How To Power Medical Nanorobots: Energy Harvesting Approaches
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nanorobot Energy Background and Objectives
Medical nanorobots represent a revolutionary frontier in healthcare, promising targeted drug delivery, precise diagnostics, and minimally invasive surgical interventions. However, the challenge of powering these microscopic devices within the human body remains a significant hurdle in their development and deployment. The quest for efficient and biocompatible energy sources for nanorobots has become a critical focus in the field of nanomedicine.
The evolution of nanorobot technology can be traced back to the conceptual foundations laid by Richard Feynman in his visionary 1959 lecture, "There's Plenty of Room at the Bottom." Since then, advancements in nanotechnology, materials science, and bioengineering have brought us closer to realizing the potential of medical nanorobots. The progression from theoretical concepts to practical applications has been marked by key milestones in miniaturization, biocompatibility, and control mechanisms.
Current technological trends in nanorobot energy harvesting are focused on leveraging the unique properties of the human body's internal environment. Researchers are exploring various approaches, including harnessing chemical energy from bodily fluids, converting mechanical energy from blood flow or cellular movements, and utilizing electromagnetic fields for wireless power transmission. These innovative methods aim to overcome the limitations of traditional power sources, such as batteries, which are often too large or potentially toxic for in vivo applications.
The primary objective in powering medical nanorobots is to develop sustainable, long-lasting, and safe energy harvesting techniques that can operate autonomously within the human body. This goal encompasses several key aspects: maximizing energy efficiency to ensure prolonged operation, minimizing the size of power components to maintain the nanorobot's microscopic scale, and ensuring biocompatibility to prevent adverse reactions in the host organism.
Another crucial objective is to create versatile energy solutions that can adapt to different physiological environments and medical applications. This adaptability is essential given the diverse potential uses of nanorobots, from navigating blood vessels to operating within specific organs or tissues. Additionally, researchers aim to develop energy harvesting methods that can be seamlessly integrated into the nanorobot's design without compromising its primary functions or mobility.
As the field progresses, the ultimate aim is to achieve a symbiotic relationship between nanorobots and the human body, where energy harvesting becomes an integral part of the nanorobot's interaction with its biological environment. This vision includes the possibility of nanorobots that can not only power themselves but also potentially contribute to the body's energy balance or assist in local tissue regeneration.
The evolution of nanorobot technology can be traced back to the conceptual foundations laid by Richard Feynman in his visionary 1959 lecture, "There's Plenty of Room at the Bottom." Since then, advancements in nanotechnology, materials science, and bioengineering have brought us closer to realizing the potential of medical nanorobots. The progression from theoretical concepts to practical applications has been marked by key milestones in miniaturization, biocompatibility, and control mechanisms.
Current technological trends in nanorobot energy harvesting are focused on leveraging the unique properties of the human body's internal environment. Researchers are exploring various approaches, including harnessing chemical energy from bodily fluids, converting mechanical energy from blood flow or cellular movements, and utilizing electromagnetic fields for wireless power transmission. These innovative methods aim to overcome the limitations of traditional power sources, such as batteries, which are often too large or potentially toxic for in vivo applications.
The primary objective in powering medical nanorobots is to develop sustainable, long-lasting, and safe energy harvesting techniques that can operate autonomously within the human body. This goal encompasses several key aspects: maximizing energy efficiency to ensure prolonged operation, minimizing the size of power components to maintain the nanorobot's microscopic scale, and ensuring biocompatibility to prevent adverse reactions in the host organism.
Another crucial objective is to create versatile energy solutions that can adapt to different physiological environments and medical applications. This adaptability is essential given the diverse potential uses of nanorobots, from navigating blood vessels to operating within specific organs or tissues. Additionally, researchers aim to develop energy harvesting methods that can be seamlessly integrated into the nanorobot's design without compromising its primary functions or mobility.
As the field progresses, the ultimate aim is to achieve a symbiotic relationship between nanorobots and the human body, where energy harvesting becomes an integral part of the nanorobot's interaction with its biological environment. This vision includes the possibility of nanorobots that can not only power themselves but also potentially contribute to the body's energy balance or assist in local tissue regeneration.
Market Demand for Powered Medical Nanorobots
The market demand for powered medical nanorobots is experiencing significant growth, driven by the increasing prevalence of chronic diseases and the need for targeted drug delivery systems. As healthcare systems worldwide face challenges in treating complex conditions, the potential of nanorobots to revolutionize medical treatments has garnered substantial interest from both the medical community and investors.
The global market for medical nanorobots is projected to expand rapidly in the coming years, with a particular focus on applications in cancer treatment, drug delivery, and minimally invasive surgeries. The ability of nanorobots to navigate through the human body and perform precise interventions at the cellular level has opened up new possibilities for personalized medicine and improved patient outcomes.
One of the key drivers of market demand is the potential for nanorobots to enhance the efficacy of cancer treatments. By delivering therapeutic agents directly to tumor sites, nanorobots can minimize damage to healthy tissues and reduce the side effects associated with traditional chemotherapy. This targeted approach has the potential to significantly improve patient quality of life and treatment success rates.
In addition to cancer treatment, the pharmaceutical industry has shown keen interest in nanorobots for drug delivery applications. The ability to precisely control the release of medications within the body could lead to more effective treatments for a wide range of conditions, from cardiovascular diseases to neurological disorders. This potential has spurred investment in research and development efforts aimed at bringing nanorobot-based drug delivery systems to market.
The aging population in many developed countries has also contributed to the growing demand for medical nanorobots. As the incidence of age-related diseases increases, there is a pressing need for innovative treatment approaches that can address complex health issues with minimal invasiveness. Nanorobots offer the promise of targeted interventions that could potentially slow or reverse the progression of degenerative conditions.
However, the market demand for powered medical nanorobots is not without challenges. Concerns regarding the safety and long-term effects of nanorobots in the human body need to be addressed through rigorous clinical trials and regulatory approvals. Additionally, the high cost of development and production may initially limit the widespread adoption of nanorobot-based treatments.
Despite these challenges, the potential benefits of powered medical nanorobots continue to drive market demand. As research progresses and technological barriers are overcome, the integration of nanorobots into mainstream medical practice is expected to accelerate, opening up new avenues for treatment and diagnosis across various medical specialties.
The global market for medical nanorobots is projected to expand rapidly in the coming years, with a particular focus on applications in cancer treatment, drug delivery, and minimally invasive surgeries. The ability of nanorobots to navigate through the human body and perform precise interventions at the cellular level has opened up new possibilities for personalized medicine and improved patient outcomes.
One of the key drivers of market demand is the potential for nanorobots to enhance the efficacy of cancer treatments. By delivering therapeutic agents directly to tumor sites, nanorobots can minimize damage to healthy tissues and reduce the side effects associated with traditional chemotherapy. This targeted approach has the potential to significantly improve patient quality of life and treatment success rates.
In addition to cancer treatment, the pharmaceutical industry has shown keen interest in nanorobots for drug delivery applications. The ability to precisely control the release of medications within the body could lead to more effective treatments for a wide range of conditions, from cardiovascular diseases to neurological disorders. This potential has spurred investment in research and development efforts aimed at bringing nanorobot-based drug delivery systems to market.
The aging population in many developed countries has also contributed to the growing demand for medical nanorobots. As the incidence of age-related diseases increases, there is a pressing need for innovative treatment approaches that can address complex health issues with minimal invasiveness. Nanorobots offer the promise of targeted interventions that could potentially slow or reverse the progression of degenerative conditions.
However, the market demand for powered medical nanorobots is not without challenges. Concerns regarding the safety and long-term effects of nanorobots in the human body need to be addressed through rigorous clinical trials and regulatory approvals. Additionally, the high cost of development and production may initially limit the widespread adoption of nanorobot-based treatments.
Despite these challenges, the potential benefits of powered medical nanorobots continue to drive market demand. As research progresses and technological barriers are overcome, the integration of nanorobots into mainstream medical practice is expected to accelerate, opening up new avenues for treatment and diagnosis across various medical specialties.
Energy Harvesting Challenges for Nanorobots
Energy harvesting for medical nanorobots presents significant challenges due to their microscopic size and the complex biological environment in which they operate. One of the primary obstacles is the limited space available for energy harvesting components, which restricts the use of conventional power sources. The nanoscale dimensions of these robots necessitate innovative approaches to energy capture and storage that can function effectively at the molecular level.
The biological environment itself poses unique challenges for energy harvesting. The human body's internal systems are dynamic and can vary greatly in terms of temperature, pH levels, and chemical composition. This variability makes it difficult to design consistent and reliable energy harvesting mechanisms. Additionally, the presence of bodily fluids and tissues can interfere with traditional energy harvesting methods, requiring solutions that are both biocompatible and capable of operating in these complex conditions.
Another significant challenge is the need for continuous and sufficient power supply. Medical nanorobots often require sustained operation for extended periods to perform their intended functions, such as drug delivery or diagnostic imaging. Ensuring a stable and adequate energy supply without frequent recharging or replacement is crucial for the practical application of these devices in medical treatments.
The efficiency of energy conversion at the nanoscale is also a major concern. Many conventional energy harvesting techniques suffer from reduced efficiency when scaled down to nanometer dimensions. This necessitates the development of novel materials and structures that can maintain high energy conversion rates at extremely small scales.
Biocompatibility and safety considerations add another layer of complexity to the energy harvesting challenge. Any energy harvesting mechanism must not only be effective but also safe for use within the human body. This includes avoiding the generation of harmful byproducts, preventing unwanted interactions with biological systems, and ensuring that the energy harvesting process itself does not cause damage to surrounding tissues.
Lastly, the integration of energy harvesting systems with the functional components of nanorobots presents significant engineering challenges. Designers must balance the space and resources allocated for energy harvesting with those required for the robot's primary functions, such as sensing, actuation, or drug delivery. This integration must be achieved while maintaining the overall size and functionality of the nanorobot, requiring advanced fabrication techniques and multifunctional materials.
The biological environment itself poses unique challenges for energy harvesting. The human body's internal systems are dynamic and can vary greatly in terms of temperature, pH levels, and chemical composition. This variability makes it difficult to design consistent and reliable energy harvesting mechanisms. Additionally, the presence of bodily fluids and tissues can interfere with traditional energy harvesting methods, requiring solutions that are both biocompatible and capable of operating in these complex conditions.
Another significant challenge is the need for continuous and sufficient power supply. Medical nanorobots often require sustained operation for extended periods to perform their intended functions, such as drug delivery or diagnostic imaging. Ensuring a stable and adequate energy supply without frequent recharging or replacement is crucial for the practical application of these devices in medical treatments.
The efficiency of energy conversion at the nanoscale is also a major concern. Many conventional energy harvesting techniques suffer from reduced efficiency when scaled down to nanometer dimensions. This necessitates the development of novel materials and structures that can maintain high energy conversion rates at extremely small scales.
Biocompatibility and safety considerations add another layer of complexity to the energy harvesting challenge. Any energy harvesting mechanism must not only be effective but also safe for use within the human body. This includes avoiding the generation of harmful byproducts, preventing unwanted interactions with biological systems, and ensuring that the energy harvesting process itself does not cause damage to surrounding tissues.
Lastly, the integration of energy harvesting systems with the functional components of nanorobots presents significant engineering challenges. Designers must balance the space and resources allocated for energy harvesting with those required for the robot's primary functions, such as sensing, actuation, or drug delivery. This integration must be achieved while maintaining the overall size and functionality of the nanorobot, requiring advanced fabrication techniques and multifunctional materials.
Current Energy Harvesting Solutions for Nanorobots
01 Energy harvesting for medical nanorobots
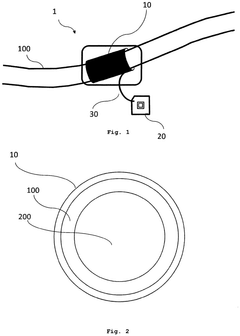
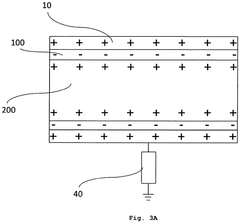
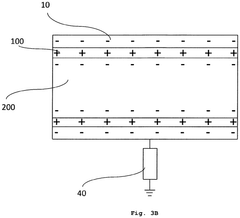
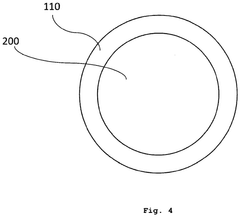
Medical nanorobots can harvest energy from their surrounding environment to power their operations. This may include utilizing electromagnetic fields, chemical reactions, or mechanical vibrations within the body to generate electricity. Such energy harvesting techniques enable nanorobots to operate autonomously for extended periods without external power sources.- Energy harvesting for medical nanorobots: Medical nanorobots can utilize various energy harvesting techniques to power their operations. These may include converting mechanical energy from blood flow, harnessing chemical energy from bodily fluids, or using external electromagnetic fields. Such energy harvesting methods enable nanorobots to operate autonomously within the body for extended periods.
- Wireless power transmission for nanorobots: Wireless power transmission technologies can be employed to provide energy to medical nanorobots. This may involve using electromagnetic waves, ultrasound, or near-field coupling to transfer power from an external source to the nanorobots inside the body. This approach allows for continuous operation without the need for internal power storage.
- Nanoscale energy storage systems: Innovative energy storage systems at the nanoscale can be integrated into medical nanorobots. These may include advanced battery technologies, supercapacitors, or novel materials capable of storing and releasing energy efficiently. Such systems enable nanorobots to carry their own power supply for autonomous operation.
- Energy-efficient nanorobot propulsion: Developing energy-efficient propulsion mechanisms for medical nanorobots is crucial for maximizing their operational lifespan. This may involve biomimetic designs, chemical propulsion systems, or magnetic field-driven locomotion. These propulsion methods aim to minimize energy consumption while enabling precise navigation within the body.
- Energy management and optimization algorithms: Sophisticated energy management and optimization algorithms can be implemented in medical nanorobots to enhance their energy efficiency. These algorithms may dynamically adjust power consumption based on task priorities, environmental conditions, and available energy sources. Such intelligent energy management extends the operational capabilities of nanorobots in medical applications.
02 Wireless power transmission to nanorobots
Wireless power transmission systems can be used to provide energy to medical nanorobots inside the body. This may involve using electromagnetic waves, ultrasound, or other forms of radiation to transfer energy from an external source to the nanorobots. This approach allows for continuous operation of nanorobots without the need for internal power storage.Expand Specific Solutions03 Nanorobots with integrated energy storage
Medical nanorobots can be designed with integrated energy storage systems, such as nanoscale batteries or supercapacitors. These storage systems allow nanorobots to carry their own energy supply, enabling them to operate independently for a certain period before requiring recharging or replacement.Expand Specific Solutions04 Biofuel cells for powering nanorobots
Biofuel cells can be incorporated into medical nanorobots to generate electricity from biological substances present in the body. These cells can utilize glucose, oxygen, or other biomolecules as fuel sources, converting chemical energy into electrical energy to power the nanorobots' functions.Expand Specific Solutions05 Energy-efficient nanorobot design
Developing energy-efficient designs for medical nanorobots is crucial for maximizing their operational lifespan and effectiveness. This includes optimizing the nanorobots' structure, materials, and operational algorithms to minimize energy consumption while maintaining functionality. Energy-efficient designs may also incorporate smart power management systems to allocate energy resources effectively.Expand Specific Solutions
Key Players in Medical Nanorobotics
The energy harvesting approaches for powering medical nanorobots are in an early developmental stage, with a rapidly growing market potential. The technology's maturity is still evolving, as evidenced by ongoing research at institutions like The Johns Hopkins University and InCube Labs. Companies such as Medtronic and Smith & Nephew are likely exploring integration possibilities, while startups like Cairdac SA are pioneering self-sustaining energy solutions for medical devices. The competitive landscape is diverse, with both established medical technology firms and innovative research institutions contributing to advancements. As the field progresses, we can expect increased collaboration between academia and industry to overcome technical challenges and bring viable nanorobot energy harvesting solutions to market.
The Johns Hopkins University
Technical Solution: The Johns Hopkins University has developed a pioneering approach to powering medical nanorobots through energy harvesting. Their method focuses on utilizing the body's own biochemical processes to generate power. The nanorobots are designed with specialized enzymes that can catalyze reactions with glucose and other molecules present in the bloodstream, effectively converting chemical energy into electrical energy[1]. This biofuel cell approach allows for continuous power generation as long as the nanorobots remain in the body. Additionally, the university has explored piezoelectric nanomaterials that can harvest energy from the mechanical forces within blood vessels, such as blood flow and pressure variations[3].
Strengths: Sustainable power source using the body's own resources; Potential for long-term operation without external charging. Weaknesses: Power output may vary depending on the availability of glucose or other substrates; Potential immune response to the enzymatic components.
InCube Labs LLC
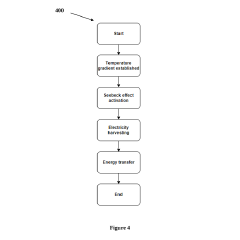
Technical Solution: InCube Labs has developed an innovative approach to powering medical nanorobots through a combination of wireless power transfer and energy harvesting techniques. Their system utilizes miniaturized electromagnetic coils embedded within the nanorobots, capable of capturing energy from externally applied magnetic fields[2]. This allows for periodic recharging of the nanorobots without the need for removal from the body. Additionally, InCube Labs has integrated thermoelectric generators into their nanorobot design, harnessing the temperature difference between the body's core and its surface to generate a small but constant power supply[4]. This dual-mode power system ensures a more reliable and consistent energy source for extended nanorobot operation.
Strengths: Ability to recharge nanorobots externally; Continuous power generation from body heat. Weaknesses: Requires periodic external magnetic field application; Limited power output from thermoelectric generation.
Core Innovations in Nanorobot Power Systems
Energy harvesting system
PatentPendingIN202411027194A
Innovation
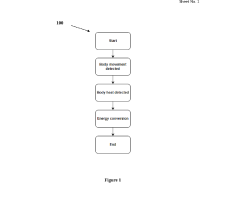
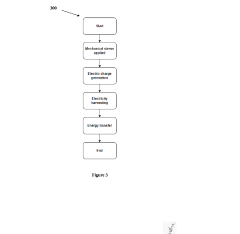
- A biocompatible energy harvesting system that converts kinetic energy from body movements and thermal energy into electrical energy using piezoelectric nano-generators and thermoelectric generators, integrated with an energy management circuit and encapsulated in a biocompatible matrix for stable power delivery.
A power generation device for harvesting energy through a fluid-solid triboelectric nanogenerator
PatentPendingEP4582139A1
Innovation
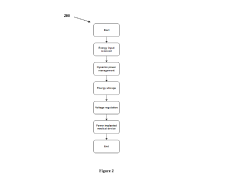
- A power generation device utilizing a fluid-solid triboelectric nanogenerator that harvests energy from body fluids, such as blood, urine, or sweat, to power medical devices without the need for batteries, using conductive components and polymers or metals to induce charges from fluid flow.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount when developing energy harvesting approaches for medical nanorobots. The materials used in these devices must be carefully selected to ensure they do not trigger adverse immune responses or cause toxicity in the human body. Biocompatible materials such as gold, titanium, and certain polymers are often preferred for their low reactivity and minimal impact on biological systems.
The size and shape of nanorobots also play a crucial role in their biocompatibility. Particles with dimensions below 100 nm can potentially cross biological barriers and interact with cellular components in unpredictable ways. Therefore, the energy harvesting mechanisms must be designed to maintain the overall size of the nanorobot within safe limits while still providing sufficient power.
Surface modifications of nanorobots are essential to enhance their biocompatibility and reduce the risk of aggregation or unwanted interactions with proteins and other biomolecules. Techniques such as PEGylation (coating with polyethylene glycol) can help create a "stealth" effect, minimizing immune system recognition and prolonging circulation time in the body.
The energy harvesting process itself must not generate harmful byproducts or cause local tissue damage. For instance, methods that rely on chemical reactions should be carefully controlled to prevent pH changes or the release of toxic substances. Similarly, approaches using mechanical energy must be designed to avoid causing physical trauma to surrounding tissues.
Long-term effects of nanorobots and their energy harvesting systems on the body must be thoroughly investigated. This includes studying potential accumulation in organs, chronic immune responses, and any interference with normal physiological processes. Biodegradability of the nanorobots after their intended function is completed is another important consideration to prevent long-term retention in the body.
Regulatory compliance is a critical aspect of safety considerations. Energy harvesting systems for medical nanorobots must adhere to strict guidelines set by regulatory bodies such as the FDA and EMA. This involves extensive pre-clinical and clinical testing to demonstrate both efficacy and safety before any potential human use.
Lastly, the potential for malfunction or unintended activation of nanorobots due to their energy harvesting mechanisms must be addressed. Fail-safe systems and redundancies should be incorporated to prevent any harmful autonomous actions in case of power surges or other unexpected energy fluctuations within the body.
The size and shape of nanorobots also play a crucial role in their biocompatibility. Particles with dimensions below 100 nm can potentially cross biological barriers and interact with cellular components in unpredictable ways. Therefore, the energy harvesting mechanisms must be designed to maintain the overall size of the nanorobot within safe limits while still providing sufficient power.
Surface modifications of nanorobots are essential to enhance their biocompatibility and reduce the risk of aggregation or unwanted interactions with proteins and other biomolecules. Techniques such as PEGylation (coating with polyethylene glycol) can help create a "stealth" effect, minimizing immune system recognition and prolonging circulation time in the body.
The energy harvesting process itself must not generate harmful byproducts or cause local tissue damage. For instance, methods that rely on chemical reactions should be carefully controlled to prevent pH changes or the release of toxic substances. Similarly, approaches using mechanical energy must be designed to avoid causing physical trauma to surrounding tissues.
Long-term effects of nanorobots and their energy harvesting systems on the body must be thoroughly investigated. This includes studying potential accumulation in organs, chronic immune responses, and any interference with normal physiological processes. Biodegradability of the nanorobots after their intended function is completed is another important consideration to prevent long-term retention in the body.
Regulatory compliance is a critical aspect of safety considerations. Energy harvesting systems for medical nanorobots must adhere to strict guidelines set by regulatory bodies such as the FDA and EMA. This involves extensive pre-clinical and clinical testing to demonstrate both efficacy and safety before any potential human use.
Lastly, the potential for malfunction or unintended activation of nanorobots due to their energy harvesting mechanisms must be addressed. Fail-safe systems and redundancies should be incorporated to prevent any harmful autonomous actions in case of power surges or other unexpected energy fluctuations within the body.
Regulatory Framework for Medical Nanorobots
The regulatory framework for medical nanorobots is a critical aspect of their development and deployment in healthcare. As these microscopic devices are designed to operate within the human body, stringent regulations are necessary to ensure patient safety and efficacy of treatment. Currently, there is no specific regulatory framework dedicated to medical nanorobots, but existing regulations for medical devices and nanotechnology are being adapted to address this emerging field.
In the United States, the Food and Drug Administration (FDA) is the primary regulatory body responsible for overseeing medical nanorobots. The FDA's approach to regulating these devices falls under its existing framework for medical devices, with additional considerations for nanotechnology-specific risks. The agency has established a Nanotechnology Task Force to address the unique challenges posed by nanoscale materials and devices in medical applications.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which came into effect in 2021. These regulations include provisions for nanomaterials and could be extended to cover medical nanorobots. The European Medicines Agency (EMA) also plays a role in regulating nanomedicine products, including potential nanorobotic applications.
Key regulatory considerations for medical nanorobots include safety assessment, quality control, and performance evaluation. Regulatory bodies are particularly concerned with the potential long-term effects of nanorobots in the human body, their biodegradability, and the risk of unintended interactions with biological systems. As such, extensive pre-clinical and clinical testing protocols are being developed to address these concerns.
International collaboration is crucial in establishing a harmonized regulatory approach for medical nanorobots. Organizations such as the International Organization for Standardization (ISO) and the Organisation for Economic Co-operation and Development (OECD) are working on developing standards and guidelines for nanotechnology in healthcare, which will likely influence the regulatory framework for nanorobots.
As the field of medical nanorobotics advances, regulatory agencies are expected to develop more specific guidelines and requirements. This may include new classification systems for nanorobots based on their intended use, risk level, and technological complexity. Additionally, regulations regarding the manufacturing processes, quality control measures, and post-market surveillance of nanorobotic devices are likely to be established.
The ethical implications of medical nanorobots are also being considered in regulatory discussions. Issues such as patient privacy, data security, and the potential for unintended uses of nanorobotic technology are driving the development of ethical guidelines that will inform future regulations.
In the United States, the Food and Drug Administration (FDA) is the primary regulatory body responsible for overseeing medical nanorobots. The FDA's approach to regulating these devices falls under its existing framework for medical devices, with additional considerations for nanotechnology-specific risks. The agency has established a Nanotechnology Task Force to address the unique challenges posed by nanoscale materials and devices in medical applications.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which came into effect in 2021. These regulations include provisions for nanomaterials and could be extended to cover medical nanorobots. The European Medicines Agency (EMA) also plays a role in regulating nanomedicine products, including potential nanorobotic applications.
Key regulatory considerations for medical nanorobots include safety assessment, quality control, and performance evaluation. Regulatory bodies are particularly concerned with the potential long-term effects of nanorobots in the human body, their biodegradability, and the risk of unintended interactions with biological systems. As such, extensive pre-clinical and clinical testing protocols are being developed to address these concerns.
International collaboration is crucial in establishing a harmonized regulatory approach for medical nanorobots. Organizations such as the International Organization for Standardization (ISO) and the Organisation for Economic Co-operation and Development (OECD) are working on developing standards and guidelines for nanotechnology in healthcare, which will likely influence the regulatory framework for nanorobots.
As the field of medical nanorobotics advances, regulatory agencies are expected to develop more specific guidelines and requirements. This may include new classification systems for nanorobots based on their intended use, risk level, and technological complexity. Additionally, regulations regarding the manufacturing processes, quality control measures, and post-market surveillance of nanorobotic devices are likely to be established.
The ethical implications of medical nanorobots are also being considered in regulatory discussions. Issues such as patient privacy, data security, and the potential for unintended uses of nanorobotic technology are driving the development of ethical guidelines that will inform future regulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!