Nanorobots Crossing The Blood-Brain Barrier: Safety Protocols
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nanorobot BBB Safety Goals
The primary goal of safety protocols for nanorobots crossing the blood-brain barrier (BBB) is to ensure the safe and effective delivery of therapeutic agents to the brain while minimizing potential risks and side effects. This objective encompasses several key aspects that must be carefully addressed in the development and implementation of nanorobot-based drug delivery systems.
One crucial safety goal is to maintain the integrity of the BBB during nanorobot passage. The BBB serves as a protective barrier, regulating the entry of substances into the brain. Nanorobots must be designed to cross this barrier without causing damage or disruption to its structure and function. This involves developing mechanisms for controlled and reversible opening of tight junctions between endothelial cells, allowing nanorobots to pass through without compromising the barrier's overall protective role.
Another important safety objective is to ensure the biocompatibility of nanorobots within the brain environment. The materials used in nanorobot construction must be non-toxic, non-immunogenic, and capable of biodegradation or safe elimination from the body. This goal requires extensive research into biocompatible materials and surface modifications that can minimize potential inflammatory responses or adverse reactions in brain tissue.
Precise targeting and controlled drug release are also critical safety goals. Nanorobots should be engineered to specifically target diseased areas or cells within the brain, minimizing off-target effects and potential damage to healthy tissue. Additionally, the release of therapeutic agents must be carefully controlled to ensure optimal dosing and prevent toxicity due to overdose or premature release.
The prevention of nanorobot aggregation and potential obstruction of blood vessels is another key safety consideration. Protocols must be developed to ensure that nanorobots remain dispersed and do not form clumps that could impede blood flow or cause ischemic events in the brain.
Long-term safety monitoring is an essential goal in nanorobot-based therapies. This involves developing methods for tracking nanorobot distribution, assessing their degradation or elimination from the body, and monitoring potential long-term effects on brain function and overall health. Such monitoring systems are crucial for identifying and addressing any unforeseen complications that may arise from nanorobot use.
Lastly, the development of fail-safe mechanisms and emergency protocols is a critical safety goal. These should include methods for rapid deactivation or removal of nanorobots in case of adverse reactions or unexpected complications, ensuring that any potential risks can be quickly mitigated.
One crucial safety goal is to maintain the integrity of the BBB during nanorobot passage. The BBB serves as a protective barrier, regulating the entry of substances into the brain. Nanorobots must be designed to cross this barrier without causing damage or disruption to its structure and function. This involves developing mechanisms for controlled and reversible opening of tight junctions between endothelial cells, allowing nanorobots to pass through without compromising the barrier's overall protective role.
Another important safety objective is to ensure the biocompatibility of nanorobots within the brain environment. The materials used in nanorobot construction must be non-toxic, non-immunogenic, and capable of biodegradation or safe elimination from the body. This goal requires extensive research into biocompatible materials and surface modifications that can minimize potential inflammatory responses or adverse reactions in brain tissue.
Precise targeting and controlled drug release are also critical safety goals. Nanorobots should be engineered to specifically target diseased areas or cells within the brain, minimizing off-target effects and potential damage to healthy tissue. Additionally, the release of therapeutic agents must be carefully controlled to ensure optimal dosing and prevent toxicity due to overdose or premature release.
The prevention of nanorobot aggregation and potential obstruction of blood vessels is another key safety consideration. Protocols must be developed to ensure that nanorobots remain dispersed and do not form clumps that could impede blood flow or cause ischemic events in the brain.
Long-term safety monitoring is an essential goal in nanorobot-based therapies. This involves developing methods for tracking nanorobot distribution, assessing their degradation or elimination from the body, and monitoring potential long-term effects on brain function and overall health. Such monitoring systems are crucial for identifying and addressing any unforeseen complications that may arise from nanorobot use.
Lastly, the development of fail-safe mechanisms and emergency protocols is a critical safety goal. These should include methods for rapid deactivation or removal of nanorobots in case of adverse reactions or unexpected complications, ensuring that any potential risks can be quickly mitigated.
Market Demand Analysis
The market demand for safety protocols in nanorobots crossing the blood-brain barrier is driven by the growing interest in targeted drug delivery and precision medicine. As nanotechnology advances, the potential for nanorobots to revolutionize medical treatments, particularly in neurological disorders, has sparked significant research and development efforts.
The global nanomedicine market, which encompasses nanorobots and related technologies, is experiencing rapid growth. This expansion is fueled by the increasing prevalence of chronic diseases, the need for more effective drug delivery systems, and the potential for personalized medicine. The ability of nanorobots to cross the blood-brain barrier offers unprecedented opportunities for treating brain-related conditions, including cancers, neurodegenerative diseases, and psychiatric disorders.
Healthcare providers and pharmaceutical companies are showing keen interest in nanorobot technologies that can safely navigate the blood-brain barrier. This interest stems from the potential to improve treatment efficacy while minimizing side effects, a crucial factor in neurological therapies. The demand for such technologies is particularly high in regions with aging populations, where the incidence of neurodegenerative diseases is on the rise.
Investors and venture capitalists are also recognizing the market potential of safe nanorobot technologies. Funding for research and development in this field has seen a significant uptick in recent years, reflecting the anticipated market growth and the transformative potential of these technologies in healthcare.
The regulatory landscape is evolving to accommodate these emerging technologies, with regulatory bodies worldwide working to establish guidelines for the development and use of nanorobots in medical applications. This regulatory attention underscores the growing market demand and the need for robust safety protocols.
Patient advocacy groups and healthcare organizations are increasingly calling for innovative treatments for neurological conditions, further driving the demand for safe and effective nanorobot technologies. The potential for improved quality of life and better treatment outcomes is a significant factor in this demand.
However, the market also faces challenges. Concerns about the long-term effects of nanorobots in the human body, particularly in the brain, necessitate rigorous safety protocols. This has created a parallel market for advanced safety testing and monitoring systems, as well as for specialized training programs for healthcare professionals.
In conclusion, the market demand for safety protocols in nanorobots crossing the blood-brain barrier is robust and multifaceted. It is driven by medical needs, technological advancements, and the promise of revolutionary treatments. As research progresses and safety protocols are refined, the market is expected to expand further, potentially reshaping the landscape of neurological treatments and precision medicine.
The global nanomedicine market, which encompasses nanorobots and related technologies, is experiencing rapid growth. This expansion is fueled by the increasing prevalence of chronic diseases, the need for more effective drug delivery systems, and the potential for personalized medicine. The ability of nanorobots to cross the blood-brain barrier offers unprecedented opportunities for treating brain-related conditions, including cancers, neurodegenerative diseases, and psychiatric disorders.
Healthcare providers and pharmaceutical companies are showing keen interest in nanorobot technologies that can safely navigate the blood-brain barrier. This interest stems from the potential to improve treatment efficacy while minimizing side effects, a crucial factor in neurological therapies. The demand for such technologies is particularly high in regions with aging populations, where the incidence of neurodegenerative diseases is on the rise.
Investors and venture capitalists are also recognizing the market potential of safe nanorobot technologies. Funding for research and development in this field has seen a significant uptick in recent years, reflecting the anticipated market growth and the transformative potential of these technologies in healthcare.
The regulatory landscape is evolving to accommodate these emerging technologies, with regulatory bodies worldwide working to establish guidelines for the development and use of nanorobots in medical applications. This regulatory attention underscores the growing market demand and the need for robust safety protocols.
Patient advocacy groups and healthcare organizations are increasingly calling for innovative treatments for neurological conditions, further driving the demand for safe and effective nanorobot technologies. The potential for improved quality of life and better treatment outcomes is a significant factor in this demand.
However, the market also faces challenges. Concerns about the long-term effects of nanorobots in the human body, particularly in the brain, necessitate rigorous safety protocols. This has created a parallel market for advanced safety testing and monitoring systems, as well as for specialized training programs for healthcare professionals.
In conclusion, the market demand for safety protocols in nanorobots crossing the blood-brain barrier is robust and multifaceted. It is driven by medical needs, technological advancements, and the promise of revolutionary treatments. As research progresses and safety protocols are refined, the market is expected to expand further, potentially reshaping the landscape of neurological treatments and precision medicine.
BBB Crossing Challenges
The blood-brain barrier (BBB) presents a significant challenge for nanorobot-based drug delivery and therapeutic interventions in the central nervous system. This highly selective semipermeable border of endothelial cells prevents most substances from entering the brain, protecting it from potentially harmful agents. However, this protective mechanism also hinders the delivery of beneficial treatments for neurological disorders.
Nanorobots designed to cross the BBB face several obstacles. Firstly, the tight junctions between endothelial cells form a physical barrier that restricts paracellular transport. These junctions are composed of various proteins, including claudins, occludins, and junctional adhesion molecules, which create a seal between adjacent cells. Nanorobots must navigate through or around these tight junctions without disrupting the BBB's integrity.
Secondly, the BBB exhibits low transcellular permeability due to the limited presence of transport vesicles and fenestrations in brain endothelial cells. This characteristic impedes the passage of nanorobots through the cellular membrane, requiring innovative strategies for transcytosis or alternative transport mechanisms.
The presence of efflux transporters, such as P-glycoprotein and multidrug resistance-associated proteins, poses another challenge. These proteins actively pump out foreign substances that manage to enter the endothelial cells, potentially expelling nanorobots before they can reach the brain parenchyma.
Furthermore, the BBB's enzymatic barrier, consisting of various metabolizing enzymes, may degrade or alter nanorobots during their passage. This enzymatic activity can compromise the functionality and safety of the nanorobots, necessitating protective measures or enzyme-resistant designs.
The immune response of the central nervous system adds another layer of complexity. Microglia and astrocytes, the resident immune cells of the brain, may recognize nanorobots as foreign entities and initiate an inflammatory response. This immune reaction could lead to the clearance of nanorobots or cause unintended damage to surrounding neural tissue.
Lastly, the heterogeneity of the BBB across different brain regions and its dynamic nature in response to various physiological and pathological conditions present additional challenges. Nanorobots must be adaptable to these variations to ensure consistent and reliable crossing of the BBB throughout the brain.
Addressing these challenges requires a multidisciplinary approach, combining nanotechnology, materials science, neurobiology, and pharmacology. Researchers must develop innovative strategies to enhance BBB permeability without compromising its protective function, design nanorobots capable of targeted and controlled BBB crossing, and ensure the safety and efficacy of these interventions in the complex environment of the central nervous system.
Nanorobots designed to cross the BBB face several obstacles. Firstly, the tight junctions between endothelial cells form a physical barrier that restricts paracellular transport. These junctions are composed of various proteins, including claudins, occludins, and junctional adhesion molecules, which create a seal between adjacent cells. Nanorobots must navigate through or around these tight junctions without disrupting the BBB's integrity.
Secondly, the BBB exhibits low transcellular permeability due to the limited presence of transport vesicles and fenestrations in brain endothelial cells. This characteristic impedes the passage of nanorobots through the cellular membrane, requiring innovative strategies for transcytosis or alternative transport mechanisms.
The presence of efflux transporters, such as P-glycoprotein and multidrug resistance-associated proteins, poses another challenge. These proteins actively pump out foreign substances that manage to enter the endothelial cells, potentially expelling nanorobots before they can reach the brain parenchyma.
Furthermore, the BBB's enzymatic barrier, consisting of various metabolizing enzymes, may degrade or alter nanorobots during their passage. This enzymatic activity can compromise the functionality and safety of the nanorobots, necessitating protective measures or enzyme-resistant designs.
The immune response of the central nervous system adds another layer of complexity. Microglia and astrocytes, the resident immune cells of the brain, may recognize nanorobots as foreign entities and initiate an inflammatory response. This immune reaction could lead to the clearance of nanorobots or cause unintended damage to surrounding neural tissue.
Lastly, the heterogeneity of the BBB across different brain regions and its dynamic nature in response to various physiological and pathological conditions present additional challenges. Nanorobots must be adaptable to these variations to ensure consistent and reliable crossing of the BBB throughout the brain.
Addressing these challenges requires a multidisciplinary approach, combining nanotechnology, materials science, neurobiology, and pharmacology. Researchers must develop innovative strategies to enhance BBB permeability without compromising its protective function, design nanorobots capable of targeted and controlled BBB crossing, and ensure the safety and efficacy of these interventions in the complex environment of the central nervous system.
Current BBB Safety Protocols
01 Safety protocols for nanorobot design and operation
Implementing robust safety protocols in the design and operation of nanorobots is crucial. This includes fail-safe mechanisms, controlled navigation systems, and strict operational guidelines to prevent unintended interactions with biological systems. Advanced control algorithms and real-time monitoring systems are employed to ensure nanorobots function within predefined safety parameters.- Safety protocols for nanorobot design and operation: Implementing robust safety protocols in the design and operation of nanorobots is crucial. This includes fail-safe mechanisms, controlled navigation systems, and strict operational guidelines to prevent unintended interactions with biological systems. Advanced monitoring and control systems are integrated to ensure nanorobots function within predefined safety parameters.
- Biocompatibility and biodegradability of nanorobots: Developing nanorobots using biocompatible and biodegradable materials is essential for minimizing potential adverse effects on the human body. This approach ensures that nanorobots can safely dissolve or be eliminated from the body after completing their intended tasks, reducing long-term safety concerns.
- Electromagnetic shielding for nanorobot protection: Implementing electromagnetic shielding techniques in nanorobot design helps protect these devices from external electromagnetic interference. This enhances their operational stability and safety, preventing unintended actions or malfunctions that could pose risks to the host organism.
- Nanorobot swarm behavior control and safety: Developing advanced algorithms and control systems for managing nanorobot swarm behavior is crucial for safety. These systems ensure coordinated action, prevent uncontrolled replication, and maintain swarm cohesion, reducing risks associated with large-scale nanorobot deployments in biological environments.
- Ethical and regulatory frameworks for nanorobot deployment: Establishing comprehensive ethical guidelines and regulatory frameworks for nanorobot development and deployment is essential. This includes addressing privacy concerns, defining acceptable use cases, and implementing strict testing and approval processes to ensure the safe integration of nanorobotic technologies in medical and other applications.
02 Biocompatibility and biodegradability of nanorobots
Developing nanorobots using biocompatible and biodegradable materials is essential for minimizing potential adverse effects on the human body. This approach ensures that nanorobots can safely interact with biological tissues and be naturally eliminated from the body after completing their tasks, reducing long-term health risks.Expand Specific Solutions03 Nanorobot detection and tracking systems
Implementing advanced detection and tracking systems for nanorobots is crucial for safety monitoring. These systems use various imaging technologies and sensors to precisely locate and monitor nanorobots within the body, enabling real-time assessment of their behavior and potential interactions with biological systems.Expand Specific Solutions04 Ethical and regulatory frameworks for nanorobot use
Establishing comprehensive ethical and regulatory frameworks is essential for ensuring the safe development and application of nanorobots. These frameworks address issues such as privacy, consent, and potential misuse, while also setting standards for clinical trials and long-term safety assessments of nanorobot technologies.Expand Specific Solutions05 Environmental impact and containment of nanorobots
Assessing and mitigating the potential environmental impact of nanorobots is crucial for overall safety. This includes developing containment strategies to prevent unintended release into the environment, as well as studying the long-term effects of nanorobots on ecosystems and implementing proper disposal methods.Expand Specific Solutions
Key Nanorobot Developers
The research on safety protocols for nanorobots crossing the blood-brain barrier is in an early developmental stage, with a growing market potential due to its applications in targeted drug delivery and neurological treatments. The technology is still emerging, with varying levels of maturity among key players. Academic institutions like Zhejiang University, Peking University, and California Institute of Technology are conducting fundamental research, while companies such as Stryker Corp. and Genentech, Inc. are exploring practical applications. Government agencies and research organizations like Institut Pasteur and CNRS are also contributing to the field's advancement. The competitive landscape is diverse, with collaborations between academia and industry driving innovation in this complex and promising area of nanotechnology.
Genentech, Inc.
Technical Solution: Genentech has pioneered a safety protocol for nanorobots crossing the BBB based on their expertise in antibody engineering. Their nanorobots are coated with engineered antibody fragments that specifically bind to transporters on the BBB, facilitating a receptor-mediated transcytosis[1]. The nanorobots are programmed with a time-limited active state, after which they automatically switch to an inert form[3]. Genentech has also developed a proprietary "molecular GPS" system that allows real-time tracking of nanorobot distribution in the brain using non-invasive imaging techniques[5]. This system enables precise control over nanorobot activity and location.
Strengths: Highly specific BBB targeting, built-in deactivation mechanism, advanced real-time monitoring capabilities. Weaknesses: Potential for immunogenicity due to antibody-based approach, may require frequent dosing due to time-limited activity.
Massachusetts Institute of Technology
Technical Solution: MIT has developed a novel approach for nanorobot safety when crossing the blood-brain barrier (BBB). Their system utilizes biodegradable nanoparticles coated with a specialized polymer that mimics the surface of red blood cells[1]. This coating allows the nanorobots to evade the immune system and cross the BBB more efficiently. The nanorobots are equipped with real-time biosensors that monitor local tissue conditions and adjust their behavior accordingly[3]. Additionally, MIT researchers have implemented a fail-safe mechanism that causes the nanorobots to disintegrate if they detect potential harmful interactions with brain tissue[5].
Strengths: Advanced biomimicry for improved BBB penetration, real-time adaptive behavior, and built-in safety mechanisms. Weaknesses: Potential complexity in manufacturing and scaling up production, possible long-term effects of biodegradable materials in the brain still under investigation.
Core BBB Safety Innovations
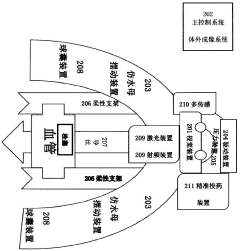
Nanoscale drug carrier capable of passing through blood-brain barrier
PatentActiveUS20200138960A1
Innovation
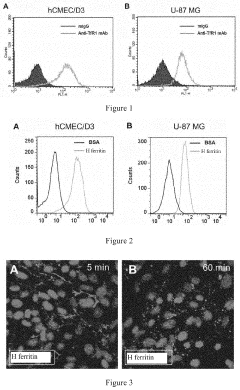
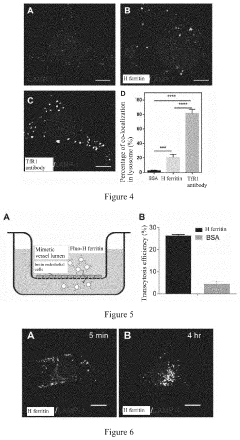
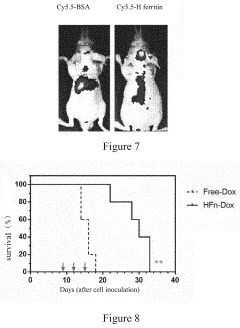
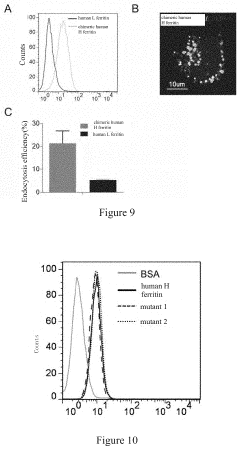
- A nanoscale drug delivery system using an all-heavy-chain human ferritin or its functional fragments, which self-assemble into a cage protein capable of binding to the Transferrin Receptor 1 (TfR1) on brain endothelial cells, allowing receptor-mediated transcytosis across the BBB, thereby targeting brain lesions.
Intravascular NANO robot apparatus, optimized control system, and method
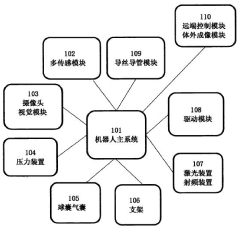
PatentPendingAU2022244782A1
Innovation
- An intravascular nano-robot device equipped with a visual recognition module, multi-sensing module, and ablative tools like lasers and radio frequency devices, guided by neural networks for real-time monitoring and treatment, allowing for precise recognition and treatment of vascular diseases without invasive trauma.
Regulatory Framework
The regulatory framework surrounding nanorobots crossing the blood-brain barrier (BBB) is a complex and evolving landscape. As this technology advances, regulatory bodies worldwide are grappling with the need to establish comprehensive guidelines that ensure safety while fostering innovation. Currently, there is no unified global regulatory approach specifically tailored to nanorobots crossing the BBB, but existing frameworks for nanomedicine and medical devices serve as a foundation.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of nanorobots through its Center for Devices and Radiological Health (CDRH) and the Center for Drug Evaluation and Research (CDER). The FDA has issued guidance documents on nanotechnology applications in medical products, which provide a basis for evaluating the safety and efficacy of nanorobots. However, specific protocols for BBB crossing are still in development.
The European Medicines Agency (EMA) has also been proactive in addressing nanotechnology-based medical applications. Their guidelines on nanomedicines cover aspects of quality, safety, and efficacy, which can be applied to nanorobots. The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation may also come into play when considering the materials used in nanorobot construction.
International organizations such as the International Organization for Standardization (ISO) and the Organisation for Economic Co-operation and Development (OECD) have developed standards and guidelines for nanotechnology safety assessment. These provide a framework for evaluating potential risks associated with nanoparticles, which can be adapted for nanorobots crossing the BBB.
Key regulatory considerations for nanorobots include biocompatibility, biodegradability, and potential long-term effects on brain function. Regulatory bodies are particularly concerned with the ability to control and monitor nanorobots once they have crossed the BBB, as well as their potential interactions with brain tissue and neurotransmitters.
As research progresses, it is anticipated that more specific regulatory guidelines will emerge. Collaborative efforts between regulatory agencies, researchers, and industry stakeholders are crucial in developing appropriate safety protocols. These protocols will likely encompass pre-clinical testing requirements, clinical trial designs specific to BBB-crossing nanorobots, and post-market surveillance strategies.
The regulatory framework must also address ethical considerations, such as privacy concerns related to potential data collection by nanorobots in the brain. As the technology advances, regulations will need to evolve to keep pace with new discoveries and potential applications, ensuring a balance between innovation and patient safety.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of nanorobots through its Center for Devices and Radiological Health (CDRH) and the Center for Drug Evaluation and Research (CDER). The FDA has issued guidance documents on nanotechnology applications in medical products, which provide a basis for evaluating the safety and efficacy of nanorobots. However, specific protocols for BBB crossing are still in development.
The European Medicines Agency (EMA) has also been proactive in addressing nanotechnology-based medical applications. Their guidelines on nanomedicines cover aspects of quality, safety, and efficacy, which can be applied to nanorobots. The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation may also come into play when considering the materials used in nanorobot construction.
International organizations such as the International Organization for Standardization (ISO) and the Organisation for Economic Co-operation and Development (OECD) have developed standards and guidelines for nanotechnology safety assessment. These provide a framework for evaluating potential risks associated with nanoparticles, which can be adapted for nanorobots crossing the BBB.
Key regulatory considerations for nanorobots include biocompatibility, biodegradability, and potential long-term effects on brain function. Regulatory bodies are particularly concerned with the ability to control and monitor nanorobots once they have crossed the BBB, as well as their potential interactions with brain tissue and neurotransmitters.
As research progresses, it is anticipated that more specific regulatory guidelines will emerge. Collaborative efforts between regulatory agencies, researchers, and industry stakeholders are crucial in developing appropriate safety protocols. These protocols will likely encompass pre-clinical testing requirements, clinical trial designs specific to BBB-crossing nanorobots, and post-market surveillance strategies.
The regulatory framework must also address ethical considerations, such as privacy concerns related to potential data collection by nanorobots in the brain. As the technology advances, regulations will need to evolve to keep pace with new discoveries and potential applications, ensuring a balance between innovation and patient safety.
Ethical Considerations
The development of nanorobots capable of crossing the blood-brain barrier presents significant ethical considerations that must be carefully addressed. One primary concern is the potential for unintended consequences on brain function and cognitive processes. As these nanorobots interact with the delicate neural networks, there is a risk of altering brain chemistry or neural pathways in ways that could affect an individual's personality, memory, or decision-making abilities. This raises questions about personal identity and autonomy, as well as the ethical implications of potentially modifying human cognition.
Privacy and data security are also critical ethical issues in this field. Nanorobots operating within the brain could potentially access and transmit sensitive neural information. Ensuring the protection of this data from unauthorized access or misuse is paramount. Additionally, there are concerns about the potential for these technologies to be used for surveillance or mind control, which would have profound implications for individual freedom and human rights.
The issue of informed consent is particularly complex when dealing with nanorobots that can cross the blood-brain barrier. Patients must be fully aware of the potential risks and long-term effects of such interventions, which may not be entirely predictable. This raises questions about how to effectively communicate these risks and obtain truly informed consent, especially in cases where the technology might be used to treat cognitive disorders that could affect a patient's decision-making capacity.
Equity and access to these advanced technologies are also important ethical considerations. As with many cutting-edge medical treatments, there is a risk that nanorobot therapies could exacerbate existing healthcare disparities, with only wealthy individuals or nations having access to these potentially life-changing interventions. Ensuring fair distribution and access to such technologies is crucial to prevent further widening of global health inequalities.
The potential for dual-use or misuse of nanorobots capable of crossing the blood-brain barrier must also be considered. While the primary intent may be therapeutic, these technologies could potentially be weaponized or used for enhancement purposes beyond medical necessity. This raises ethical questions about the limits of human enhancement and the potential for creating unfair advantages or societal divisions.
Lastly, the long-term effects of introducing artificial nanostructures into the brain environment are not fully understood. There are ethical concerns about the potential for these nanorobots to cause unforeseen changes in brain structure or function over time, possibly leading to new neurological conditions or altering the course of human cognitive evolution. Rigorous long-term studies and ongoing ethical oversight will be essential to address these concerns and ensure the responsible development and application of this technology.
Privacy and data security are also critical ethical issues in this field. Nanorobots operating within the brain could potentially access and transmit sensitive neural information. Ensuring the protection of this data from unauthorized access or misuse is paramount. Additionally, there are concerns about the potential for these technologies to be used for surveillance or mind control, which would have profound implications for individual freedom and human rights.
The issue of informed consent is particularly complex when dealing with nanorobots that can cross the blood-brain barrier. Patients must be fully aware of the potential risks and long-term effects of such interventions, which may not be entirely predictable. This raises questions about how to effectively communicate these risks and obtain truly informed consent, especially in cases where the technology might be used to treat cognitive disorders that could affect a patient's decision-making capacity.
Equity and access to these advanced technologies are also important ethical considerations. As with many cutting-edge medical treatments, there is a risk that nanorobot therapies could exacerbate existing healthcare disparities, with only wealthy individuals or nations having access to these potentially life-changing interventions. Ensuring fair distribution and access to such technologies is crucial to prevent further widening of global health inequalities.
The potential for dual-use or misuse of nanorobots capable of crossing the blood-brain barrier must also be considered. While the primary intent may be therapeutic, these technologies could potentially be weaponized or used for enhancement purposes beyond medical necessity. This raises ethical questions about the limits of human enhancement and the potential for creating unfair advantages or societal divisions.
Lastly, the long-term effects of introducing artificial nanostructures into the brain environment are not fully understood. There are ethical concerns about the potential for these nanorobots to cause unforeseen changes in brain structure or function over time, possibly leading to new neurological conditions or altering the course of human cognitive evolution. Rigorous long-term studies and ongoing ethical oversight will be essential to address these concerns and ensure the responsible development and application of this technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!