Nanorobotics For Tumor Ablation: Dosimetry And Delivery Metrics
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nanorobotics Background
Nanorobotics represents a cutting-edge field at the intersection of nanotechnology, robotics, and medicine. This emerging technology involves the design, fabrication, and control of nanoscale devices capable of performing specific tasks at the molecular level. In the context of tumor ablation, nanorobotics offers a revolutionary approach to targeted cancer treatment.
The concept of nanorobotics dates back to the 1950s when physicist Richard Feynman first proposed the idea of manipulating matter at the atomic scale. However, it wasn't until the late 20th and early 21st centuries that significant progress was made in realizing this vision. The development of advanced microscopy techniques, such as atomic force microscopy and scanning tunneling microscopy, played a crucial role in enabling the manipulation and visualization of nanoscale structures.
In recent years, the field of nanorobotics has experienced rapid growth, driven by advancements in materials science, bioengineering, and computational technologies. Researchers have successfully created various types of nanorobots, including DNA-based structures, magnetic nanoparticles, and synthetic molecular machines. These nanorobots can be programmed to perform specific functions, such as drug delivery, tissue repair, and diagnostic imaging.
The application of nanorobotics in tumor ablation represents a promising frontier in cancer treatment. Traditional tumor ablation methods, such as radiofrequency ablation and cryoablation, often lack precision and may damage surrounding healthy tissues. Nanorobots, on the other hand, offer the potential for highly targeted and minimally invasive tumor destruction.
In the context of tumor ablation, nanorobots can be designed to selectively target cancer cells, deliver therapeutic agents, and induce localized heating or mechanical disruption of tumor tissues. This approach aims to maximize treatment efficacy while minimizing side effects and collateral damage to healthy cells. The ability to precisely control the dosage and delivery of therapeutic agents at the nanoscale level is a key advantage of nanorobotics in cancer treatment.
The development of nanorobotics for tumor ablation involves interdisciplinary collaboration among experts in nanotechnology, oncology, materials science, and biomedical engineering. Researchers are exploring various nanorobot designs, including DNA origami structures, magnetically controlled nanoparticles, and stimuli-responsive nanomaterials. These nanorobots can be engineered to respond to external stimuli such as light, magnetic fields, or chemical signals, enabling precise control over their movement and activation within the body.
The concept of nanorobotics dates back to the 1950s when physicist Richard Feynman first proposed the idea of manipulating matter at the atomic scale. However, it wasn't until the late 20th and early 21st centuries that significant progress was made in realizing this vision. The development of advanced microscopy techniques, such as atomic force microscopy and scanning tunneling microscopy, played a crucial role in enabling the manipulation and visualization of nanoscale structures.
In recent years, the field of nanorobotics has experienced rapid growth, driven by advancements in materials science, bioengineering, and computational technologies. Researchers have successfully created various types of nanorobots, including DNA-based structures, magnetic nanoparticles, and synthetic molecular machines. These nanorobots can be programmed to perform specific functions, such as drug delivery, tissue repair, and diagnostic imaging.
The application of nanorobotics in tumor ablation represents a promising frontier in cancer treatment. Traditional tumor ablation methods, such as radiofrequency ablation and cryoablation, often lack precision and may damage surrounding healthy tissues. Nanorobots, on the other hand, offer the potential for highly targeted and minimally invasive tumor destruction.
In the context of tumor ablation, nanorobots can be designed to selectively target cancer cells, deliver therapeutic agents, and induce localized heating or mechanical disruption of tumor tissues. This approach aims to maximize treatment efficacy while minimizing side effects and collateral damage to healthy cells. The ability to precisely control the dosage and delivery of therapeutic agents at the nanoscale level is a key advantage of nanorobotics in cancer treatment.
The development of nanorobotics for tumor ablation involves interdisciplinary collaboration among experts in nanotechnology, oncology, materials science, and biomedical engineering. Researchers are exploring various nanorobot designs, including DNA origami structures, magnetically controlled nanoparticles, and stimuli-responsive nanomaterials. These nanorobots can be engineered to respond to external stimuli such as light, magnetic fields, or chemical signals, enabling precise control over their movement and activation within the body.
Tumor Ablation Market
The tumor ablation market has been experiencing significant growth in recent years, driven by the increasing prevalence of cancer worldwide and the growing demand for minimally invasive treatment options. This market encompasses various ablation technologies, including radiofrequency, microwave, cryoablation, and emerging nanorobotics-based approaches. The global tumor ablation market was valued at approximately $1.5 billion in 2020 and is projected to reach $3.2 billion by 2027, with a compound annual growth rate (CAGR) of around 11.5% during this period.
The market is primarily segmented by technology, application, and geography. Radiofrequency ablation currently holds the largest market share due to its established efficacy and widespread adoption. However, newer technologies such as nanorobotics for tumor ablation are gaining traction due to their potential for improved precision and reduced side effects. The application segment is dominated by liver cancer, followed by lung, kidney, and bone cancers.
Geographically, North America leads the tumor ablation market, accounting for about 40% of the global share. This dominance is attributed to the high prevalence of cancer, advanced healthcare infrastructure, and favorable reimbursement policies. Europe follows closely, while the Asia-Pacific region is expected to witness the fastest growth due to improving healthcare access and rising cancer incidence rates.
Key market drivers include the increasing preference for minimally invasive procedures, technological advancements in ablation devices, and the growing geriatric population. The rise in cancer cases, particularly liver and lung cancers, is also fueling market growth. However, the high cost of ablation procedures and the lack of skilled professionals in developing regions pose challenges to market expansion.
The competitive landscape of the tumor ablation market is characterized by the presence of several major players, including Medtronic, Boston Scientific Corporation, AngioDynamics, and Johnson & Johnson. These companies are investing heavily in research and development to introduce innovative ablation technologies and expand their product portfolios. The emergence of nanorobotics-based ablation techniques presents both opportunities and challenges for established players, potentially reshaping the competitive dynamics of the market in the coming years.
As the field of nanorobotics for tumor ablation advances, it is expected to create new growth opportunities within the broader tumor ablation market. The ability to deliver targeted therapy at the nanoscale level could revolutionize cancer treatment, potentially offering improved efficacy and reduced side effects compared to traditional ablation methods. This emerging technology is likely to attract significant investment and research focus, potentially leading to the development of novel ablation devices and techniques in the near future.
The market is primarily segmented by technology, application, and geography. Radiofrequency ablation currently holds the largest market share due to its established efficacy and widespread adoption. However, newer technologies such as nanorobotics for tumor ablation are gaining traction due to their potential for improved precision and reduced side effects. The application segment is dominated by liver cancer, followed by lung, kidney, and bone cancers.
Geographically, North America leads the tumor ablation market, accounting for about 40% of the global share. This dominance is attributed to the high prevalence of cancer, advanced healthcare infrastructure, and favorable reimbursement policies. Europe follows closely, while the Asia-Pacific region is expected to witness the fastest growth due to improving healthcare access and rising cancer incidence rates.
Key market drivers include the increasing preference for minimally invasive procedures, technological advancements in ablation devices, and the growing geriatric population. The rise in cancer cases, particularly liver and lung cancers, is also fueling market growth. However, the high cost of ablation procedures and the lack of skilled professionals in developing regions pose challenges to market expansion.
The competitive landscape of the tumor ablation market is characterized by the presence of several major players, including Medtronic, Boston Scientific Corporation, AngioDynamics, and Johnson & Johnson. These companies are investing heavily in research and development to introduce innovative ablation technologies and expand their product portfolios. The emergence of nanorobotics-based ablation techniques presents both opportunities and challenges for established players, potentially reshaping the competitive dynamics of the market in the coming years.
As the field of nanorobotics for tumor ablation advances, it is expected to create new growth opportunities within the broader tumor ablation market. The ability to deliver targeted therapy at the nanoscale level could revolutionize cancer treatment, potentially offering improved efficacy and reduced side effects compared to traditional ablation methods. This emerging technology is likely to attract significant investment and research focus, potentially leading to the development of novel ablation devices and techniques in the near future.
Nanorobotics Challenges
Nanorobotics for tumor ablation faces several significant challenges that must be addressed to realize its full potential in cancer treatment. One of the primary obstacles is the precise control and navigation of nanorobots within the complex and dynamic environment of the human body. The ability to accurately guide these microscopic devices to specific tumor sites while avoiding healthy tissues remains a formidable task.
Another critical challenge lies in the development of effective propulsion mechanisms for nanorobots. Current methods, such as chemical propulsion or magnetic field-driven locomotion, have limitations in terms of speed, efficiency, and biocompatibility. Researchers are exploring novel approaches, including biomimetic propulsion systems inspired by natural microorganisms, to overcome these hurdles.
The issue of biocompatibility and potential toxicity of nanorobots is also a significant concern. Materials used in nanorobot construction must be carefully selected to ensure they do not trigger adverse immune responses or cause unintended damage to healthy cells. Additionally, the long-term effects of nanorobots on the human body are not yet fully understood, necessitating extensive research and clinical trials.
Power supply and energy management present another set of challenges for nanorobotics in tumor ablation. Developing miniaturized power sources that can sustain nanorobot operations for extended periods without compromising safety or efficacy is crucial. Researchers are investigating various options, including wireless power transfer and energy harvesting from the biological environment.
The accurate dosimetry and delivery of therapeutic agents or energy for tumor ablation pose significant technical hurdles. Ensuring precise and controlled release of drugs or application of heat at the tumor site while minimizing collateral damage to surrounding tissues is essential. This requires advanced sensing and feedback mechanisms to be integrated into nanorobotic systems.
Manufacturing and scalability challenges also exist in the field of nanorobotics. Current fabrication techniques for nanorobots are often complex, time-consuming, and expensive, limiting their potential for large-scale production and clinical application. Developing more efficient and cost-effective manufacturing processes is crucial for the widespread adoption of nanorobotic tumor ablation technologies.
Lastly, regulatory and ethical considerations present significant hurdles in the development and implementation of nanorobotics for tumor ablation. Establishing clear guidelines for safety, efficacy, and ethical use of nanorobots in medical applications is essential. This requires close collaboration between researchers, clinicians, ethicists, and regulatory bodies to address potential risks and ensure responsible development of this promising technology.
Another critical challenge lies in the development of effective propulsion mechanisms for nanorobots. Current methods, such as chemical propulsion or magnetic field-driven locomotion, have limitations in terms of speed, efficiency, and biocompatibility. Researchers are exploring novel approaches, including biomimetic propulsion systems inspired by natural microorganisms, to overcome these hurdles.
The issue of biocompatibility and potential toxicity of nanorobots is also a significant concern. Materials used in nanorobot construction must be carefully selected to ensure they do not trigger adverse immune responses or cause unintended damage to healthy cells. Additionally, the long-term effects of nanorobots on the human body are not yet fully understood, necessitating extensive research and clinical trials.
Power supply and energy management present another set of challenges for nanorobotics in tumor ablation. Developing miniaturized power sources that can sustain nanorobot operations for extended periods without compromising safety or efficacy is crucial. Researchers are investigating various options, including wireless power transfer and energy harvesting from the biological environment.
The accurate dosimetry and delivery of therapeutic agents or energy for tumor ablation pose significant technical hurdles. Ensuring precise and controlled release of drugs or application of heat at the tumor site while minimizing collateral damage to surrounding tissues is essential. This requires advanced sensing and feedback mechanisms to be integrated into nanorobotic systems.
Manufacturing and scalability challenges also exist in the field of nanorobotics. Current fabrication techniques for nanorobots are often complex, time-consuming, and expensive, limiting their potential for large-scale production and clinical application. Developing more efficient and cost-effective manufacturing processes is crucial for the widespread adoption of nanorobotic tumor ablation technologies.
Lastly, regulatory and ethical considerations present significant hurdles in the development and implementation of nanorobotics for tumor ablation. Establishing clear guidelines for safety, efficacy, and ethical use of nanorobots in medical applications is essential. This requires close collaboration between researchers, clinicians, ethicists, and regulatory bodies to address potential risks and ensure responsible development of this promising technology.
Current Ablation Methods
01 Nanorobotics for targeted drug delivery
Nanorobots are designed for precise drug delivery, allowing for controlled dosage and targeted release of therapeutic agents. These microscopic devices can navigate through the body, delivering medications to specific sites, potentially improving treatment efficacy and reducing side effects. The technology incorporates advanced sensing and navigation systems to ensure accurate delivery metrics.- Nanorobotic drug delivery systems: Advanced nanorobotic systems are being developed for precise drug delivery. These systems can navigate through the body, target specific cells or tissues, and release therapeutic agents in controlled doses. This technology promises to improve treatment efficacy while minimizing side effects by delivering drugs exactly where they are needed.
- Dosimetry techniques for nanorobotics: Novel dosimetry methods are being developed to accurately measure and control the amount of therapeutic agents delivered by nanorobots. These techniques involve advanced sensors and imaging technologies to monitor drug concentrations in real-time, ensuring optimal dosage and reducing the risk of over- or under-medication.
- Nanorobot navigation and targeting: Innovative navigation and targeting systems are being integrated into nanorobots to enhance their ability to reach specific locations within the body. These systems utilize various guidance mechanisms, including magnetic fields, chemical gradients, and biological markers, to ensure precise delivery of therapeutic agents to target sites.
- Performance metrics for nanorobotic delivery: Researchers are developing comprehensive performance metrics to evaluate the effectiveness of nanorobotic delivery systems. These metrics include factors such as targeting accuracy, drug release kinetics, biocompatibility, and clearance rates. By standardizing these metrics, the field aims to improve the comparison and optimization of different nanorobotic platforms.
- Integration of AI and machine learning in nanorobotics: Artificial intelligence and machine learning algorithms are being incorporated into nanorobotic systems to enhance their decision-making capabilities and adaptability. These advanced computational techniques enable nanorobots to process complex biological data in real-time, adjust their behavior based on the local environment, and optimize drug delivery strategies for individual patients.
02 Dosimetry systems for nanorobotics
Specialized dosimetry systems are developed to measure and monitor the radiation exposure or drug concentrations delivered by nanorobots. These systems ensure precise control over the dosage administered, crucial for maintaining safety and efficacy in nanorobotics-based treatments. Advanced sensors and imaging techniques are integrated to provide real-time dosimetry data.Expand Specific Solutions03 Nanorobot navigation and tracking
Advanced navigation and tracking systems are implemented in nanorobotics to ensure accurate delivery to target sites. These systems utilize various technologies such as magnetic guidance, optical tracking, or radio frequency identification to monitor the position and movement of nanorobots within the body. This enables precise control over delivery metrics and enhances the overall effectiveness of nanorobotics-based interventions.Expand Specific Solutions04 Integration of AI and machine learning in nanorobotics
Artificial intelligence and machine learning algorithms are incorporated into nanorobotics systems to optimize dosimetry and delivery metrics. These technologies enable adaptive decision-making, allowing nanorobots to adjust their behavior based on real-time physiological data and environmental conditions. This integration enhances the precision and efficiency of nanorobot-mediated treatments.Expand Specific Solutions05 Nanorobotics interface with biological systems
Research focuses on developing nanorobots that can effectively interface with biological systems for improved dosimetry and delivery. This includes designing nanorobots with biocompatible materials and surface modifications that allow for seamless interaction with cells and tissues. The goal is to enhance the accuracy of dosage delivery and minimize potential adverse reactions in the body.Expand Specific Solutions
Key Nanorobotics Players
The nanorobotics field for tumor ablation is in its early developmental stages, with significant potential for growth. The market size is expanding as research progresses, but commercialization remains limited. Technologically, it's still emerging, with key players like Memorial Sloan Kettering Cancer Center, King's College London, and AngioDynamics leading research efforts. Universities such as Fudan and Northwestern are contributing to advancements, while companies like NH TherAguix and Spago Nanomedical are developing nanoparticle-based solutions. The involvement of diverse institutions indicates growing interest, but practical applications are still evolving.
King's College London
Technical Solution: King's College London has developed a nanorobotics platform for tumor ablation that utilizes magnetically guided nanoparticles. Their approach combines magnetic resonance imaging (MRI) for real-time guidance with nanoparticles that can be heated remotely to destroy tumor cells. The system employs precise dosimetry control, allowing for targeted delivery of thermal energy to cancerous tissues while minimizing damage to surrounding healthy cells[1][3]. The nanorobots are engineered to navigate through complex vascular networks, overcoming biological barriers to reach tumor sites effectively. Additionally, they have integrated advanced imaging techniques to monitor nanoparticle distribution and assess treatment efficacy in real-time[5].
Strengths: Precise targeting, real-time monitoring, and minimally invasive approach. Weaknesses: Potential for off-target effects and challenges in scaling up production for clinical use.
Memorial Sloan Kettering Cancer Center
Technical Solution: Memorial Sloan Kettering Cancer Center has pioneered a nanorobotics-based approach for tumor ablation that utilizes DNA origami nanorobots. These nanorobots are designed to selectively target cancer cells and deliver therapeutic payloads. The center has developed sophisticated dosimetry models that account for tumor heterogeneity and microenvironment factors, ensuring optimal drug delivery[2]. Their system incorporates smart sensing capabilities, allowing nanorobots to detect specific molecular markers and adjust their behavior accordingly. The delivery metrics are enhanced through the use of a multi-stage targeting system, which improves penetration into solid tumors and reduces systemic toxicity[4][6].
Strengths: Highly specific targeting, adaptable payload delivery, and potential for personalized treatment. Weaknesses: Complexity in manufacturing and potential immunogenicity of DNA-based structures.
Core Nanorobot Designs
Nanobots with embedded biosensors
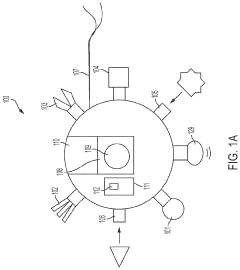
PatentActiveUS20220225942A1
Innovation
- Development of medical nanobots equipped with embedded biosensors that transmit data for real-time anatomic localization, diagnosis, and therapeutic intervention, utilizing a transmitter/receiver system and anatomic localizers to guide the nanobots to specific locations within the body for accurate data collection and treatment.
Application of nanorobotics in high-density pharmaceutical assay process
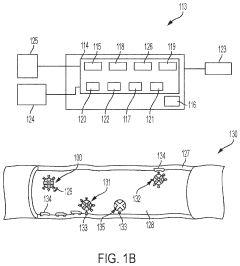
PatentPendingIN202231069269A
Innovation
- Development of a visual and haptic interface using scanning electron microscopy (SEM) and atomic force microscopy (AFM), combined with virtual reality techniques, to enhance operator interaction with nanorobots, and the use of carbon-based nanocomposites like diamond or diamondoid/fullerene for medical nanorobots, which are designed and manufactured in desktop nanofactories to ensure safety and effectiveness.
Nanorobotics Regulations
The development and deployment of nanorobotics for tumor ablation necessitate a comprehensive regulatory framework to ensure safety, efficacy, and ethical use. Currently, regulatory bodies worldwide are grappling with the unique challenges posed by this emerging technology. The U.S. Food and Drug Administration (FDA) has taken initial steps by establishing a Nanotechnology Task Force to address the regulatory challenges of nanomaterials and nanodevices in medical applications.
In the European Union, the European Medicines Agency (EMA) has developed guidelines for the evaluation of nanomedicines, which include considerations for nanorobotic systems. These guidelines emphasize the importance of characterizing the physicochemical properties of nanorobots and assessing their potential interactions with biological systems.
Regulatory frameworks for nanorobotics must address several key areas. First, the manufacturing process of nanorobots requires stringent quality control measures to ensure consistency and safety. Regulatory bodies are developing Good Manufacturing Practice (GMP) guidelines specific to nanorobotics production.
Second, preclinical testing protocols for nanorobots are being established to evaluate their biodistribution, pharmacokinetics, and potential toxicity. These protocols must account for the unique properties of nanorobots, such as their ability to navigate through biological barriers and interact with cellular structures.
Third, clinical trial designs for nanorobotic tumor ablation therapies are being adapted to address the specific challenges of this technology. This includes developing appropriate endpoints to assess efficacy and safety, as well as long-term follow-up protocols to monitor potential delayed effects.
Regulatory bodies are also focusing on the ethical implications of nanorobotics in cancer treatment. Guidelines are being developed to ensure informed consent processes adequately communicate the risks and benefits of nanorobotic interventions to patients.
International collaboration is crucial in developing harmonized regulatory approaches for nanorobotics. Organizations such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) are working to create global standards for the evaluation and approval of nanorobotic medical devices.
As the field of nanorobotics for tumor ablation advances, regulatory frameworks will need to evolve rapidly to keep pace with technological developments. This will require ongoing dialogue between researchers, industry stakeholders, and regulatory agencies to ensure that regulations support innovation while safeguarding patient safety and public health.
In the European Union, the European Medicines Agency (EMA) has developed guidelines for the evaluation of nanomedicines, which include considerations for nanorobotic systems. These guidelines emphasize the importance of characterizing the physicochemical properties of nanorobots and assessing their potential interactions with biological systems.
Regulatory frameworks for nanorobotics must address several key areas. First, the manufacturing process of nanorobots requires stringent quality control measures to ensure consistency and safety. Regulatory bodies are developing Good Manufacturing Practice (GMP) guidelines specific to nanorobotics production.
Second, preclinical testing protocols for nanorobots are being established to evaluate their biodistribution, pharmacokinetics, and potential toxicity. These protocols must account for the unique properties of nanorobots, such as their ability to navigate through biological barriers and interact with cellular structures.
Third, clinical trial designs for nanorobotic tumor ablation therapies are being adapted to address the specific challenges of this technology. This includes developing appropriate endpoints to assess efficacy and safety, as well as long-term follow-up protocols to monitor potential delayed effects.
Regulatory bodies are also focusing on the ethical implications of nanorobotics in cancer treatment. Guidelines are being developed to ensure informed consent processes adequately communicate the risks and benefits of nanorobotic interventions to patients.
International collaboration is crucial in developing harmonized regulatory approaches for nanorobotics. Organizations such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) are working to create global standards for the evaluation and approval of nanorobotic medical devices.
As the field of nanorobotics for tumor ablation advances, regulatory frameworks will need to evolve rapidly to keep pace with technological developments. This will require ongoing dialogue between researchers, industry stakeholders, and regulatory agencies to ensure that regulations support innovation while safeguarding patient safety and public health.
Nanorobot Biocompatibility
Nanorobot biocompatibility is a critical aspect in the development and application of nanorobotics for tumor ablation. The interaction between nanorobots and biological systems must be carefully evaluated to ensure safety and efficacy. One of the primary concerns is the potential immune response triggered by the introduction of nanorobots into the body. Researchers have been exploring various surface modifications and coatings to minimize immune recognition and enhance biocompatibility.
The size and shape of nanorobots play a crucial role in their biocompatibility. Optimal dimensions are essential to avoid rapid clearance by the reticuloendothelial system while maintaining the ability to navigate through blood vessels and reach target tumor sites. Studies have shown that nanorobots with sizes ranging from 10 to 100 nanometers exhibit favorable biodistribution and tumor penetration properties.
Material selection is another key factor in nanorobot biocompatibility. Biodegradable materials such as poly(lactic-co-glycolic acid) (PLGA) and chitosan have gained attention due to their ability to break down into non-toxic components after completing their therapeutic function. Additionally, inorganic materials like gold and iron oxide nanoparticles have demonstrated promising results in terms of biocompatibility and functionality for tumor ablation applications.
The surface charge of nanorobots significantly influences their interaction with biological systems. Neutral or slightly negatively charged nanorobots tend to exhibit longer circulation times and reduced non-specific cellular uptake compared to positively charged counterparts. Researchers have been investigating various surface functionalization strategies to optimize the charge distribution and enhance biocompatibility.
Long-term effects of nanorobots on biological systems remain a subject of ongoing research. Chronic exposure studies are essential to evaluate potential accumulation in organs and tissues, as well as any delayed immune responses or toxicity. Advanced imaging techniques, such as intravital microscopy and whole-body imaging, are being employed to track nanorobot distribution and assess their long-term impact on organ function.
Biocompatibility assessment protocols for nanorobots are continually evolving. In vitro studies using cell cultures and tissue models provide initial insights into cytotoxicity and cellular interactions. However, in vivo studies in animal models are crucial for evaluating systemic effects, biodistribution, and potential off-target interactions. Standardized testing methods and regulatory guidelines specific to nanorobotics are being developed to ensure consistent and reliable biocompatibility evaluations across different research groups and applications.
The size and shape of nanorobots play a crucial role in their biocompatibility. Optimal dimensions are essential to avoid rapid clearance by the reticuloendothelial system while maintaining the ability to navigate through blood vessels and reach target tumor sites. Studies have shown that nanorobots with sizes ranging from 10 to 100 nanometers exhibit favorable biodistribution and tumor penetration properties.
Material selection is another key factor in nanorobot biocompatibility. Biodegradable materials such as poly(lactic-co-glycolic acid) (PLGA) and chitosan have gained attention due to their ability to break down into non-toxic components after completing their therapeutic function. Additionally, inorganic materials like gold and iron oxide nanoparticles have demonstrated promising results in terms of biocompatibility and functionality for tumor ablation applications.
The surface charge of nanorobots significantly influences their interaction with biological systems. Neutral or slightly negatively charged nanorobots tend to exhibit longer circulation times and reduced non-specific cellular uptake compared to positively charged counterparts. Researchers have been investigating various surface functionalization strategies to optimize the charge distribution and enhance biocompatibility.
Long-term effects of nanorobots on biological systems remain a subject of ongoing research. Chronic exposure studies are essential to evaluate potential accumulation in organs and tissues, as well as any delayed immune responses or toxicity. Advanced imaging techniques, such as intravital microscopy and whole-body imaging, are being employed to track nanorobot distribution and assess their long-term impact on organ function.
Biocompatibility assessment protocols for nanorobots are continually evolving. In vitro studies using cell cultures and tissue models provide initial insights into cytotoxicity and cellular interactions. However, in vivo studies in animal models are crucial for evaluating systemic effects, biodistribution, and potential off-target interactions. Standardized testing methods and regulatory guidelines specific to nanorobotics are being developed to ensure consistent and reliable biocompatibility evaluations across different research groups and applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!