factor in maintaining quality in large-scale electrophoresis operations. Stable temperature and humidity conditions must be maintained throughout the process to prevent variations that could affect gel performance or sample integrity.
Finally, implementing a robust data management system is essential for tracking samples, recording run parameters, and storing results. This system should be capable of integrating with other laboratory information management systems (LIMS) to ensure seamless data flow and traceability.
One key element of quality control in large-scale electrophoresis is the standardization of sample preparation. This involves developing and adhering to strict protocols for sample collection, storage, and processing. Automated liquid handling systems can be employed to minimize human error and ensure precise sample loading.
Gel preparation and casting also require stringent quality control measures. Automated gel casting systems can help maintain consistency in gel thickness and composition. Regular calibration and maintenance of these systems are crucial for optimal performance.
The electrophoresis process itself must be closely monitored. Advanced imaging systems can be integrated to track the progress of sample migration in real-time. This allows for immediate detection of anomalies and enables timely interventions to prevent run failures.
Post-electrophoresis analysis is another critical area for quality control. Automated gel imaging and analysis software can be utilized to ensure objective and consistent interpretation of results. These systems can be programmed to flag any deviations from expected patterns or intensities.
Implementing a comprehensive quality management system (QMS) is essential for large-scale operations. This should include regular equipment calibration, maintenance schedules, and thorough documentation of all procedures and results. The QMS should also incorporate periodic proficiency testing and inter-laboratory comparisons to ensure the reliability of results.
Training and competency assessment of personnel are vital components of quality control. Regular training sessions and assessments can help maintain a high level of expertise among staff and ensure adherence to standard operating procedures.
Environmental control is another crucial factor in maintaining quality in large-scale electrophoresis operations. Stable temperature and humidity conditions must be maintained throughout the process to prevent variations that could affect gel performance or sample integrity.
Finally, implementing a robust data management system is essential for tracking samples, recording run parameters, and storing results. This system should be capable of integrating with other laboratory information management systems (LIMS) to ensure seamless data flow and traceability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!