How To Implement Fail-Safe Mechanisms In Medical Nanorobots
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nanorobot Safety Goals
The primary safety goal for medical nanorobots is to ensure their reliable and secure operation within the human body while minimizing potential risks to patients. This objective encompasses several key aspects that must be addressed in the development and implementation of fail-safe mechanisms.
One crucial safety goal is to prevent uncontrolled replication or aggregation of nanorobots. This is essential to avoid potential harm to the patient's tissues or organs. Implementing strict control mechanisms and self-destruct protocols can help mitigate this risk, ensuring that nanorobots remain within their designated operational parameters.
Another critical safety objective is to maintain the integrity of the nanorobots' programming and functionality. This includes protecting against external interference, such as electromagnetic fields or hacking attempts, which could compromise the nanorobots' intended operations. Robust encryption and authentication protocols must be integrated to safeguard the nanorobots' control systems.
Biocompatibility is a paramount safety concern for medical nanorobots. The materials used in their construction must not trigger adverse immune responses or cause toxicity in the patient's body. Additionally, the nanorobots should be designed to avoid causing physical damage to cellular structures or disrupting normal physiological processes during their navigation and operation within the body.
Precise navigation and targeting capabilities are essential safety goals for nanorobots. They must be able to accurately locate their intended sites of action while avoiding unintended interactions with healthy tissues or organs. This requires sophisticated sensing and guidance systems that can adapt to the complex and dynamic environment of the human body.
The ability to monitor and control nanorobot function in real-time is another critical safety objective. This includes implementing mechanisms for remote activation, deactivation, and adjustment of nanorobot behavior. Such capabilities are vital for responding to unexpected situations or adverse reactions during treatment.
Lastly, a key safety goal is to ensure the complete and safe removal or biodegradation of nanorobots after they have completed their intended tasks. This prevents potential long-term complications or accumulation of foreign materials in the patient's body. Developing reliable methods for nanorobot retrieval or designing them to be naturally eliminated by the body's systems is crucial for overall patient safety.
One crucial safety goal is to prevent uncontrolled replication or aggregation of nanorobots. This is essential to avoid potential harm to the patient's tissues or organs. Implementing strict control mechanisms and self-destruct protocols can help mitigate this risk, ensuring that nanorobots remain within their designated operational parameters.
Another critical safety objective is to maintain the integrity of the nanorobots' programming and functionality. This includes protecting against external interference, such as electromagnetic fields or hacking attempts, which could compromise the nanorobots' intended operations. Robust encryption and authentication protocols must be integrated to safeguard the nanorobots' control systems.
Biocompatibility is a paramount safety concern for medical nanorobots. The materials used in their construction must not trigger adverse immune responses or cause toxicity in the patient's body. Additionally, the nanorobots should be designed to avoid causing physical damage to cellular structures or disrupting normal physiological processes during their navigation and operation within the body.
Precise navigation and targeting capabilities are essential safety goals for nanorobots. They must be able to accurately locate their intended sites of action while avoiding unintended interactions with healthy tissues or organs. This requires sophisticated sensing and guidance systems that can adapt to the complex and dynamic environment of the human body.
The ability to monitor and control nanorobot function in real-time is another critical safety objective. This includes implementing mechanisms for remote activation, deactivation, and adjustment of nanorobot behavior. Such capabilities are vital for responding to unexpected situations or adverse reactions during treatment.
Lastly, a key safety goal is to ensure the complete and safe removal or biodegradation of nanorobots after they have completed their intended tasks. This prevents potential long-term complications or accumulation of foreign materials in the patient's body. Developing reliable methods for nanorobot retrieval or designing them to be naturally eliminated by the body's systems is crucial for overall patient safety.
Medical Nanorobot Demand
The demand for medical nanorobots has been steadily increasing in recent years, driven by the growing need for targeted and minimally invasive medical interventions. These microscopic devices hold immense potential in revolutionizing healthcare by offering precise drug delivery, early disease detection, and localized treatments. The market for medical nanorobots is expected to expand significantly as the technology matures and becomes more widely adopted in clinical settings.
One of the primary drivers of demand is the rising prevalence of chronic diseases such as cancer, cardiovascular disorders, and neurological conditions. Medical nanorobots offer a promising approach to addressing these complex health issues by providing targeted therapies that can potentially reduce side effects and improve treatment efficacy. The aging global population and the subsequent increase in age-related diseases further contribute to the growing demand for innovative medical solutions like nanorobots.
The pharmaceutical industry has shown considerable interest in leveraging nanorobot technology for drug delivery applications. This interest stems from the potential to enhance drug efficacy, reduce dosage requirements, and minimize adverse effects. As a result, major pharmaceutical companies are investing in research and development of nanorobot-based drug delivery systems, further fueling market demand.
In the field of diagnostics, medical nanorobots offer the possibility of early disease detection at the molecular level. This capability is particularly valuable in oncology, where early diagnosis can significantly improve patient outcomes. The demand for such advanced diagnostic tools is expected to grow as healthcare systems worldwide emphasize preventive care and early intervention strategies.
The potential of medical nanorobots in surgical applications is another factor driving market demand. These devices could enable minimally invasive procedures, reducing patient recovery times and healthcare costs. Surgeons and medical institutions are increasingly interested in adopting nanorobot-assisted surgical techniques, creating a growing market for these advanced medical devices.
However, the demand for medical nanorobots is tempered by several factors, including regulatory challenges, safety concerns, and the need for extensive clinical trials. The implementation of fail-safe mechanisms in these devices is crucial to address safety issues and gain regulatory approval, which in turn will boost market confidence and accelerate adoption rates.
As research progresses and more applications are discovered, the demand for medical nanorobots is expected to expand into new areas of healthcare, including regenerative medicine, gene therapy, and personalized medicine. This diversification of applications will likely contribute to sustained market growth and continued investment in nanorobot technology development.
One of the primary drivers of demand is the rising prevalence of chronic diseases such as cancer, cardiovascular disorders, and neurological conditions. Medical nanorobots offer a promising approach to addressing these complex health issues by providing targeted therapies that can potentially reduce side effects and improve treatment efficacy. The aging global population and the subsequent increase in age-related diseases further contribute to the growing demand for innovative medical solutions like nanorobots.
The pharmaceutical industry has shown considerable interest in leveraging nanorobot technology for drug delivery applications. This interest stems from the potential to enhance drug efficacy, reduce dosage requirements, and minimize adverse effects. As a result, major pharmaceutical companies are investing in research and development of nanorobot-based drug delivery systems, further fueling market demand.
In the field of diagnostics, medical nanorobots offer the possibility of early disease detection at the molecular level. This capability is particularly valuable in oncology, where early diagnosis can significantly improve patient outcomes. The demand for such advanced diagnostic tools is expected to grow as healthcare systems worldwide emphasize preventive care and early intervention strategies.
The potential of medical nanorobots in surgical applications is another factor driving market demand. These devices could enable minimally invasive procedures, reducing patient recovery times and healthcare costs. Surgeons and medical institutions are increasingly interested in adopting nanorobot-assisted surgical techniques, creating a growing market for these advanced medical devices.
However, the demand for medical nanorobots is tempered by several factors, including regulatory challenges, safety concerns, and the need for extensive clinical trials. The implementation of fail-safe mechanisms in these devices is crucial to address safety issues and gain regulatory approval, which in turn will boost market confidence and accelerate adoption rates.
As research progresses and more applications are discovered, the demand for medical nanorobots is expected to expand into new areas of healthcare, including regenerative medicine, gene therapy, and personalized medicine. This diversification of applications will likely contribute to sustained market growth and continued investment in nanorobot technology development.
Fail-Safe Challenges
Implementing fail-safe mechanisms in medical nanorobots presents several significant challenges due to the unique nature of these microscopic devices and their critical applications. One of the primary obstacles is the limited computational power and memory available on nanorobots, which restricts the complexity of fail-safe algorithms that can be implemented. This constraint necessitates the development of highly efficient and streamlined safety protocols that can operate within these severe resource limitations.
Another major challenge is the unpredictable environment in which medical nanorobots operate. The human body is a complex and dynamic system, with varying conditions across different tissues and organs. Designing fail-safe mechanisms that can adapt to these diverse environments while maintaining reliability is a formidable task. Factors such as pH levels, temperature fluctuations, and immune system responses can all potentially interfere with nanorobot operations, requiring robust fail-safe systems that can function across a wide range of conditions.
The potential for unintended interactions between nanorobots and biological systems poses another significant challenge. Fail-safe mechanisms must be designed to prevent nanorobots from causing harm to healthy tissues or triggering adverse immune responses. This requires sophisticated sensing and decision-making capabilities to distinguish between target areas and non-target tissues, as well as the ability to quickly deactivate or change behavior when potential risks are detected.
Communication between nanorobots and external control systems is another critical aspect that presents challenges for implementing fail-safe mechanisms. The ability to remotely monitor and control nanorobots is essential for safety, but achieving reliable communication at the nanoscale within the human body is technically challenging. Developing fail-safe protocols that can operate autonomously when communication is lost or compromised is crucial.
The issue of nanorobot degradation and malfunction over time also poses significant challenges for fail-safe design. As these devices operate within the body, they may be subject to wear, damage, or unexpected chemical interactions. Fail-safe mechanisms must be able to detect and respond to gradual degradation or sudden failures, ensuring that nanorobots can either self-repair, safely shut down, or be removed from the body before they pose any risk to the patient.
Lastly, the ethical and regulatory challenges surrounding the implementation of fail-safe mechanisms in medical nanorobots cannot be overlooked. Ensuring that these safety systems meet stringent medical standards and comply with evolving regulations is a complex task. There is a need for transparent and verifiable fail-safe protocols that can be thoroughly tested and validated before deployment in human patients, which presents both technical and procedural challenges in the development process.
Another major challenge is the unpredictable environment in which medical nanorobots operate. The human body is a complex and dynamic system, with varying conditions across different tissues and organs. Designing fail-safe mechanisms that can adapt to these diverse environments while maintaining reliability is a formidable task. Factors such as pH levels, temperature fluctuations, and immune system responses can all potentially interfere with nanorobot operations, requiring robust fail-safe systems that can function across a wide range of conditions.
The potential for unintended interactions between nanorobots and biological systems poses another significant challenge. Fail-safe mechanisms must be designed to prevent nanorobots from causing harm to healthy tissues or triggering adverse immune responses. This requires sophisticated sensing and decision-making capabilities to distinguish between target areas and non-target tissues, as well as the ability to quickly deactivate or change behavior when potential risks are detected.
Communication between nanorobots and external control systems is another critical aspect that presents challenges for implementing fail-safe mechanisms. The ability to remotely monitor and control nanorobots is essential for safety, but achieving reliable communication at the nanoscale within the human body is technically challenging. Developing fail-safe protocols that can operate autonomously when communication is lost or compromised is crucial.
The issue of nanorobot degradation and malfunction over time also poses significant challenges for fail-safe design. As these devices operate within the body, they may be subject to wear, damage, or unexpected chemical interactions. Fail-safe mechanisms must be able to detect and respond to gradual degradation or sudden failures, ensuring that nanorobots can either self-repair, safely shut down, or be removed from the body before they pose any risk to the patient.
Lastly, the ethical and regulatory challenges surrounding the implementation of fail-safe mechanisms in medical nanorobots cannot be overlooked. Ensuring that these safety systems meet stringent medical standards and comply with evolving regulations is a complex task. There is a need for transparent and verifiable fail-safe protocols that can be thoroughly tested and validated before deployment in human patients, which presents both technical and procedural challenges in the development process.
Current Fail-Safe Solutions
01 Self-destruction mechanisms
Medical nanorobots can be equipped with self-destruction mechanisms that activate under specific conditions, such as when the nanorobot malfunctions or completes its intended task. This fail-safe ensures that the nanorobots do not remain in the body indefinitely or cause unintended harm. The self-destruction process can involve breaking down the nanorobot into harmless components that can be safely eliminated from the body.- Self-destruction mechanisms: Medical nanorobots can be designed with self-destruction mechanisms that activate under specific conditions. These fail-safe features ensure that the nanorobots can be safely eliminated from the body when their mission is complete or if they malfunction. The mechanisms may include programmed disintegration, chemical breakdown, or physical disassembly triggered by external stimuli or internal timers.
- Remote control and deactivation: Fail-safe mechanisms for medical nanorobots can include remote control and deactivation capabilities. These systems allow operators to monitor and control the nanorobots from outside the body, enabling immediate shutdown or reprogramming if necessary. Remote control can be achieved through various methods such as electromagnetic signals, ultrasound, or optical communication.
- Biocompatible materials and biodegradation: Medical nanorobots can be constructed using biocompatible materials that naturally degrade over time within the body. This approach ensures that even if other fail-safe mechanisms fail, the nanorobots will eventually break down into harmless components that can be safely eliminated. The use of biodegradable materials also reduces the risk of long-term side effects or complications.
- Sensor-based safety systems: Incorporating advanced sensor systems into medical nanorobots can enhance their safety profile. These sensors can detect changes in the surrounding environment, such as pH levels, temperature, or specific biomolecules, and trigger appropriate responses. If abnormal conditions are detected, the nanorobots can automatically cease operations, change their behavior, or initiate self-destruction protocols.
- Swarm intelligence and collective fail-safe: Implementing swarm intelligence in medical nanorobot systems can provide an additional layer of safety. In this approach, individual nanorobots communicate and coordinate their actions as a collective. If one or more units malfunction, the swarm can adapt its behavior, isolate faulty units, or collectively decide to abort the mission. This distributed decision-making process enhances the overall reliability and safety of the nanorobot system.
02 Remote deactivation systems
Fail-safe mechanisms for medical nanorobots can include remote deactivation systems that allow external control over the nanorobots' functions. These systems enable healthcare professionals to shut down or alter the behavior of nanorobots in real-time if any issues arise during treatment. Remote deactivation can be achieved through various methods, such as electromagnetic signals or chemical triggers.Expand Specific Solutions03 Biodegradable materials
Incorporating biodegradable materials in the construction of medical nanorobots serves as a passive fail-safe mechanism. These materials naturally break down over time within the body, ensuring that the nanorobots do not persist indefinitely. The degradation process can be designed to occur after the nanorobots have completed their intended function, minimizing the risk of long-term side effects or complications.Expand Specific Solutions04 Sensor-based safety protocols
Medical nanorobots can be equipped with advanced sensors that continuously monitor their environment and internal functions. These sensors can trigger fail-safe protocols if they detect abnormal conditions, such as unexpected changes in body chemistry or mechanical failures. The safety protocols may include immediate cessation of activity, self-destruction, or signaling for external intervention.Expand Specific Solutions05 Swarm intelligence and redundancy
Implementing swarm intelligence and redundancy in medical nanorobot systems can serve as a fail-safe mechanism. By deploying multiple nanorobots that can communicate and coordinate their actions, the system becomes more resilient to individual failures. If one nanorobot malfunctions, others can compensate or assist in its removal, ensuring the overall success and safety of the treatment.Expand Specific Solutions
Key Nanorobotics Players
The implementation of fail-safe mechanisms in medical nanorobots is an emerging field at the intersection of nanotechnology and healthcare. The market is in its early stages, with significant potential for growth as the technology matures. Key players in this space include established medical device companies like Medtronic and Siemens Healthineers, as well as specialized robotics firms such as KUKA and Medical Microinstruments. Research institutions like Arizona State University and The Chinese University of Hong Kong are also contributing to advancements. The technology is still in the research and development phase, with most companies focusing on proof-of-concept studies and early-stage clinical trials. As the field progresses, we can expect increased collaboration between academia, industry, and healthcare providers to address safety and regulatory challenges.
Medtronic, Inc.
Technical Solution: Medtronic's approach to implementing fail-safe mechanisms in medical nanorobots focuses on multi-layered redundancy and real-time monitoring. Their system incorporates triple-redundant control circuits, ensuring that if one fails, the others can maintain operation[1]. They've developed a proprietary "NanoGuard" protocol that continuously monitors nanorobot function and can initiate immediate shutdown if anomalies are detected[2]. Additionally, Medtronic has implemented a bio-compatible self-destruct mechanism that can be triggered remotely or automatically in case of severe malfunction, ensuring patient safety[3]. The nanorobots are also designed with a passive "fail-safe" mode, where they default to an inert state if power or control signals are lost, preventing unintended actions in the body[4].
Strengths: Comprehensive safety approach, industry-leading redundancy systems, and remote control capabilities. Weaknesses: Complexity may lead to higher costs and potential for unforeseen interactions between safety systems.
Siemens Healthineers AG
Technical Solution: Siemens Healthineers has developed a comprehensive fail-safe system for medical nanorobots that combines hardware redundancy with advanced software controls. Their approach, called "NanoShield," incorporates multiple layers of safety mechanisms. At the hardware level, each nanorobot is equipped with redundant power sources and control circuits[10]. The software layer includes a sophisticated error detection and correction system that can identify and rectify minor malfunctions in real-time. For more serious issues, Siemens has implemented a "graceful degradation" protocol, where nanorobots can continue to function at reduced capacity rather than shutting down completely[11]. Additionally, their system includes a secure, blockchain-based logging mechanism that records all nanorobot actions and status updates, allowing for detailed post-operation analysis and continuous improvement of safety protocols[12].
Strengths: Robust combination of hardware and software safety measures, innovative use of blockchain for accountability. Weaknesses: The complex system may require significant processing power, potentially limiting the size and capabilities of the nanorobots.
Core Fail-Safe Innovations
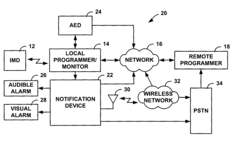
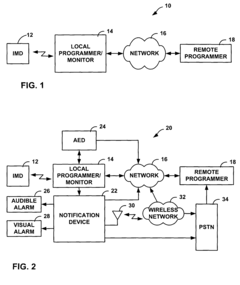
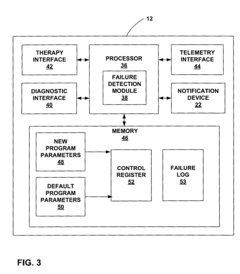
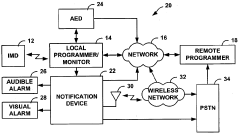
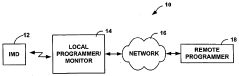
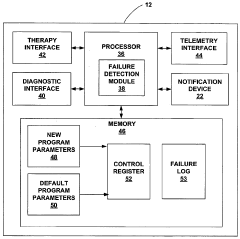
Fail-safe programming for implantable medical device
PatentActiveUS20050049656A1
Innovation
- A fail-safe mode is implemented that includes notifying personnel of session failures, modifying programming parameters to revert to default settings, and delivering therapies to ensure patient safety, thereby preventing harmful consequences from improper programming.
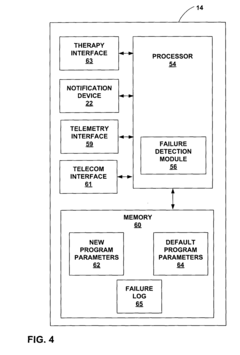
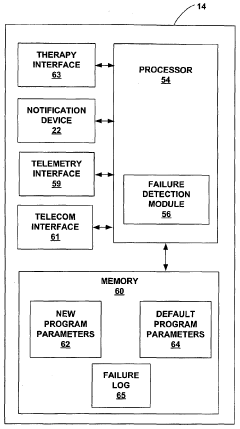
Fail-safe programming for implantable medical device
PatentWO2005021092A1
Innovation
- A fail-safe mode is implemented that includes notifying personnel of session failures, modifying programming parameters to revert to default settings, and delivering therapy to ensure patient safety, utilizing a system with a communication interface and processor to invoke fail-safe operations during adverse events.
Nanorobot Regulatory Framework
The regulatory framework for medical nanorobots is a critical aspect of their development and implementation, particularly concerning fail-safe mechanisms. As these microscopic devices are designed to operate within the human body, stringent regulations are necessary to ensure patient safety and efficacy of treatment. The framework must address various aspects, including design standards, manufacturing processes, clinical testing protocols, and post-market surveillance.
One key component of the regulatory framework is the establishment of specific guidelines for fail-safe mechanisms in medical nanorobots. These guidelines should outline the minimum requirements for redundancy systems, error detection algorithms, and emergency shutdown procedures. Regulatory bodies, such as the FDA in the United States or the EMA in Europe, would need to collaborate with experts in nanotechnology, robotics, and medicine to develop these standards.
The framework should also include a comprehensive risk assessment process for nanorobot technologies. This would involve identifying potential failure modes, evaluating their likelihood and severity, and implementing appropriate mitigation strategies. Manufacturers would be required to demonstrate compliance with these risk assessment protocols before obtaining approval for clinical use.
Another crucial aspect of the regulatory framework is the establishment of a robust reporting system for adverse events and malfunctions. This system would enable healthcare providers and patients to report any issues encountered with nanorobots, allowing for rapid identification and resolution of potential safety concerns. The framework should also mandate regular software updates and remote monitoring capabilities to ensure the ongoing safety and effectiveness of deployed nanorobots.
Ethical considerations must also be addressed within the regulatory framework. This includes guidelines for patient consent, data privacy, and the potential long-term effects of nanorobot use. The framework should establish clear boundaries for the use of nanorobots in medical applications, ensuring that their deployment aligns with established ethical principles in healthcare.
Lastly, the regulatory framework should outline requirements for clinical trials specific to nanorobot technologies. These trials would need to demonstrate not only the efficacy of the nanorobots in treating specific conditions but also the reliability and effectiveness of their fail-safe mechanisms under various physiological conditions. The framework should specify the necessary endpoints and safety measures for such trials, ensuring that potential risks are thoroughly evaluated before widespread adoption.
One key component of the regulatory framework is the establishment of specific guidelines for fail-safe mechanisms in medical nanorobots. These guidelines should outline the minimum requirements for redundancy systems, error detection algorithms, and emergency shutdown procedures. Regulatory bodies, such as the FDA in the United States or the EMA in Europe, would need to collaborate with experts in nanotechnology, robotics, and medicine to develop these standards.
The framework should also include a comprehensive risk assessment process for nanorobot technologies. This would involve identifying potential failure modes, evaluating their likelihood and severity, and implementing appropriate mitigation strategies. Manufacturers would be required to demonstrate compliance with these risk assessment protocols before obtaining approval for clinical use.
Another crucial aspect of the regulatory framework is the establishment of a robust reporting system for adverse events and malfunctions. This system would enable healthcare providers and patients to report any issues encountered with nanorobots, allowing for rapid identification and resolution of potential safety concerns. The framework should also mandate regular software updates and remote monitoring capabilities to ensure the ongoing safety and effectiveness of deployed nanorobots.
Ethical considerations must also be addressed within the regulatory framework. This includes guidelines for patient consent, data privacy, and the potential long-term effects of nanorobot use. The framework should establish clear boundaries for the use of nanorobots in medical applications, ensuring that their deployment aligns with established ethical principles in healthcare.
Lastly, the regulatory framework should outline requirements for clinical trials specific to nanorobot technologies. These trials would need to demonstrate not only the efficacy of the nanorobots in treating specific conditions but also the reliability and effectiveness of their fail-safe mechanisms under various physiological conditions. The framework should specify the necessary endpoints and safety measures for such trials, ensuring that potential risks are thoroughly evaluated before widespread adoption.
Nanorobot Ethical Issues
The integration of nanorobots into medical applications raises significant ethical concerns that must be carefully addressed. One primary issue is the potential for unintended harm to patients due to nanorobot malfunction or unforeseen interactions within the human body. This necessitates rigorous safety protocols and fail-safe mechanisms to prevent adverse effects. Privacy and data security also present ethical challenges, as nanorobots may collect sensitive medical information that could be vulnerable to unauthorized access or misuse.
The concept of human enhancement through nanorobotics introduces complex ethical questions regarding fairness, equality, and the definition of human nature. If nanorobots can significantly improve cognitive or physical capabilities, it may create societal divisions between those who have access to such technologies and those who do not. This raises concerns about exacerbating existing social inequalities and potentially altering the course of human evolution.
Autonomy and informed consent are crucial ethical considerations in the deployment of medical nanorobots. Patients must be fully informed about the risks, benefits, and potential long-term consequences of nanorobot treatments. The level of control patients have over nanorobots within their bodies and the extent to which these devices can make autonomous decisions about medical interventions must be clearly defined and agreed upon.
The potential for dual-use applications of nanorobotics technology also presents ethical dilemmas. While developed for medical purposes, nanorobots could potentially be repurposed for harmful activities, such as biological warfare or unauthorized surveillance. This necessitates strict regulations and oversight to prevent misuse and ensure that the technology is used solely for beneficial medical applications.
Environmental impact is another ethical concern, as the production and disposal of nanorobots may have unforeseen consequences on ecosystems. The potential for nanorobots to escape into the environment and interact with non-target organisms must be carefully evaluated and mitigated. Additionally, the long-term effects of nanorobot components on the human body and the environment require extensive research to ensure their safety and sustainability.
Addressing these ethical issues requires a multidisciplinary approach involving scientists, ethicists, policymakers, and the public. Developing comprehensive ethical guidelines, establishing regulatory frameworks, and fostering open dialogue about the implications of nanorobotics in medicine are essential steps in ensuring responsible development and deployment of this transformative technology.
The concept of human enhancement through nanorobotics introduces complex ethical questions regarding fairness, equality, and the definition of human nature. If nanorobots can significantly improve cognitive or physical capabilities, it may create societal divisions between those who have access to such technologies and those who do not. This raises concerns about exacerbating existing social inequalities and potentially altering the course of human evolution.
Autonomy and informed consent are crucial ethical considerations in the deployment of medical nanorobots. Patients must be fully informed about the risks, benefits, and potential long-term consequences of nanorobot treatments. The level of control patients have over nanorobots within their bodies and the extent to which these devices can make autonomous decisions about medical interventions must be clearly defined and agreed upon.
The potential for dual-use applications of nanorobotics technology also presents ethical dilemmas. While developed for medical purposes, nanorobots could potentially be repurposed for harmful activities, such as biological warfare or unauthorized surveillance. This necessitates strict regulations and oversight to prevent misuse and ensure that the technology is used solely for beneficial medical applications.
Environmental impact is another ethical concern, as the production and disposal of nanorobots may have unforeseen consequences on ecosystems. The potential for nanorobots to escape into the environment and interact with non-target organisms must be carefully evaluated and mitigated. Additionally, the long-term effects of nanorobot components on the human body and the environment require extensive research to ensure their safety and sustainability.
Addressing these ethical issues requires a multidisciplinary approach involving scientists, ethicists, policymakers, and the public. Developing comprehensive ethical guidelines, establishing regulatory frameworks, and fostering open dialogue about the implications of nanorobotics in medicine are essential steps in ensuring responsible development and deployment of this transformative technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!