Nanorobotics Regulatory Pathways: Preclinical Evidence Checklist
AUG 21, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nanorobotics Landscape
The field of nanorobotics has witnessed significant advancements in recent years, with potential applications spanning across healthcare, environmental remediation, and advanced manufacturing. Nanorobots, typically ranging from 1 to 100 nanometers in size, are engineered to perform specific tasks at the molecular or cellular level. The landscape of nanorobotics is characterized by a convergence of multiple disciplines, including nanotechnology, robotics, bioengineering, and materials science.
Key players in this domain include academic institutions, research laboratories, and innovative biotech companies. Notable contributors include MIT, Harvard University, ETH Zurich, and companies like Genentech and Nanorobotics Corporation. These entities are driving progress in areas such as targeted drug delivery, minimally invasive surgery, and environmental sensing.
The current state of nanorobotics technology encompasses various approaches to design and fabrication. DNA origami has emerged as a promising method for creating nanoscale structures with precise control over shape and function. Another significant development is the use of magnetic fields to guide and control nanorobots, particularly in medical applications for targeted therapy.
Challenges in the nanorobotics landscape include the need for more sophisticated control mechanisms, improved biocompatibility, and enhanced power sources for sustained operation. Additionally, the integration of artificial intelligence and machine learning algorithms is becoming increasingly important for autonomous navigation and decision-making capabilities of nanorobots.
Regulatory frameworks for nanorobotics are still evolving, with agencies like the FDA and EMA working to establish guidelines for safety assessment and clinical translation. The development of standardized protocols for preclinical evidence gathering is crucial for advancing nanorobotics towards real-world applications.
Looking ahead, the nanorobotics landscape is poised for further innovation in areas such as swarm behavior, self-assembly, and bio-hybrid systems. These advancements hold promise for revolutionizing personalized medicine, environmental monitoring, and nanoscale manufacturing processes.
Key players in this domain include academic institutions, research laboratories, and innovative biotech companies. Notable contributors include MIT, Harvard University, ETH Zurich, and companies like Genentech and Nanorobotics Corporation. These entities are driving progress in areas such as targeted drug delivery, minimally invasive surgery, and environmental sensing.
The current state of nanorobotics technology encompasses various approaches to design and fabrication. DNA origami has emerged as a promising method for creating nanoscale structures with precise control over shape and function. Another significant development is the use of magnetic fields to guide and control nanorobots, particularly in medical applications for targeted therapy.
Challenges in the nanorobotics landscape include the need for more sophisticated control mechanisms, improved biocompatibility, and enhanced power sources for sustained operation. Additionally, the integration of artificial intelligence and machine learning algorithms is becoming increasingly important for autonomous navigation and decision-making capabilities of nanorobots.
Regulatory frameworks for nanorobotics are still evolving, with agencies like the FDA and EMA working to establish guidelines for safety assessment and clinical translation. The development of standardized protocols for preclinical evidence gathering is crucial for advancing nanorobotics towards real-world applications.
Looking ahead, the nanorobotics landscape is poised for further innovation in areas such as swarm behavior, self-assembly, and bio-hybrid systems. These advancements hold promise for revolutionizing personalized medicine, environmental monitoring, and nanoscale manufacturing processes.
Market Potential
The market potential for nanorobotics in healthcare is substantial and rapidly expanding. As the field of nanotechnology continues to advance, the integration of nanorobots into medical applications presents a promising frontier for diagnosis, treatment, and prevention of diseases. The global nanorobotics market is expected to grow significantly in the coming years, driven by increasing investments in research and development, rising prevalence of chronic diseases, and growing demand for minimally invasive surgical procedures.
One of the primary areas of market potential for nanorobotics lies in targeted drug delivery systems. Nanorobots can be engineered to navigate through the bloodstream and deliver therapeutic agents directly to specific cells or tissues, potentially revolutionizing cancer treatment and management of other complex diseases. This targeted approach could significantly reduce side effects associated with traditional drug delivery methods and improve treatment efficacy.
Another promising application is in diagnostics and imaging. Nanorobots equipped with sensors and imaging capabilities could provide real-time, high-resolution information about cellular and molecular processes within the body. This technology has the potential to enable earlier detection of diseases, more accurate diagnoses, and personalized treatment strategies.
In the field of surgery, nanorobots could facilitate minimally invasive procedures with unprecedented precision. These microscopic devices could be used to perform delicate operations at the cellular level, potentially reducing recovery times and improving surgical outcomes. The market for such applications is expected to grow as healthcare providers seek more efficient and less invasive treatment options.
The aging population and increasing prevalence of chronic diseases worldwide are also driving factors for the nanorobotics market. As healthcare systems face mounting pressures to provide effective, long-term care for conditions such as cardiovascular diseases, neurodegenerative disorders, and diabetes, nanorobotics offers innovative solutions for monitoring, managing, and treating these conditions.
However, the market potential of nanorobotics is not without challenges. Regulatory hurdles, ethical considerations, and the need for extensive clinical trials may slow the adoption of these technologies in clinical practice. Additionally, the high costs associated with research, development, and manufacturing of nanorobots could initially limit their accessibility and widespread use.
Despite these challenges, the long-term market potential for nanorobotics in healthcare remains promising. As regulatory pathways become more defined and preclinical evidence accumulates, the integration of nanorobots into medical practice is likely to accelerate. This could lead to new market opportunities for pharmaceutical companies, medical device manufacturers, and healthcare providers, ultimately transforming patient care and treatment outcomes.
One of the primary areas of market potential for nanorobotics lies in targeted drug delivery systems. Nanorobots can be engineered to navigate through the bloodstream and deliver therapeutic agents directly to specific cells or tissues, potentially revolutionizing cancer treatment and management of other complex diseases. This targeted approach could significantly reduce side effects associated with traditional drug delivery methods and improve treatment efficacy.
Another promising application is in diagnostics and imaging. Nanorobots equipped with sensors and imaging capabilities could provide real-time, high-resolution information about cellular and molecular processes within the body. This technology has the potential to enable earlier detection of diseases, more accurate diagnoses, and personalized treatment strategies.
In the field of surgery, nanorobots could facilitate minimally invasive procedures with unprecedented precision. These microscopic devices could be used to perform delicate operations at the cellular level, potentially reducing recovery times and improving surgical outcomes. The market for such applications is expected to grow as healthcare providers seek more efficient and less invasive treatment options.
The aging population and increasing prevalence of chronic diseases worldwide are also driving factors for the nanorobotics market. As healthcare systems face mounting pressures to provide effective, long-term care for conditions such as cardiovascular diseases, neurodegenerative disorders, and diabetes, nanorobotics offers innovative solutions for monitoring, managing, and treating these conditions.
However, the market potential of nanorobotics is not without challenges. Regulatory hurdles, ethical considerations, and the need for extensive clinical trials may slow the adoption of these technologies in clinical practice. Additionally, the high costs associated with research, development, and manufacturing of nanorobots could initially limit their accessibility and widespread use.
Despite these challenges, the long-term market potential for nanorobotics in healthcare remains promising. As regulatory pathways become more defined and preclinical evidence accumulates, the integration of nanorobots into medical practice is likely to accelerate. This could lead to new market opportunities for pharmaceutical companies, medical device manufacturers, and healthcare providers, ultimately transforming patient care and treatment outcomes.
Technical Hurdles
The development of nanorobotics presents several significant technical hurdles that must be overcome to ensure safe and effective implementation in preclinical settings. One of the primary challenges is the precise control and navigation of nanorobots within biological systems. The complex and dynamic nature of the human body makes it difficult to accurately guide these microscopic devices to their intended targets without causing unintended interactions or damage to surrounding tissues.
Another major obstacle is the power supply for nanorobots. Traditional energy sources are too large and impractical for use at the nanoscale. Researchers are exploring various alternatives, such as harvesting energy from the biological environment or using external fields to power the devices. However, each approach comes with its own set of limitations and potential risks that need to be carefully evaluated.
The materials used in nanorobot construction pose another significant challenge. These materials must be biocompatible, durable enough to withstand the harsh biological environment, and capable of performing the intended functions. Additionally, they should be designed to avoid triggering immune responses or causing long-term toxicity. Developing materials that meet all these criteria while maintaining the necessary nanoscale dimensions is a complex task that requires extensive research and testing.
Communication between nanorobots and external control systems is another critical hurdle. Establishing reliable and secure methods for transmitting data and commands to and from nanodevices within the body is essential for their effective operation. This involves overcoming issues related to signal attenuation, interference from biological tissues, and potential security risks associated with wireless communication.
The manufacturing and quality control of nanorobots present unique challenges due to their extremely small size. Ensuring consistent production and verifying the functionality of each device requires the development of new manufacturing techniques and quality assurance protocols. This is particularly important for regulatory compliance and ensuring the safety and efficacy of nanorobotic interventions.
Lastly, the integration of multiple functionalities into a single nanorobot while maintaining its miniature size is a significant technical challenge. Researchers must find innovative ways to combine sensing, actuation, communication, and therapeutic capabilities within the constraints of nanoscale dimensions. This often requires interdisciplinary collaboration and the development of novel approaches to miniaturization and functional integration.
Another major obstacle is the power supply for nanorobots. Traditional energy sources are too large and impractical for use at the nanoscale. Researchers are exploring various alternatives, such as harvesting energy from the biological environment or using external fields to power the devices. However, each approach comes with its own set of limitations and potential risks that need to be carefully evaluated.
The materials used in nanorobot construction pose another significant challenge. These materials must be biocompatible, durable enough to withstand the harsh biological environment, and capable of performing the intended functions. Additionally, they should be designed to avoid triggering immune responses or causing long-term toxicity. Developing materials that meet all these criteria while maintaining the necessary nanoscale dimensions is a complex task that requires extensive research and testing.
Communication between nanorobots and external control systems is another critical hurdle. Establishing reliable and secure methods for transmitting data and commands to and from nanodevices within the body is essential for their effective operation. This involves overcoming issues related to signal attenuation, interference from biological tissues, and potential security risks associated with wireless communication.
The manufacturing and quality control of nanorobots present unique challenges due to their extremely small size. Ensuring consistent production and verifying the functionality of each device requires the development of new manufacturing techniques and quality assurance protocols. This is particularly important for regulatory compliance and ensuring the safety and efficacy of nanorobotic interventions.
Lastly, the integration of multiple functionalities into a single nanorobot while maintaining its miniature size is a significant technical challenge. Researchers must find innovative ways to combine sensing, actuation, communication, and therapeutic capabilities within the constraints of nanoscale dimensions. This often requires interdisciplinary collaboration and the development of novel approaches to miniaturization and functional integration.
Current Approaches
01 Nanorobot design and fabrication
Research focuses on the design and fabrication of nanorobots for medical applications. This includes developing nanoscale components, integrating various functionalities, and ensuring biocompatibility. Preclinical evidence demonstrates the potential of these nanorobots in targeted drug delivery, diagnostics, and minimally invasive procedures.- Nanorobot design and fabrication: Research focuses on the design and fabrication of nanorobots for medical applications. This includes developing nanoscale components, integrating various functionalities, and ensuring biocompatibility. Preclinical evidence involves testing these nanorobots in controlled laboratory environments to assess their performance and potential for targeted drug delivery, diagnostics, and therapeutic interventions.
- In vitro and in vivo testing of nanorobots: Preclinical studies involve both in vitro and in vivo testing of nanorobots. In vitro experiments are conducted to evaluate the behavior and efficacy of nanorobots in simulated biological environments. In vivo studies in animal models provide evidence of nanorobot performance in living systems, assessing factors such as biodistribution, targeting efficiency, and potential side effects.
- Nanorobot control and navigation systems: Development of control and navigation systems for nanorobots is crucial for their effective operation in biological environments. Preclinical evidence includes testing various methods for guiding nanorobots to target sites, such as magnetic fields, chemical gradients, or autonomous navigation algorithms. These systems are evaluated for precision, reliability, and adaptability in complex biological systems.
- Nanorobot-tissue interaction studies: Preclinical research investigates the interactions between nanorobots and biological tissues. This includes studying how nanorobots navigate through different tissue types, their ability to penetrate barriers like the blood-brain barrier, and potential immune responses. Evidence from these studies helps in optimizing nanorobot design and assessing their safety for potential clinical applications.
- Nanorobot imaging and tracking techniques: Developing and validating imaging and tracking techniques for nanorobots is essential for preclinical studies. This involves using advanced imaging modalities to visualize nanorobots in real-time within biological systems. Preclinical evidence demonstrates the effectiveness of various imaging methods in monitoring nanorobot movement, distribution, and functionality, which is crucial for assessing their performance and safety.
02 In vivo imaging and tracking of nanorobots
Advanced imaging techniques are being developed to visualize and track nanorobots in living organisms. These methods provide crucial preclinical evidence on nanorobot behavior, distribution, and efficacy in biological systems. Real-time monitoring enables researchers to optimize nanorobot performance and assess potential risks.Expand Specific Solutions03 Nanorobot-based drug delivery systems
Preclinical studies are exploring the use of nanorobots for targeted drug delivery. These systems aim to improve therapeutic efficacy while minimizing side effects. Evidence suggests that nanorobots can navigate through complex biological environments and release drugs at specific sites, potentially revolutionizing treatment for various diseases.Expand Specific Solutions04 Nanorobots for cancer diagnosis and treatment
Preclinical research is investigating the application of nanorobots in cancer diagnosis and treatment. These nanodevices can potentially detect cancer cells, deliver targeted therapies, and monitor treatment response. Early evidence shows promising results in improving cancer detection accuracy and treatment efficacy.Expand Specific Solutions05 Safety and biocompatibility assessment of nanorobots
Preclinical studies are focusing on evaluating the safety and biocompatibility of nanorobots in biological systems. This includes assessing potential toxicity, immune responses, and long-term effects. Evidence from these studies is crucial for advancing nanorobotics towards clinical applications and regulatory approval.Expand Specific Solutions
Industry Leaders
The nanorobotics regulatory landscape is in its early stages, reflecting the emerging nature of this technology. The market size is still relatively small but growing rapidly as research progresses. Technological maturity varies across different applications, with some areas more advanced than others. Key players like Hunan Morning Nano Robot Co., Mazor Robotics, and Auris Health are driving innovation in medical nanorobotics, while research institutions such as Caltech, MIT, and the Agency for Science, Technology & Research are contributing to fundamental advancements. The competitive landscape is characterized by a mix of specialized startups, established medical device companies, and academic institutions, each focusing on specific aspects of nanorobotics development and application.
California Institute of Technology
Technical Solution: California Institute of Technology (Caltech) has been at the forefront of nanorobotics research, focusing on developing regulatory pathways for preclinical evidence. Their approach involves creating a comprehensive checklist for evaluating nanorobots' safety and efficacy. Caltech's researchers have developed a multi-stage testing protocol that includes in vitro studies, ex vivo tissue models, and in vivo animal studies [1]. They utilize advanced imaging techniques, such as high-resolution electron microscopy and fluorescence imaging, to track nanorobot behavior in biological systems [3]. Caltech's checklist emphasizes the importance of assessing nanorobot biodistribution, clearance mechanisms, and potential off-target effects [5]. Their research also includes developing standardized methods for characterizing nanorobot physicochemical properties and their interactions with biological barriers.
Strengths: Comprehensive approach covering multiple aspects of nanorobot evaluation; utilization of cutting-edge imaging technologies. Weaknesses: Potential challenges in translating complex preclinical data to regulatory standards; time-consuming and resource-intensive process.
Agency for Science, Technology & Research
Technical Solution: The Agency for Science, Technology & Research (A*STAR) in Singapore has developed a robust regulatory pathway for nanorobotics, focusing on a preclinical evidence checklist that emphasizes interdisciplinary collaboration. Their approach combines expertise from nanotechnology, robotics, and biomedical sciences to create a comprehensive evaluation framework. A*STAR's checklist includes advanced in vitro 3D tissue models that mimic complex organ structures, allowing for more accurate assessment of nanorobot behavior in human-like environments [8]. They have also developed novel biomarker panels specifically designed to detect subtle physiological changes induced by nanorobots, enhancing the sensitivity of safety assessments [10]. A*STAR's regulatory pathway incorporates real-time monitoring systems for tracking nanorobot distribution and activity in live animal models, utilizing advanced imaging techniques such as photoacoustic tomography and Raman spectroscopy [12]. Their approach also includes guidelines for evaluating the potential environmental impact of nanorobots, addressing concerns about their release into ecosystems.
Strengths: Strong interdisciplinary approach; advanced in vitro 3D tissue models for accurate assessment. Weaknesses: Potential challenges in scaling up complex evaluation methods for large-scale production; may require significant resources for implementation.
Key Innovations
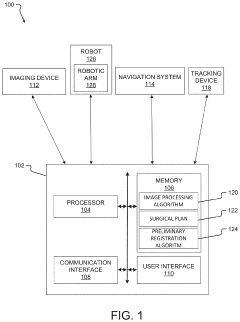
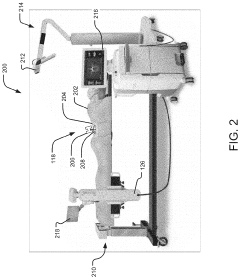
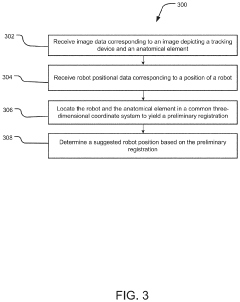
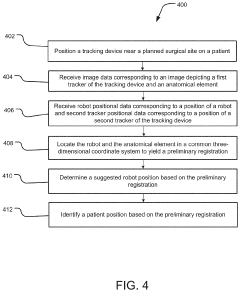
System and method for preliminary registration
PatentActiveUS20220031397A1
Innovation
- A preliminary registration method that uses image data and positional data to locate a robot and anatomical elements in a common 3D coordinate system, determining a suggested robot position based on the robot's reach and surgical plan, allowing for optimized incision planning and robot mounting without the need for full registration accuracy.
System and method for preliminary registration
PatentActiveUS20220031397A1
Innovation
- A preliminary registration method that uses image data and positional data to locate a robot and anatomical elements in a common 3D coordinate system, determining a suggested robot position based on the robot's reach and surgical plan, allowing for optimized incision planning and robot mounting without the need for full registration accuracy.
Regulatory Framework
The regulatory framework for nanorobotics is a complex and evolving landscape that requires careful consideration of multiple factors. As nanorobotics technology advances, regulatory bodies worldwide are grappling with the challenge of developing appropriate guidelines and standards to ensure safety and efficacy while fostering innovation.
At the international level, organizations such as the International Organization for Standardization (ISO) and the Organisation for Economic Co-operation and Development (OECD) are working to establish harmonized approaches to nanorobotics regulation. These efforts aim to create a common understanding of terminology, risk assessment methodologies, and testing protocols specific to nanorobotic devices.
In the United States, the Food and Drug Administration (FDA) has taken a lead role in addressing the regulatory challenges posed by nanorobotics. The agency has established a Nanotechnology Task Force to develop guidance documents and regulatory strategies tailored to nanoscale materials and devices. The FDA's approach emphasizes a case-by-case evaluation of nanorobotic products, considering their unique properties and potential risks.
The European Union has implemented the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, which includes provisions for nanomaterials. While not specifically designed for nanorobotics, this framework provides a foundation for assessing the safety of nanoscale components used in robotic systems. Additionally, the European Medicines Agency (EMA) has published guidelines on the use of nanomaterials in medical products, which may have implications for nanorobotic devices intended for therapeutic applications.
Regulatory bodies are increasingly focusing on the need for standardized preclinical evidence requirements for nanorobotics. This includes developing specific protocols for assessing the biocompatibility, biodistribution, and potential long-term effects of nanorobotic systems in biological environments. The establishment of a comprehensive preclinical evidence checklist is crucial for ensuring that nanorobotic devices meet stringent safety and performance standards before advancing to clinical trials.
Key areas of regulatory concern for nanorobotics include the potential for unintended interactions with biological systems, the long-term fate of nanorobotic components in the body, and the challenges associated with monitoring and controlling nanoscale devices in vivo. Regulatory frameworks must also address ethical considerations, such as privacy concerns and the potential for unintended consequences of nanorobotic interventions.
As the field of nanorobotics continues to advance, it is likely that regulatory frameworks will need to evolve rapidly to keep pace with technological developments. This may require the establishment of new regulatory categories and the development of novel assessment methodologies specifically tailored to the unique characteristics of nanorobotic systems.
At the international level, organizations such as the International Organization for Standardization (ISO) and the Organisation for Economic Co-operation and Development (OECD) are working to establish harmonized approaches to nanorobotics regulation. These efforts aim to create a common understanding of terminology, risk assessment methodologies, and testing protocols specific to nanorobotic devices.
In the United States, the Food and Drug Administration (FDA) has taken a lead role in addressing the regulatory challenges posed by nanorobotics. The agency has established a Nanotechnology Task Force to develop guidance documents and regulatory strategies tailored to nanoscale materials and devices. The FDA's approach emphasizes a case-by-case evaluation of nanorobotic products, considering their unique properties and potential risks.
The European Union has implemented the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, which includes provisions for nanomaterials. While not specifically designed for nanorobotics, this framework provides a foundation for assessing the safety of nanoscale components used in robotic systems. Additionally, the European Medicines Agency (EMA) has published guidelines on the use of nanomaterials in medical products, which may have implications for nanorobotic devices intended for therapeutic applications.
Regulatory bodies are increasingly focusing on the need for standardized preclinical evidence requirements for nanorobotics. This includes developing specific protocols for assessing the biocompatibility, biodistribution, and potential long-term effects of nanorobotic systems in biological environments. The establishment of a comprehensive preclinical evidence checklist is crucial for ensuring that nanorobotic devices meet stringent safety and performance standards before advancing to clinical trials.
Key areas of regulatory concern for nanorobotics include the potential for unintended interactions with biological systems, the long-term fate of nanorobotic components in the body, and the challenges associated with monitoring and controlling nanoscale devices in vivo. Regulatory frameworks must also address ethical considerations, such as privacy concerns and the potential for unintended consequences of nanorobotic interventions.
As the field of nanorobotics continues to advance, it is likely that regulatory frameworks will need to evolve rapidly to keep pace with technological developments. This may require the establishment of new regulatory categories and the development of novel assessment methodologies specifically tailored to the unique characteristics of nanorobotic systems.
Safety Considerations
Safety considerations are paramount in the development and implementation of nanorobotics for medical applications. The miniature size and unique properties of nanorobots present novel challenges that must be thoroughly addressed to ensure patient safety and regulatory compliance. A comprehensive safety assessment framework for nanorobotics should encompass several key areas.
Firstly, biocompatibility and biodegradability are crucial factors. Nanorobots must be designed using materials that do not elicit adverse immune responses or cause toxicity in the human body. The potential for accumulation in organs and tissues must be carefully evaluated, with a focus on long-term effects. Additionally, the degradation products of nanorobots should be non-toxic and easily eliminated from the body.
Secondly, the potential for unintended interactions with biological systems must be thoroughly investigated. This includes assessing the impact of nanorobots on cellular functions, protein interactions, and gene expression. The risk of nanorobots crossing biological barriers, such as the blood-brain barrier, should be evaluated to prevent unintended consequences in sensitive areas of the body.
Thirdly, the control and navigation of nanorobots within the body pose unique safety challenges. Robust mechanisms must be in place to ensure precise targeting and to prevent off-target effects. The potential for nanorobots to aggregate or form unintended structures in vivo must be addressed, as this could lead to complications such as embolisms or tissue damage.
Fourthly, the electromagnetic properties of nanorobots and their potential interactions with medical imaging technologies must be carefully considered. Compatibility with MRI, CT scans, and other diagnostic tools is essential to prevent interference or safety hazards during medical procedures.
Lastly, the development of standardized safety protocols and testing methodologies specific to nanorobotics is crucial. This includes establishing guidelines for preclinical studies, defining appropriate animal models, and determining relevant endpoints for safety assessments. Collaboration between regulatory bodies, researchers, and industry stakeholders is necessary to create a comprehensive regulatory framework that addresses the unique challenges posed by nanorobotics in healthcare.
Firstly, biocompatibility and biodegradability are crucial factors. Nanorobots must be designed using materials that do not elicit adverse immune responses or cause toxicity in the human body. The potential for accumulation in organs and tissues must be carefully evaluated, with a focus on long-term effects. Additionally, the degradation products of nanorobots should be non-toxic and easily eliminated from the body.
Secondly, the potential for unintended interactions with biological systems must be thoroughly investigated. This includes assessing the impact of nanorobots on cellular functions, protein interactions, and gene expression. The risk of nanorobots crossing biological barriers, such as the blood-brain barrier, should be evaluated to prevent unintended consequences in sensitive areas of the body.
Thirdly, the control and navigation of nanorobots within the body pose unique safety challenges. Robust mechanisms must be in place to ensure precise targeting and to prevent off-target effects. The potential for nanorobots to aggregate or form unintended structures in vivo must be addressed, as this could lead to complications such as embolisms or tissue damage.
Fourthly, the electromagnetic properties of nanorobots and their potential interactions with medical imaging technologies must be carefully considered. Compatibility with MRI, CT scans, and other diagnostic tools is essential to prevent interference or safety hazards during medical procedures.
Lastly, the development of standardized safety protocols and testing methodologies specific to nanorobotics is crucial. This includes establishing guidelines for preclinical studies, defining appropriate animal models, and determining relevant endpoints for safety assessments. Collaboration between regulatory bodies, researchers, and industry stakeholders is necessary to create a comprehensive regulatory framework that addresses the unique challenges posed by nanorobotics in healthcare.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!