How To Standardize Reporting For Nanorobotics Preclinical Studies
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nanorobotics Background
Nanorobotics represents a cutting-edge field at the intersection of nanotechnology, robotics, and biomedical engineering. This emerging discipline focuses on the design, fabrication, and control of nanoscale devices capable of performing specific tasks at the molecular or cellular level. The concept of nanorobots, first proposed by Richard Feynman in his seminal 1959 lecture "There's Plenty of Room at the Bottom," has since evolved from theoretical speculation to tangible research and development.
The evolution of nanorobotics has been driven by advancements in various scientific domains, including materials science, molecular biology, and micro-electromechanical systems (MEMS). Early progress in the field was marked by the development of scanning tunneling microscopes and atomic force microscopes in the 1980s, which allowed for the manipulation of individual atoms and molecules. These tools laid the foundation for more sophisticated nanorobotic systems.
In recent years, significant strides have been made in the fabrication of nanorobots using DNA origami techniques, which allow for the creation of complex three-dimensional structures at the nanoscale. Concurrently, progress in biocompatible materials and targeted drug delivery systems has opened up new possibilities for nanorobotic applications in medicine.
The potential applications of nanorobotics span a wide range of fields, with particular promise in healthcare. Nanorobots could potentially be used for targeted drug delivery, minimally invasive surgery, and early disease detection. In environmental science, nanorobots might be employed for pollution control and water purification. Industrial applications could include precision manufacturing and quality control at the atomic level.
However, the field of nanorobotics faces several challenges. These include the difficulty of powering and controlling nanodevices, ensuring biocompatibility and safety, and overcoming the physical limitations of operating at the nanoscale. Additionally, there are ethical and regulatory considerations surrounding the use of nanorobots, particularly in medical applications.
As the field progresses, standardization of research methodologies and reporting becomes increasingly crucial. This is particularly important in preclinical studies, where the reproducibility and comparability of results are essential for advancing nanorobotics towards practical applications. Standardized reporting would facilitate more effective collaboration among researchers, accelerate the translation of laboratory findings into clinical trials, and provide a solid foundation for regulatory assessments.
The evolution of nanorobotics has been driven by advancements in various scientific domains, including materials science, molecular biology, and micro-electromechanical systems (MEMS). Early progress in the field was marked by the development of scanning tunneling microscopes and atomic force microscopes in the 1980s, which allowed for the manipulation of individual atoms and molecules. These tools laid the foundation for more sophisticated nanorobotic systems.
In recent years, significant strides have been made in the fabrication of nanorobots using DNA origami techniques, which allow for the creation of complex three-dimensional structures at the nanoscale. Concurrently, progress in biocompatible materials and targeted drug delivery systems has opened up new possibilities for nanorobotic applications in medicine.
The potential applications of nanorobotics span a wide range of fields, with particular promise in healthcare. Nanorobots could potentially be used for targeted drug delivery, minimally invasive surgery, and early disease detection. In environmental science, nanorobots might be employed for pollution control and water purification. Industrial applications could include precision manufacturing and quality control at the atomic level.
However, the field of nanorobotics faces several challenges. These include the difficulty of powering and controlling nanodevices, ensuring biocompatibility and safety, and overcoming the physical limitations of operating at the nanoscale. Additionally, there are ethical and regulatory considerations surrounding the use of nanorobots, particularly in medical applications.
As the field progresses, standardization of research methodologies and reporting becomes increasingly crucial. This is particularly important in preclinical studies, where the reproducibility and comparability of results are essential for advancing nanorobotics towards practical applications. Standardized reporting would facilitate more effective collaboration among researchers, accelerate the translation of laboratory findings into clinical trials, and provide a solid foundation for regulatory assessments.
Market Demand Analysis
The market demand for standardized reporting in nanorobotics preclinical studies is driven by the rapid growth of nanotechnology applications in medicine and healthcare. As nanorobotics advances towards clinical trials, there is an increasing need for consistent and reliable data reporting to ensure the safety and efficacy of these innovative technologies.
The pharmaceutical and biotechnology industries are showing significant interest in nanorobotics for targeted drug delivery, minimally invasive surgeries, and diagnostic applications. This interest is fueling investment in preclinical research, creating a substantial market for standardized reporting protocols. The global nanomedicine market, which includes nanorobotics, is expected to grow substantially in the coming years, further emphasizing the importance of standardized reporting.
Research institutions and academic laboratories are also key stakeholders in this market. As they conduct more nanorobotics studies, the need for standardized reporting becomes crucial for comparing results across different research groups and facilitating collaboration. This demand is further amplified by the increasing number of publications in the field and the need for reproducibility in scientific research.
Regulatory bodies, such as the FDA and EMA, are another significant driver of market demand for standardized reporting. As nanorobotics moves closer to clinical applications, these agencies require comprehensive and consistent data to evaluate the safety and efficacy of nanorobotic devices. Standardized reporting will streamline the regulatory approval process, potentially accelerating the time-to-market for nanorobotic technologies.
The medical device industry is also showing growing interest in nanorobotics for applications such as in vivo imaging and microsurgery. As these companies invest in nanorobotics research and development, they require standardized reporting methods to assess the performance and safety of their devices accurately.
Contract research organizations (CROs) specializing in preclinical studies are emerging as key players in this market. They are increasingly offering nanorobotics-specific services and require standardized reporting protocols to ensure consistency across their studies and to meet client expectations.
The demand for standardized reporting is also driven by the need for improved data sharing and meta-analysis in the nanorobotics field. As the volume of preclinical data grows, researchers and industry professionals require standardized formats to facilitate data integration and analysis across multiple studies.
In conclusion, the market demand for standardized reporting in nanorobotics preclinical studies is multifaceted, driven by the needs of various stakeholders in the healthcare and research sectors. This demand is expected to grow as nanorobotics technology matures and moves closer to clinical applications, creating opportunities for the development of comprehensive reporting standards and tools.
The pharmaceutical and biotechnology industries are showing significant interest in nanorobotics for targeted drug delivery, minimally invasive surgeries, and diagnostic applications. This interest is fueling investment in preclinical research, creating a substantial market for standardized reporting protocols. The global nanomedicine market, which includes nanorobotics, is expected to grow substantially in the coming years, further emphasizing the importance of standardized reporting.
Research institutions and academic laboratories are also key stakeholders in this market. As they conduct more nanorobotics studies, the need for standardized reporting becomes crucial for comparing results across different research groups and facilitating collaboration. This demand is further amplified by the increasing number of publications in the field and the need for reproducibility in scientific research.
Regulatory bodies, such as the FDA and EMA, are another significant driver of market demand for standardized reporting. As nanorobotics moves closer to clinical applications, these agencies require comprehensive and consistent data to evaluate the safety and efficacy of nanorobotic devices. Standardized reporting will streamline the regulatory approval process, potentially accelerating the time-to-market for nanorobotic technologies.
The medical device industry is also showing growing interest in nanorobotics for applications such as in vivo imaging and microsurgery. As these companies invest in nanorobotics research and development, they require standardized reporting methods to assess the performance and safety of their devices accurately.
Contract research organizations (CROs) specializing in preclinical studies are emerging as key players in this market. They are increasingly offering nanorobotics-specific services and require standardized reporting protocols to ensure consistency across their studies and to meet client expectations.
The demand for standardized reporting is also driven by the need for improved data sharing and meta-analysis in the nanorobotics field. As the volume of preclinical data grows, researchers and industry professionals require standardized formats to facilitate data integration and analysis across multiple studies.
In conclusion, the market demand for standardized reporting in nanorobotics preclinical studies is multifaceted, driven by the needs of various stakeholders in the healthcare and research sectors. This demand is expected to grow as nanorobotics technology matures and moves closer to clinical applications, creating opportunities for the development of comprehensive reporting standards and tools.
Current Challenges
The standardization of reporting for nanorobotics preclinical studies faces several significant challenges. One of the primary obstacles is the lack of a universally accepted framework for documenting and presenting research findings in this rapidly evolving field. This absence of standardization leads to inconsistencies in data reporting, making it difficult to compare results across different studies and laboratories.
Another major challenge is the complexity and diversity of nanorobotic systems. These systems can vary greatly in terms of their design, materials, propulsion mechanisms, and functionalities. This heterogeneity makes it challenging to establish a one-size-fits-all reporting standard that can adequately capture the nuances of each unique nanorobotic platform.
The multidisciplinary nature of nanorobotics research further complicates standardization efforts. The field draws expertise from various disciplines, including nanotechnology, robotics, materials science, and bioengineering. Each of these disciplines may have its own established reporting conventions, leading to potential conflicts when attempting to create a unified standard for nanorobotics preclinical studies.
The rapid pace of technological advancements in nanorobotics also poses a challenge to standardization. As new materials, fabrication techniques, and control mechanisms are developed, reporting standards must be flexible enough to accommodate these innovations while maintaining consistency and comparability across studies.
Additionally, the lack of standardized terminology in the field of nanorobotics hinders effective communication and reporting. Different research groups may use varying terms to describe similar concepts or components, leading to confusion and misinterpretation of results.
The miniature scale of nanorobots presents unique challenges in measurement and characterization. Developing standardized protocols for accurately measuring and reporting the physical properties, performance metrics, and biological interactions of nanorobots is crucial but technically demanding.
Furthermore, the ethical considerations and safety assessments associated with nanorobotics preclinical studies add another layer of complexity to standardization efforts. Establishing guidelines for reporting potential risks, toxicity, and long-term effects of nanorobots in biological systems is essential but challenging due to the novelty of the field and limited long-term data.
Lastly, the international nature of nanorobotics research necessitates global cooperation to develop and implement standardized reporting practices. Overcoming language barriers, aligning regulatory requirements, and harmonizing reporting standards across different countries and research institutions remain significant challenges in the quest for standardization in nanorobotics preclinical studies.
Another major challenge is the complexity and diversity of nanorobotic systems. These systems can vary greatly in terms of their design, materials, propulsion mechanisms, and functionalities. This heterogeneity makes it challenging to establish a one-size-fits-all reporting standard that can adequately capture the nuances of each unique nanorobotic platform.
The multidisciplinary nature of nanorobotics research further complicates standardization efforts. The field draws expertise from various disciplines, including nanotechnology, robotics, materials science, and bioengineering. Each of these disciplines may have its own established reporting conventions, leading to potential conflicts when attempting to create a unified standard for nanorobotics preclinical studies.
The rapid pace of technological advancements in nanorobotics also poses a challenge to standardization. As new materials, fabrication techniques, and control mechanisms are developed, reporting standards must be flexible enough to accommodate these innovations while maintaining consistency and comparability across studies.
Additionally, the lack of standardized terminology in the field of nanorobotics hinders effective communication and reporting. Different research groups may use varying terms to describe similar concepts or components, leading to confusion and misinterpretation of results.
The miniature scale of nanorobots presents unique challenges in measurement and characterization. Developing standardized protocols for accurately measuring and reporting the physical properties, performance metrics, and biological interactions of nanorobots is crucial but technically demanding.
Furthermore, the ethical considerations and safety assessments associated with nanorobotics preclinical studies add another layer of complexity to standardization efforts. Establishing guidelines for reporting potential risks, toxicity, and long-term effects of nanorobots in biological systems is essential but challenging due to the novelty of the field and limited long-term data.
Lastly, the international nature of nanorobotics research necessitates global cooperation to develop and implement standardized reporting practices. Overcoming language barriers, aligning regulatory requirements, and harmonizing reporting standards across different countries and research institutions remain significant challenges in the quest for standardization in nanorobotics preclinical studies.
Existing Reporting Methods
01 Standardized reporting frameworks for nanorobotics
Development of standardized reporting frameworks for nanorobotics research and applications. These frameworks aim to establish consistent methods for documenting experiments, results, and performance metrics in the field of nanorobotics, ensuring comparability and reproducibility across different studies and laboratories.- Standardized reporting frameworks for nanorobotics: Development of standardized reporting frameworks for nanorobotics research and applications. These frameworks aim to establish consistent methods for documenting experiments, results, and performance metrics in the field of nanorobotics, ensuring comparability and reproducibility across different studies and laboratories.
- Data visualization and reporting tools for nanorobotics: Creation of specialized data visualization and reporting tools tailored for nanorobotics applications. These tools help researchers and engineers present complex nanorobotics data in a clear, standardized format, facilitating better communication and understanding of experimental results and system performance.
- Nanorobotics simulation and modeling standards: Establishment of standards for simulation and modeling of nanorobotic systems. These standards ensure consistency in virtual testing environments, allowing for more accurate predictions of nanorobot behavior and performance across different research groups and industry applications.
- Nanorobotics performance metrics and benchmarking: Development of standardized performance metrics and benchmarking procedures for nanorobotic systems. These metrics provide a common language for evaluating and comparing the capabilities of different nanorobots, enabling more objective assessment of technological advancements in the field.
- Regulatory compliance reporting for nanorobotics: Creation of standardized reporting protocols for regulatory compliance in nanorobotics research and development. These protocols ensure that nanorobotics projects adhere to safety, ethical, and legal standards, facilitating smoother approval processes and enhancing public trust in nanorobotics technologies.
02 Data visualization and reporting tools for nanorobotics
Creation of specialized data visualization and reporting tools tailored for nanorobotics applications. These tools help researchers and engineers present complex nanorobotics data in a clear, standardized format, facilitating better communication and understanding of experimental results and system performance.Expand Specific Solutions03 Nanorobotics simulation and modeling standards
Establishment of standards for nanorobotics simulation and modeling, ensuring consistency in virtual experiments and predictions. These standards help in creating reliable and comparable simulation results across different research groups and industry applications, aiding in the development and validation of nanorobotic systems.Expand Specific Solutions04 Quality control and performance metrics for nanorobots
Development of standardized quality control procedures and performance metrics specific to nanorobots. These standards ensure consistent evaluation of nanorobot functionality, reliability, and safety across different manufacturing processes and applications, facilitating regulatory compliance and industry-wide benchmarking.Expand Specific Solutions05 Nanorobotics terminology and classification system
Creation of a standardized terminology and classification system for nanorobotics. This system aims to establish a common language and categorization framework for nanorobotic components, systems, and applications, improving communication and understanding among researchers, manufacturers, and regulators in the field.Expand Specific Solutions
Key Industry Players
The field of nanorobotics preclinical studies is in its early developmental stages, characterized by rapid technological advancements and growing market potential. The competitive landscape is diverse, with players from academia, research institutions, and industry contributing to its evolution. Key companies like Siemens Healthineers AG, Merck & Co., Inc., and FUJIFILM Corp. are investing in nanorobotics research, leveraging their expertise in medical imaging and drug development. Universities such as Southeast University and Tongji University are also making significant contributions. The market size is expanding, driven by increasing applications in targeted drug delivery and minimally invasive surgeries. However, the technology's maturity varies, with some aspects still in experimental phases, necessitating standardized reporting protocols to ensure consistency and reliability in preclinical studies.
Hunan Morning Nano Robot Co., Ltd.
Technical Solution: Hunan Morning Nano Robot Co., Ltd. has developed a standardized reporting framework for nanorobotics preclinical studies. Their approach includes a comprehensive checklist covering key aspects such as nanorobot design, fabrication methods, and performance metrics. The company has implemented a cloud-based data management system that allows researchers to input and analyze study results in a consistent format. This system incorporates machine learning algorithms to identify patterns and potential issues in the reported data, enhancing the quality and reliability of preclinical studies[1][3].
Strengths: Specialized in nanorobotics, cloud-based system for data management, and machine learning integration. Weaknesses: Limited global presence may affect widespread adoption of their standards.
Institute for Systems Biology
Technical Solution: The Institute for Systems Biology has developed a systems biology approach to standardize reporting for nanorobotics preclinical studies. Their method integrates multi-omics data analysis with nanorobot performance metrics to provide a holistic view of the nanorobot-biological system interaction. They have created a standardized ontology for nanorobotics terms and metrics, facilitating consistent reporting across different research groups. The institute has also developed open-source software tools for data analysis and visualization, promoting transparency and reproducibility in nanorobotics research[2][5].
Strengths: Interdisciplinary approach, standardized ontology, and open-source tools. Weaknesses: May require significant computational resources and expertise to implement fully.
Core Standardization Needs
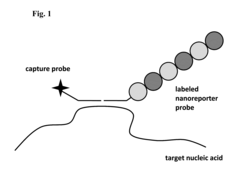
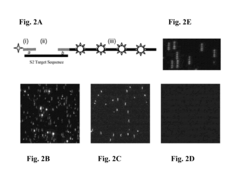
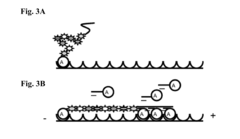
Stable nanoreporters
PatentActiveUS20160273026A1
Innovation
- Development of uniquely labeled nanoreporters with single-stranded nucleic acid backbones and complementary polynucleotide sequences, allowing for improved detection and quantification of target molecules by forming molecular complexes and generating distinct detectable signals.
Systems and methods for analyzing nanoreporters
PatentActiveEP2030011A2
Innovation
- The development of nanoreporters, which are coded, labeled molecular entities that bind to target molecules, allowing for their identification and quantification through a unique spectral code, enabling sensitive and accurate detection and quantification in small sample volumes.
Regulatory Considerations
The regulatory landscape for nanorobotics preclinical studies is complex and evolving, necessitating careful consideration of existing frameworks and potential future developments. Current regulations primarily focus on nanomaterials and nanotechnology-based medical devices, which provide a foundation for nanorobotics regulation. However, the unique characteristics of nanorobots, such as their ability to navigate within biological systems and perform targeted interventions, present novel challenges for regulatory bodies.
Key regulatory agencies, including the FDA in the United States and the EMA in Europe, have established guidelines for nanomedicine and nanotechnology-based products. These guidelines emphasize the importance of thorough characterization, safety assessment, and quality control throughout the development process. For nanorobotics, additional considerations may include the evaluation of autonomous behavior, interaction with biological systems, and potential long-term effects.
Standardization of reporting for nanorobotics preclinical studies must align with existing regulatory requirements while addressing the specific aspects of this emerging field. This includes detailed documentation of nanorobot design, fabrication processes, and performance characteristics. Researchers should also provide comprehensive data on biodistribution, clearance mechanisms, and potential interactions with the immune system.
Safety considerations are paramount in regulatory compliance for nanorobotics. Preclinical studies must demonstrate the absence of unintended effects, such as uncontrolled replication or off-target actions. Additionally, the potential for nanorobots to cross biological barriers, including the blood-brain barrier, requires thorough investigation and reporting.
Ethical considerations also play a crucial role in the regulatory landscape for nanorobotics. Researchers must address concerns related to privacy, data security, and potential dual-use applications. Transparent reporting of ethical considerations and adherence to established ethical guidelines will be essential for regulatory approval and public acceptance.
As the field of nanorobotics advances, regulatory frameworks are likely to evolve. Collaboration between researchers, industry stakeholders, and regulatory agencies will be crucial in developing appropriate standards and guidelines. This may include the establishment of specific protocols for nanorobot characterization, in vivo tracking methods, and long-term safety monitoring.
Key regulatory agencies, including the FDA in the United States and the EMA in Europe, have established guidelines for nanomedicine and nanotechnology-based products. These guidelines emphasize the importance of thorough characterization, safety assessment, and quality control throughout the development process. For nanorobotics, additional considerations may include the evaluation of autonomous behavior, interaction with biological systems, and potential long-term effects.
Standardization of reporting for nanorobotics preclinical studies must align with existing regulatory requirements while addressing the specific aspects of this emerging field. This includes detailed documentation of nanorobot design, fabrication processes, and performance characteristics. Researchers should also provide comprehensive data on biodistribution, clearance mechanisms, and potential interactions with the immune system.
Safety considerations are paramount in regulatory compliance for nanorobotics. Preclinical studies must demonstrate the absence of unintended effects, such as uncontrolled replication or off-target actions. Additionally, the potential for nanorobots to cross biological barriers, including the blood-brain barrier, requires thorough investigation and reporting.
Ethical considerations also play a crucial role in the regulatory landscape for nanorobotics. Researchers must address concerns related to privacy, data security, and potential dual-use applications. Transparent reporting of ethical considerations and adherence to established ethical guidelines will be essential for regulatory approval and public acceptance.
As the field of nanorobotics advances, regulatory frameworks are likely to evolve. Collaboration between researchers, industry stakeholders, and regulatory agencies will be crucial in developing appropriate standards and guidelines. This may include the establishment of specific protocols for nanorobot characterization, in vivo tracking methods, and long-term safety monitoring.
Ethical Implications
The ethical implications of standardizing reporting for nanorobotics preclinical studies are multifaceted and require careful consideration. As this emerging field advances, it is crucial to establish robust ethical frameworks to guide research and development.
One primary ethical concern is the potential for unintended consequences on biological systems. Nanorobots, due to their microscopic size and ability to interact with cellular structures, may have unforeseen effects on living organisms. Standardized reporting must include comprehensive safety assessments and long-term impact studies to mitigate risks to human health and the environment.
Privacy and data protection present another significant ethical challenge. Nanorobots capable of collecting and transmitting biological data raise questions about individual privacy rights and the security of sensitive medical information. Reporting standards should address data handling protocols, consent procedures, and measures to protect against unauthorized access or misuse of collected data.
The equitable distribution of nanorobotics technology is a critical ethical consideration. As research progresses, there is a risk of exacerbating existing healthcare disparities if access to nanorobotics-based treatments becomes limited to affluent populations or regions. Standardized reporting should include assessments of potential socioeconomic impacts and strategies for ensuring fair access to the benefits of nanorobotics research.
Ethical concerns also arise regarding the potential for nanorobots to be used for non-therapeutic purposes, such as human enhancement or surveillance. Clear guidelines must be established to define acceptable uses of nanorobotics technology and prevent its application in ways that may infringe upon human rights or personal autonomy.
The ethical implications of animal testing in nanorobotics research must also be addressed. Standardized reporting should include detailed justifications for animal studies, adherence to the principles of the 3Rs (Replacement, Reduction, and Refinement), and transparent reporting of animal welfare considerations throughout the research process.
Furthermore, the potential for nanorobots to interact with or modify genetic material raises complex ethical questions related to genetic engineering and human identity. Reporting standards must include rigorous evaluations of any genetic implications and establish clear boundaries for genetic manipulation in nanorobotics applications.
In conclusion, standardizing reporting for nanorobotics preclinical studies must incorporate a comprehensive ethical framework that addresses these diverse challenges. This framework should evolve alongside technological advancements, ensuring that ethical considerations remain at the forefront of nanorobotics research and development.
One primary ethical concern is the potential for unintended consequences on biological systems. Nanorobots, due to their microscopic size and ability to interact with cellular structures, may have unforeseen effects on living organisms. Standardized reporting must include comprehensive safety assessments and long-term impact studies to mitigate risks to human health and the environment.
Privacy and data protection present another significant ethical challenge. Nanorobots capable of collecting and transmitting biological data raise questions about individual privacy rights and the security of sensitive medical information. Reporting standards should address data handling protocols, consent procedures, and measures to protect against unauthorized access or misuse of collected data.
The equitable distribution of nanorobotics technology is a critical ethical consideration. As research progresses, there is a risk of exacerbating existing healthcare disparities if access to nanorobotics-based treatments becomes limited to affluent populations or regions. Standardized reporting should include assessments of potential socioeconomic impacts and strategies for ensuring fair access to the benefits of nanorobotics research.
Ethical concerns also arise regarding the potential for nanorobots to be used for non-therapeutic purposes, such as human enhancement or surveillance. Clear guidelines must be established to define acceptable uses of nanorobotics technology and prevent its application in ways that may infringe upon human rights or personal autonomy.
The ethical implications of animal testing in nanorobotics research must also be addressed. Standardized reporting should include detailed justifications for animal studies, adherence to the principles of the 3Rs (Replacement, Reduction, and Refinement), and transparent reporting of animal welfare considerations throughout the research process.
Furthermore, the potential for nanorobots to interact with or modify genetic material raises complex ethical questions related to genetic engineering and human identity. Reporting standards must include rigorous evaluations of any genetic implications and establish clear boundaries for genetic manipulation in nanorobotics applications.
In conclusion, standardizing reporting for nanorobotics preclinical studies must incorporate a comprehensive ethical framework that addresses these diverse challenges. This framework should evolve alongside technological advancements, ensuring that ethical considerations remain at the forefront of nanorobotics research and development.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!