Nanorobotics Clinical Trial Design: Endpoints And Safety Metrics
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nanorobotics Background
Nanorobotics represents a cutting-edge field at the intersection of nanotechnology, robotics, and medicine. This emerging technology involves the design, fabrication, and control of nanoscale devices capable of performing specific tasks at the molecular or cellular level. The concept of nanorobotics dates back to the 1950s when physicist Richard Feynman first proposed the idea of manipulating matter at the atomic scale.
Over the past few decades, significant advancements in nanotechnology, materials science, and bioengineering have brought nanorobotics closer to reality. These miniature machines, typically ranging from 1 to 100 nanometers in size, hold immense potential for revolutionizing various fields, particularly in healthcare and medicine.
The development of nanorobotics has been driven by the need for precise, targeted interventions at the cellular and molecular levels. Traditional medical treatments often lack specificity, leading to unwanted side effects and limited efficacy. Nanorobots offer the promise of highly targeted drug delivery, minimally invasive diagnostics, and even the ability to perform microscopic surgeries within the human body.
Key milestones in nanorobotics include the creation of DNA-based nanostructures, the development of magnetically controlled nanoparticles, and the fabrication of synthetic molecular motors. These advancements have paved the way for more sophisticated nanorobotic systems capable of sensing, processing information, and executing specific actions within biological environments.
As nanorobotics progresses towards clinical applications, the design of appropriate clinical trials becomes crucial. This involves establishing clear endpoints to measure the efficacy and safety of nanorobotic interventions. Traditional clinical trial designs may need to be adapted to account for the unique characteristics and potential risks associated with nanorobots operating within the human body.
The development of safety metrics for nanorobotics presents unique challenges due to the microscopic scale and potential for unforeseen interactions with biological systems. Researchers must consider factors such as biocompatibility, biodegradation, potential immune responses, and long-term effects of nanorobots in the body.
As the field advances, interdisciplinary collaboration between nanotechnologists, roboticists, medical professionals, and regulatory experts will be essential to address the complex challenges associated with bringing nanorobotic technologies from the laboratory to clinical practice.
Over the past few decades, significant advancements in nanotechnology, materials science, and bioengineering have brought nanorobotics closer to reality. These miniature machines, typically ranging from 1 to 100 nanometers in size, hold immense potential for revolutionizing various fields, particularly in healthcare and medicine.
The development of nanorobotics has been driven by the need for precise, targeted interventions at the cellular and molecular levels. Traditional medical treatments often lack specificity, leading to unwanted side effects and limited efficacy. Nanorobots offer the promise of highly targeted drug delivery, minimally invasive diagnostics, and even the ability to perform microscopic surgeries within the human body.
Key milestones in nanorobotics include the creation of DNA-based nanostructures, the development of magnetically controlled nanoparticles, and the fabrication of synthetic molecular motors. These advancements have paved the way for more sophisticated nanorobotic systems capable of sensing, processing information, and executing specific actions within biological environments.
As nanorobotics progresses towards clinical applications, the design of appropriate clinical trials becomes crucial. This involves establishing clear endpoints to measure the efficacy and safety of nanorobotic interventions. Traditional clinical trial designs may need to be adapted to account for the unique characteristics and potential risks associated with nanorobots operating within the human body.
The development of safety metrics for nanorobotics presents unique challenges due to the microscopic scale and potential for unforeseen interactions with biological systems. Researchers must consider factors such as biocompatibility, biodegradation, potential immune responses, and long-term effects of nanorobots in the body.
As the field advances, interdisciplinary collaboration between nanotechnologists, roboticists, medical professionals, and regulatory experts will be essential to address the complex challenges associated with bringing nanorobotic technologies from the laboratory to clinical practice.
Clinical Trial Demand
The demand for clinical trials in nanorobotics is rapidly growing as this emerging field promises revolutionary advancements in targeted drug delivery, minimally invasive surgeries, and precision diagnostics. As nanorobots become more sophisticated and their potential applications in healthcare expand, there is an increasing need for rigorous clinical trials to evaluate their safety, efficacy, and long-term effects on human health.
One of the primary drivers of clinical trial demand in nanorobotics is the potential for highly targeted cancer treatments. Nanorobots designed to deliver chemotherapy drugs directly to tumor cells while sparing healthy tissue have shown promising results in preclinical studies. This has led to a surge in interest from pharmaceutical companies and research institutions seeking to conduct human trials to validate these findings and bring nanorobot-based cancer therapies to market.
Another area driving clinical trial demand is the use of nanorobots for early disease detection and diagnosis. Nanorobots equipped with sensors capable of detecting biomarkers associated with various diseases at extremely low concentrations could revolutionize preventive medicine. Clinical trials are needed to assess the accuracy, reliability, and clinical utility of these diagnostic nanorobots across different patient populations and disease states.
The potential of nanorobots in cardiovascular medicine is also fueling clinical trial demand. Researchers are developing nanorobots capable of clearing arterial blockages, repairing damaged heart tissue, and delivering targeted therapies to specific areas of the cardiovascular system. Clinical trials are essential to evaluate the safety and efficacy of these interventions in patients with various cardiovascular conditions.
As nanorobotics technology advances, there is a growing need for clinical trials to assess the long-term safety and biocompatibility of these devices in the human body. Regulatory agencies require comprehensive data on the potential risks and side effects associated with nanorobot use, including their biodegradation, potential for immune system reactions, and any unforeseen interactions with human tissues or organs.
The complexity of nanorobotics clinical trials necessitates the development of specialized trial designs and endpoints. Researchers must establish appropriate metrics for evaluating nanorobot performance, such as targeting efficiency, drug delivery accuracy, and navigation capabilities within the body. This has led to increased collaboration between nanorobotics engineers, clinical researchers, and regulatory experts to develop standardized protocols for nanorobotics clinical trials.
As the field of nanorobotics continues to evolve, the demand for clinical trials is expected to grow exponentially. This will require significant investment in research infrastructure, specialized training for clinical trial personnel, and the development of new regulatory frameworks to address the unique challenges posed by nanorobotics in healthcare.
One of the primary drivers of clinical trial demand in nanorobotics is the potential for highly targeted cancer treatments. Nanorobots designed to deliver chemotherapy drugs directly to tumor cells while sparing healthy tissue have shown promising results in preclinical studies. This has led to a surge in interest from pharmaceutical companies and research institutions seeking to conduct human trials to validate these findings and bring nanorobot-based cancer therapies to market.
Another area driving clinical trial demand is the use of nanorobots for early disease detection and diagnosis. Nanorobots equipped with sensors capable of detecting biomarkers associated with various diseases at extremely low concentrations could revolutionize preventive medicine. Clinical trials are needed to assess the accuracy, reliability, and clinical utility of these diagnostic nanorobots across different patient populations and disease states.
The potential of nanorobots in cardiovascular medicine is also fueling clinical trial demand. Researchers are developing nanorobots capable of clearing arterial blockages, repairing damaged heart tissue, and delivering targeted therapies to specific areas of the cardiovascular system. Clinical trials are essential to evaluate the safety and efficacy of these interventions in patients with various cardiovascular conditions.
As nanorobotics technology advances, there is a growing need for clinical trials to assess the long-term safety and biocompatibility of these devices in the human body. Regulatory agencies require comprehensive data on the potential risks and side effects associated with nanorobot use, including their biodegradation, potential for immune system reactions, and any unforeseen interactions with human tissues or organs.
The complexity of nanorobotics clinical trials necessitates the development of specialized trial designs and endpoints. Researchers must establish appropriate metrics for evaluating nanorobot performance, such as targeting efficiency, drug delivery accuracy, and navigation capabilities within the body. This has led to increased collaboration between nanorobotics engineers, clinical researchers, and regulatory experts to develop standardized protocols for nanorobotics clinical trials.
As the field of nanorobotics continues to evolve, the demand for clinical trials is expected to grow exponentially. This will require significant investment in research infrastructure, specialized training for clinical trial personnel, and the development of new regulatory frameworks to address the unique challenges posed by nanorobotics in healthcare.
Current Challenges
The development of nanorobotics for clinical applications faces several significant challenges that must be addressed before widespread implementation can be realized. One of the primary obstacles is the precise control and navigation of nanorobots within the human body. The complex and dynamic nature of biological systems makes it difficult to accurately guide these microscopic devices to their intended targets without causing unintended interactions or damage to surrounding tissues.
Another major challenge lies in the design and manufacturing of nanorobots that can effectively perform their intended functions while maintaining biocompatibility and safety. The materials used must be non-toxic, non-immunogenic, and capable of withstanding the harsh environment of the human body. Additionally, ensuring the long-term stability and functionality of nanorobots in vivo remains a significant hurdle.
The development of reliable and standardized methods for assessing the safety and efficacy of nanorobots in clinical trials presents another critical challenge. Current regulatory frameworks and testing protocols are not adequately equipped to evaluate the unique risks and benefits associated with nanorobotics. This gap in regulatory guidance creates uncertainty for researchers and manufacturers, potentially slowing down the progress of clinical trials and product development.
Furthermore, the ethical implications of introducing nanorobots into the human body raise complex questions that must be carefully considered. Issues such as privacy, consent, and the potential for unintended consequences of nanorobot interventions need to be thoroughly addressed before clinical trials can proceed.
The integration of nanorobotics with existing medical technologies and treatment protocols also poses significant challenges. Developing seamless interfaces between nanorobots and current diagnostic and therapeutic tools requires extensive interdisciplinary collaboration and innovation.
Lastly, the scalability and cost-effectiveness of nanorobotics production remain significant barriers to widespread clinical adoption. Current manufacturing processes for nanorobots are often complex, time-consuming, and expensive, limiting their potential for large-scale production and accessibility in healthcare settings.
Addressing these challenges will require concerted efforts from researchers, clinicians, regulatory bodies, and industry stakeholders. Overcoming these obstacles will be crucial for realizing the full potential of nanorobotics in clinical applications and advancing the field of nanomedicine.
Another major challenge lies in the design and manufacturing of nanorobots that can effectively perform their intended functions while maintaining biocompatibility and safety. The materials used must be non-toxic, non-immunogenic, and capable of withstanding the harsh environment of the human body. Additionally, ensuring the long-term stability and functionality of nanorobots in vivo remains a significant hurdle.
The development of reliable and standardized methods for assessing the safety and efficacy of nanorobots in clinical trials presents another critical challenge. Current regulatory frameworks and testing protocols are not adequately equipped to evaluate the unique risks and benefits associated with nanorobotics. This gap in regulatory guidance creates uncertainty for researchers and manufacturers, potentially slowing down the progress of clinical trials and product development.
Furthermore, the ethical implications of introducing nanorobots into the human body raise complex questions that must be carefully considered. Issues such as privacy, consent, and the potential for unintended consequences of nanorobot interventions need to be thoroughly addressed before clinical trials can proceed.
The integration of nanorobotics with existing medical technologies and treatment protocols also poses significant challenges. Developing seamless interfaces between nanorobots and current diagnostic and therapeutic tools requires extensive interdisciplinary collaboration and innovation.
Lastly, the scalability and cost-effectiveness of nanorobotics production remain significant barriers to widespread clinical adoption. Current manufacturing processes for nanorobots are often complex, time-consuming, and expensive, limiting their potential for large-scale production and accessibility in healthcare settings.
Addressing these challenges will require concerted efforts from researchers, clinicians, regulatory bodies, and industry stakeholders. Overcoming these obstacles will be crucial for realizing the full potential of nanorobotics in clinical applications and advancing the field of nanomedicine.
Trial Design Solutions
01 Nanorobot safety monitoring and control systems
Advanced systems for monitoring and controlling nanorobots to ensure safety during operation. These systems include real-time tracking, remote shutdown capabilities, and fail-safe mechanisms to prevent unintended actions or malfunctions. They aim to maintain the integrity of nanorobots and protect the surrounding environment or biological systems.- Nanorobot safety monitoring and control systems: Advanced monitoring and control systems are crucial for ensuring the safety of nanorobots during operation. These systems include real-time tracking of nanorobot location, performance, and potential adverse effects. Safety metrics may involve measuring the nanorobots' interaction with biological tissues, their ability to complete assigned tasks without causing harm, and their successful removal or biodegradation after use.
- Nanorobot communication and data management: Effective communication between nanorobots and external control systems is essential for safety and performance. This includes developing secure protocols for data transmission, managing large volumes of data generated by nanorobot swarms, and ensuring real-time responsiveness to changing conditions within the target environment. Endpoints in this area focus on data integrity, latency reduction, and robust error handling.
- Biocompatibility and biodegradation of nanorobots: Ensuring the biocompatibility of nanorobots is crucial for their safe use in medical applications. This involves designing nanorobots with materials that do not trigger immune responses or cause toxicity. Additionally, developing nanorobots that can biodegrade or be safely removed from the body after completing their tasks is a key safety consideration. Metrics in this area may include measuring inflammatory responses, assessing long-term effects on tissues, and evaluating the efficiency of nanorobot clearance from the body.
- Nanorobot navigation and targeting accuracy: Precise navigation and targeting are critical for the safety and efficacy of nanorobots, especially in medical applications. This involves developing advanced guidance systems, improving the accuracy of nanorobot movement within complex biological environments, and ensuring that therapeutic or diagnostic actions are performed at the intended locations. Safety metrics may include measuring off-target effects, quantifying the precision of nanorobot localization, and assessing the impact on surrounding healthy tissues.
- Nanorobot swarm behavior and collective intelligence: Managing the behavior of nanorobot swarms is crucial for safety and effectiveness in complex applications. This involves developing algorithms for coordinated action, ensuring that individual nanorobots work together efficiently without causing harm, and implementing fail-safe mechanisms to prevent unintended collective behaviors. Safety endpoints may include measuring swarm cohesion, assessing the robustness of swarm decision-making, and evaluating the swarm's ability to adapt to unexpected environmental changes.
02 Endpoint detection and response for nanorobotics
Techniques for detecting and responding to various endpoints in nanorobotics applications. This includes methods for identifying when a nanorobot has reached its target, completed its task, or needs to be retrieved. Endpoint detection systems help in assessing the success of nanorobot missions and initiating appropriate responses.Expand Specific Solutions03 Nanorobot communication and data security
Protocols and systems for secure communication between nanorobots and control centers. These technologies ensure that data transmitted to and from nanorobots is protected from unauthorized access or manipulation. Encryption methods and secure channels are implemented to maintain the integrity of nanorobot operations and prevent potential security breaches.Expand Specific Solutions04 Environmental impact assessment of nanorobots
Methodologies for evaluating the potential environmental impacts of nanorobots. This includes assessing their interactions with biological systems, potential for accumulation in ecosystems, and long-term effects on various environmental factors. These assessments help in developing guidelines for the safe deployment and retrieval of nanorobots in different environments.Expand Specific Solutions05 Nanorobot performance metrics and quality control
Standardized metrics and quality control measures for evaluating nanorobot performance and safety. These include tests for precision, reliability, and efficiency of nanorobots in various applications. Quality control procedures ensure that nanorobots meet specified safety and performance standards before deployment in real-world scenarios.Expand Specific Solutions
Key Industry Players
The field of nanorobotics clinical trial design for endpoints and safety metrics is in its early developmental stages, characterized by a rapidly evolving competitive landscape. The market size is still relatively small but growing, driven by increasing interest in nanomedicine applications. Technological maturity varies among key players, with companies like Pfizer, Bristol Myers Squibb, and Janssen Pharmaceutica leading in pharmaceutical applications. Academic institutions such as Tsinghua University, California Institute of Technology, and Nanyang Technological University are contributing significant research advancements. Emerging players like BioVie and NeurMedix are focusing on specialized nanorobotics applications for neurological disorders, while established medical technology firms like Siemens Healthineers are exploring integration with existing medical imaging and diagnostic systems.
Pfizer Inc.
Technical Solution: Pfizer has developed a nanorobotics-based drug delivery system for targeted cancer therapy. Their approach utilizes DNA origami nanorobots loaded with therapeutic payloads. These nanorobots are designed to recognize specific cancer cell markers and release their cargo upon binding[1]. For clinical trial design, Pfizer employs a multi-phase approach, starting with safety assessments in animal models before progressing to human trials. They focus on endpoints such as tumor size reduction, progression-free survival, and overall survival rates. Safety metrics include monitoring for immune responses, off-target effects, and nanoparticle accumulation in non-target tissues[2][3].
Strengths: Precise targeting of cancer cells, potentially reducing side effects. Weaknesses: Complexity in manufacturing and potential immune system reactions.
Janssen Pharmaceutica NV
Technical Solution: Janssen Pharmaceutica is exploring nanorobotics for targeted drug delivery in neurological disorders. Their approach involves using magnetically guided nanorobots to cross the blood-brain barrier and deliver therapeutics to specific brain regions[4]. For clinical trial design, they emphasize the use of advanced imaging techniques to track nanorobot distribution and drug release in real-time. Key endpoints include improvements in cognitive function, reduction in disease biomarkers, and changes in brain structure as measured by MRI. Safety metrics focus on assessing potential neurotoxicity, monitoring for unexpected nanorobot accumulation, and evaluating long-term effects on brain function[5].
Strengths: Potential to overcome the blood-brain barrier for CNS drug delivery. Weaknesses: Challenges in controlling nanorobot behavior in complex brain environments.
Endpoint Innovations
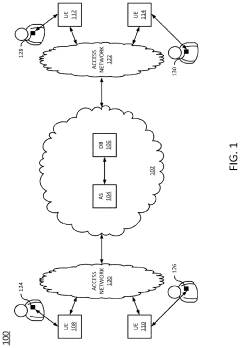
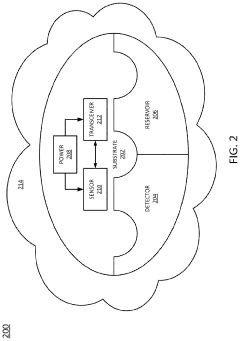
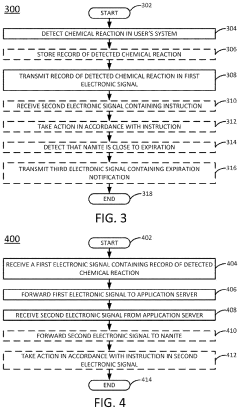
Detecting potential health risks using nanorobotics
PatentInactiveUS20210196123A1
Innovation
- The use of ingestible, non-toxic nano-scale robotics (nanorobotics or nanites) equipped with detection mechanisms and short-range transceivers to monitor biomedical conditions on-demand, communicate data, and administer treatments, including a reservoir for medication dispensing, to quickly identify and mitigate potential health risks.
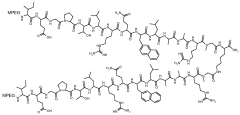
Dosing regimen
PatentWO2009148954A2
Innovation
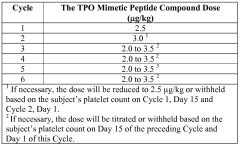
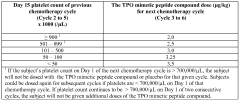
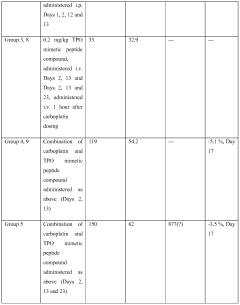
- Administration of a PEGylated TPO mimetic peptide compound within a specified time frame surrounding chemotherapy, with dosing adjusted based on individual hematological parameters to prevent anemia and thrombocytopenia, and monitoring to avoid excessive platelet counts that could lead to thrombovascular events.
Regulatory Framework
The regulatory framework for nanorobotics clinical trials is a complex and evolving landscape that requires careful consideration of existing guidelines and the development of new standards. Given the novel nature of nanorobotics, regulatory bodies such as the FDA and EMA are working to adapt current regulations and create new ones to address the unique challenges posed by this emerging technology.
One of the primary considerations in the regulatory framework is the classification of nanorobots as medical devices, drugs, or a combination product. This classification will determine the specific regulatory pathway and requirements for clinical trials. Currently, most nanorobotic systems are likely to be considered combination products, necessitating a collaborative approach between different regulatory divisions.
Safety is paramount in the regulatory framework for nanorobotics clinical trials. Regulatory bodies are particularly concerned with the potential long-term effects of nanorobots in the human body, including their biodistribution, clearance, and potential for unintended interactions with biological systems. As a result, preclinical studies must be extensive and may require longer observation periods than traditional medical devices or drugs.
The design of clinical trials for nanorobotics must adhere to Good Clinical Practice (GCP) guidelines while also incorporating specific considerations for nanotechnology. This includes developing appropriate endpoints that can accurately assess the efficacy and safety of nanorobotic interventions. Regulators are likely to require a phased approach to clinical trials, with careful monitoring and interim analyses to ensure patient safety.
Manufacturing and quality control standards for nanorobots present another regulatory challenge. Current Good Manufacturing Practice (cGMP) guidelines may need to be adapted to address the unique production processes and quality assurance requirements of nanorobotic systems. Regulatory bodies are working on developing specific guidance for the manufacturing of nanoscale medical products.
International harmonization of regulatory standards for nanorobotics is an ongoing effort. Organizations such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) are working to develop globally consistent guidelines for the evaluation of nanotechnology-based medical products. This harmonization is crucial for facilitating multinational clinical trials and ensuring consistent safety and efficacy standards across different regions.
As the field of nanorobotics advances, regulatory frameworks will need to remain flexible and adaptable. Continuous dialogue between researchers, industry, and regulatory agencies will be essential to ensure that regulations keep pace with technological developments while maintaining the highest standards of patient safety and scientific rigor in clinical trials.
One of the primary considerations in the regulatory framework is the classification of nanorobots as medical devices, drugs, or a combination product. This classification will determine the specific regulatory pathway and requirements for clinical trials. Currently, most nanorobotic systems are likely to be considered combination products, necessitating a collaborative approach between different regulatory divisions.
Safety is paramount in the regulatory framework for nanorobotics clinical trials. Regulatory bodies are particularly concerned with the potential long-term effects of nanorobots in the human body, including their biodistribution, clearance, and potential for unintended interactions with biological systems. As a result, preclinical studies must be extensive and may require longer observation periods than traditional medical devices or drugs.
The design of clinical trials for nanorobotics must adhere to Good Clinical Practice (GCP) guidelines while also incorporating specific considerations for nanotechnology. This includes developing appropriate endpoints that can accurately assess the efficacy and safety of nanorobotic interventions. Regulators are likely to require a phased approach to clinical trials, with careful monitoring and interim analyses to ensure patient safety.
Manufacturing and quality control standards for nanorobots present another regulatory challenge. Current Good Manufacturing Practice (cGMP) guidelines may need to be adapted to address the unique production processes and quality assurance requirements of nanorobotic systems. Regulatory bodies are working on developing specific guidance for the manufacturing of nanoscale medical products.
International harmonization of regulatory standards for nanorobotics is an ongoing effort. Organizations such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) are working to develop globally consistent guidelines for the evaluation of nanotechnology-based medical products. This harmonization is crucial for facilitating multinational clinical trials and ensuring consistent safety and efficacy standards across different regions.
As the field of nanorobotics advances, regulatory frameworks will need to remain flexible and adaptable. Continuous dialogue between researchers, industry, and regulatory agencies will be essential to ensure that regulations keep pace with technological developments while maintaining the highest standards of patient safety and scientific rigor in clinical trials.
Ethical Considerations
The ethical considerations surrounding nanorobotics clinical trials are complex and multifaceted, requiring careful examination and robust safeguards. One primary concern is the potential for unintended consequences and long-term effects on human health. Given the microscopic nature of nanorobots and their ability to interact with biological systems at a cellular level, there is a need for extensive pre-clinical testing and long-term follow-up studies to assess any unforeseen impacts on patients' physiology.
Patient autonomy and informed consent present another critical ethical challenge. The intricate nature of nanorobotics technology may make it difficult for participants to fully comprehend the risks and benefits involved. This necessitates the development of clear, accessible communication strategies and educational materials to ensure that patients can make truly informed decisions about their participation in clinical trials.
Privacy and data protection are also paramount ethical considerations. Nanorobots have the potential to collect vast amounts of sensitive biological data, raising concerns about data ownership, storage, and potential misuse. Robust protocols must be established to protect patient privacy and ensure that data collected during trials is used solely for its intended purpose.
The equitable distribution of potential benefits from nanorobotics research is another ethical imperative. There is a risk that this cutting-edge technology could exacerbate existing health disparities if access is limited to affluent populations or developed nations. Clinical trial designs should aim to include diverse participant groups and consider how the technology can be made accessible to a broad range of patients.
Environmental impact is an additional ethical concern that must be addressed. The production, use, and disposal of nanorobots could have unforeseen consequences on ecosystems. Clinical trial designs should incorporate assessments of environmental risks and establish protocols for the safe disposal or deactivation of nanorobots post-trial.
Lastly, the potential for nanorobotics to be used for non-therapeutic purposes, such as human enhancement, raises profound ethical questions about the boundaries of medical intervention and the nature of human identity. Clear guidelines must be established to ensure that clinical trials focus on legitimate medical applications and do not venture into ethically ambiguous territory.
In conclusion, addressing these ethical considerations requires a multidisciplinary approach, involving ethicists, scientists, policymakers, and patient advocates. Establishing a robust ethical framework for nanorobotics clinical trials is essential to ensure the responsible development of this promising technology while safeguarding patient welfare and societal values.
Patient autonomy and informed consent present another critical ethical challenge. The intricate nature of nanorobotics technology may make it difficult for participants to fully comprehend the risks and benefits involved. This necessitates the development of clear, accessible communication strategies and educational materials to ensure that patients can make truly informed decisions about their participation in clinical trials.
Privacy and data protection are also paramount ethical considerations. Nanorobots have the potential to collect vast amounts of sensitive biological data, raising concerns about data ownership, storage, and potential misuse. Robust protocols must be established to protect patient privacy and ensure that data collected during trials is used solely for its intended purpose.
The equitable distribution of potential benefits from nanorobotics research is another ethical imperative. There is a risk that this cutting-edge technology could exacerbate existing health disparities if access is limited to affluent populations or developed nations. Clinical trial designs should aim to include diverse participant groups and consider how the technology can be made accessible to a broad range of patients.
Environmental impact is an additional ethical concern that must be addressed. The production, use, and disposal of nanorobots could have unforeseen consequences on ecosystems. Clinical trial designs should incorporate assessments of environmental risks and establish protocols for the safe disposal or deactivation of nanorobots post-trial.
Lastly, the potential for nanorobotics to be used for non-therapeutic purposes, such as human enhancement, raises profound ethical questions about the boundaries of medical intervention and the nature of human identity. Clear guidelines must be established to ensure that clinical trials focus on legitimate medical applications and do not venture into ethically ambiguous territory.
In conclusion, addressing these ethical considerations requires a multidisciplinary approach, involving ethicists, scientists, policymakers, and patient advocates. Establishing a robust ethical framework for nanorobotics clinical trials is essential to ensure the responsible development of this promising technology while safeguarding patient welfare and societal values.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!