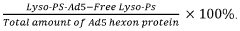How To Mitigate Immune Reactions To Medical Nanorobots
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nanorobot Immunogenicity Challenges and Objectives
Nanorobots represent a revolutionary frontier in medical technology, offering unprecedented potential for targeted drug delivery, precision surgery, and real-time diagnostics. However, their application faces a significant challenge: the human immune system's natural response to foreign entities. This immune reaction can severely impede the efficacy and safety of nanorobots, potentially rendering them ineffective or even harmful.
The primary objective in mitigating immune reactions to medical nanorobots is to develop strategies that allow these microscopic machines to operate within the human body without triggering a significant immune response. This goal encompasses several key aspects, including enhancing the biocompatibility of nanorobot materials, designing stealth mechanisms to evade immune detection, and developing methods to modulate the immune system's response.
One crucial aim is to create nanorobots that can persist in the bloodstream long enough to complete their intended tasks without being rapidly cleared by the immune system. This involves engineering surfaces that minimize protein adsorption and subsequent recognition by immune cells. Additionally, researchers are exploring ways to mimic the body's own cells, potentially using cell membrane coatings derived from the patient's own cells to camouflage the nanorobots.
Another important objective is to prevent the formation of a "protein corona" around the nanorobots. This protein layer, which forms when nanoparticles enter biological fluids, can significantly alter their properties and increase their visibility to the immune system. Developing strategies to control or prevent corona formation is essential for maintaining the intended functionality of nanorobots in vivo.
Furthermore, there is a need to understand and potentially exploit the body's own immune tolerance mechanisms. This could involve designing nanorobots that interact with regulatory T cells or other immunosuppressive components of the immune system to induce a state of local or systemic tolerance.
Lastly, a critical objective is to develop methods for real-time monitoring and control of immune responses to nanorobots. This could involve incorporating sensors into the nanorobots themselves or developing companion diagnostic tools to detect early signs of an immune reaction, allowing for timely intervention or adjustment of treatment strategies.
Achieving these objectives will require interdisciplinary collaboration between nanotechnologists, immunologists, materials scientists, and clinicians. The successful mitigation of immune reactions to medical nanorobots has the potential to unlock a new era of nanomedicine, enabling transformative therapies for a wide range of diseases and conditions.
The primary objective in mitigating immune reactions to medical nanorobots is to develop strategies that allow these microscopic machines to operate within the human body without triggering a significant immune response. This goal encompasses several key aspects, including enhancing the biocompatibility of nanorobot materials, designing stealth mechanisms to evade immune detection, and developing methods to modulate the immune system's response.
One crucial aim is to create nanorobots that can persist in the bloodstream long enough to complete their intended tasks without being rapidly cleared by the immune system. This involves engineering surfaces that minimize protein adsorption and subsequent recognition by immune cells. Additionally, researchers are exploring ways to mimic the body's own cells, potentially using cell membrane coatings derived from the patient's own cells to camouflage the nanorobots.
Another important objective is to prevent the formation of a "protein corona" around the nanorobots. This protein layer, which forms when nanoparticles enter biological fluids, can significantly alter their properties and increase their visibility to the immune system. Developing strategies to control or prevent corona formation is essential for maintaining the intended functionality of nanorobots in vivo.
Furthermore, there is a need to understand and potentially exploit the body's own immune tolerance mechanisms. This could involve designing nanorobots that interact with regulatory T cells or other immunosuppressive components of the immune system to induce a state of local or systemic tolerance.
Lastly, a critical objective is to develop methods for real-time monitoring and control of immune responses to nanorobots. This could involve incorporating sensors into the nanorobots themselves or developing companion diagnostic tools to detect early signs of an immune reaction, allowing for timely intervention or adjustment of treatment strategies.
Achieving these objectives will require interdisciplinary collaboration between nanotechnologists, immunologists, materials scientists, and clinicians. The successful mitigation of immune reactions to medical nanorobots has the potential to unlock a new era of nanomedicine, enabling transformative therapies for a wide range of diseases and conditions.
Market Analysis for Immune-Compatible Nanorobots
The market for immune-compatible nanorobots represents a rapidly growing segment within the broader field of nanomedicine. As the potential applications of medical nanorobots expand, the demand for solutions that can effectively mitigate immune reactions becomes increasingly critical. Current market estimates suggest that the global nanomedicine market, which encompasses nanorobots, is projected to reach significant value in the coming years, driven by advancements in nanotechnology and increasing investments in research and development.
The primary market drivers for immune-compatible nanorobots include the rising prevalence of chronic diseases, the need for targeted drug delivery systems, and the growing emphasis on personalized medicine. Healthcare providers and pharmaceutical companies are showing keen interest in nanorobotic technologies that can navigate the human body without triggering adverse immune responses, as these solutions promise to enhance treatment efficacy while minimizing side effects.
Geographically, North America and Europe currently lead the market for immune-compatible nanorobots, owing to their advanced healthcare infrastructure and substantial investments in nanotechnology research. However, Asia-Pacific is emerging as a rapidly growing market, fueled by increasing healthcare expenditure and rising awareness of innovative medical technologies in countries like China, Japan, and South Korea.
The market landscape is characterized by a mix of established pharmaceutical companies, biotechnology firms, and specialized nanotechnology startups. Collaborations between academic institutions and industry players are becoming increasingly common, accelerating the development of novel immune-compatible nanorobotic solutions.
Key market segments for immune-compatible nanorobots include oncology, cardiovascular diseases, neurological disorders, and infectious diseases. Among these, oncology represents the largest market share, as nanorobots offer promising solutions for targeted cancer therapies with reduced systemic toxicity.
Despite the promising outlook, several challenges persist in the market. Regulatory hurdles, concerns about long-term safety, and the high costs associated with research and development are potential barriers to market growth. Additionally, public perception and ethical considerations surrounding nanorobotic technologies may influence market adoption rates.
Looking ahead, the market for immune-compatible nanorobots is expected to witness significant technological advancements and increased commercial viability. As research progresses in areas such as biomimetic nanorobot design and smart materials, the potential applications and market opportunities for immune-compatible nanorobots are likely to expand further, potentially revolutionizing various aspects of medical treatment and diagnostics.
The primary market drivers for immune-compatible nanorobots include the rising prevalence of chronic diseases, the need for targeted drug delivery systems, and the growing emphasis on personalized medicine. Healthcare providers and pharmaceutical companies are showing keen interest in nanorobotic technologies that can navigate the human body without triggering adverse immune responses, as these solutions promise to enhance treatment efficacy while minimizing side effects.
Geographically, North America and Europe currently lead the market for immune-compatible nanorobots, owing to their advanced healthcare infrastructure and substantial investments in nanotechnology research. However, Asia-Pacific is emerging as a rapidly growing market, fueled by increasing healthcare expenditure and rising awareness of innovative medical technologies in countries like China, Japan, and South Korea.
The market landscape is characterized by a mix of established pharmaceutical companies, biotechnology firms, and specialized nanotechnology startups. Collaborations between academic institutions and industry players are becoming increasingly common, accelerating the development of novel immune-compatible nanorobotic solutions.
Key market segments for immune-compatible nanorobots include oncology, cardiovascular diseases, neurological disorders, and infectious diseases. Among these, oncology represents the largest market share, as nanorobots offer promising solutions for targeted cancer therapies with reduced systemic toxicity.
Despite the promising outlook, several challenges persist in the market. Regulatory hurdles, concerns about long-term safety, and the high costs associated with research and development are potential barriers to market growth. Additionally, public perception and ethical considerations surrounding nanorobotic technologies may influence market adoption rates.
Looking ahead, the market for immune-compatible nanorobots is expected to witness significant technological advancements and increased commercial viability. As research progresses in areas such as biomimetic nanorobot design and smart materials, the potential applications and market opportunities for immune-compatible nanorobots are likely to expand further, potentially revolutionizing various aspects of medical treatment and diagnostics.
Current State of Nanorobot Immune Evasion Techniques
The current state of nanorobot immune evasion techniques represents a critical frontier in nanomedicine, with significant progress made in recent years. Researchers have developed several strategies to mitigate immune reactions to medical nanorobots, addressing the primary challenge of avoiding detection and elimination by the body's immune system.
One of the most promising approaches is the use of stealth coatings. These coatings, often composed of hydrophilic polymers such as polyethylene glycol (PEG), create a hydration layer around the nanorobot, effectively masking it from immune recognition. PEGylation has shown considerable success in prolonging the circulation time of nanorobots in the bloodstream, reducing opsonization and subsequent phagocytosis by macrophages.
Another innovative technique involves biomimetic strategies, where nanorobots are designed to mimic the surface properties of native cells. By incorporating cell membrane components or entire cell membranes onto the nanorobot surface, researchers have created "cloaked" nanorobots that can evade immune surveillance. For instance, nanorobots coated with erythrocyte membranes have demonstrated enhanced circulation times and reduced immunogenicity.
Active camouflage mechanisms are also being explored, where nanorobots can dynamically alter their surface properties in response to their environment. This includes the development of stimuli-responsive materials that can change their conformation or charge based on pH, temperature, or other physiological cues, allowing nanorobots to adapt to different biological compartments and evade immune detection.
Immunomodulation is another avenue being investigated, where nanorobots are designed to actively suppress or redirect immune responses. This can involve the incorporation of immunosuppressive drugs or the engineering of surface ligands that interact with immune cells to induce tolerance rather than activation.
Size and shape optimization of nanorobots has also proven crucial in immune evasion. Researchers have found that certain geometries and dimensions can influence the rate of immune clearance, with smaller and more streamlined shapes generally exhibiting improved evasion capabilities.
Despite these advancements, challenges remain. The long-term effects of repeated nanorobot administration on the immune system are not fully understood, and there are concerns about potential immunotoxicity. Additionally, while current techniques have shown promise in animal models, their efficacy in human systems requires further investigation.
As the field progresses, combination strategies that integrate multiple immune evasion techniques are emerging as a promising direction. These hybrid approaches aim to create synergistic effects, potentially offering more robust and versatile immune evasion capabilities for medical nanorobots.
One of the most promising approaches is the use of stealth coatings. These coatings, often composed of hydrophilic polymers such as polyethylene glycol (PEG), create a hydration layer around the nanorobot, effectively masking it from immune recognition. PEGylation has shown considerable success in prolonging the circulation time of nanorobots in the bloodstream, reducing opsonization and subsequent phagocytosis by macrophages.
Another innovative technique involves biomimetic strategies, where nanorobots are designed to mimic the surface properties of native cells. By incorporating cell membrane components or entire cell membranes onto the nanorobot surface, researchers have created "cloaked" nanorobots that can evade immune surveillance. For instance, nanorobots coated with erythrocyte membranes have demonstrated enhanced circulation times and reduced immunogenicity.
Active camouflage mechanisms are also being explored, where nanorobots can dynamically alter their surface properties in response to their environment. This includes the development of stimuli-responsive materials that can change their conformation or charge based on pH, temperature, or other physiological cues, allowing nanorobots to adapt to different biological compartments and evade immune detection.
Immunomodulation is another avenue being investigated, where nanorobots are designed to actively suppress or redirect immune responses. This can involve the incorporation of immunosuppressive drugs or the engineering of surface ligands that interact with immune cells to induce tolerance rather than activation.
Size and shape optimization of nanorobots has also proven crucial in immune evasion. Researchers have found that certain geometries and dimensions can influence the rate of immune clearance, with smaller and more streamlined shapes generally exhibiting improved evasion capabilities.
Despite these advancements, challenges remain. The long-term effects of repeated nanorobot administration on the immune system are not fully understood, and there are concerns about potential immunotoxicity. Additionally, while current techniques have shown promise in animal models, their efficacy in human systems requires further investigation.
As the field progresses, combination strategies that integrate multiple immune evasion techniques are emerging as a promising direction. These hybrid approaches aim to create synergistic effects, potentially offering more robust and versatile immune evasion capabilities for medical nanorobots.
Existing Immune Mitigation Solutions for Nanorobots
01 Nanorobot design to minimize immune reactions
Developing medical nanorobots with surface modifications or coatings that reduce recognition by the immune system. This approach aims to prevent or minimize immune responses, allowing nanorobots to perform their intended functions without triggering unwanted reactions in the body.- Nanorobot design to minimize immune reactions: Developing medical nanorobots with surface modifications or coatings that reduce recognition by the immune system. This approach aims to prevent or minimize immune responses, allowing nanorobots to perform their intended functions without triggering an inflammatory reaction or being rapidly cleared from the body.
- Immune system-friendly materials for nanorobot construction: Utilizing biocompatible and biodegradable materials in the construction of medical nanorobots to reduce immune reactions. These materials are designed to be non-toxic and can be safely metabolized by the body, minimizing the risk of long-term immune responses or complications.
- Nanorobot-based immunomodulation: Developing nanorobots capable of actively modulating the immune system. These nanorobots can be programmed to release immunosuppressive agents or interact with immune cells to prevent unwanted immune reactions, potentially improving the efficacy of nanorobot-based treatments.
- Detection and mitigation of immune responses: Incorporating sensors and responsive mechanisms in medical nanorobots to detect and mitigate immune reactions in real-time. This approach allows nanorobots to adapt their behavior or release counter-measures when an immune response is detected, enhancing their survival and functionality in the body.
- Personalized nanorobot design based on individual immune profiles: Tailoring nanorobot designs to individual patients' immune profiles to minimize adverse reactions. This approach involves analyzing a patient's immune system characteristics and customizing the nanorobot's surface properties, materials, or functionalities to ensure optimal compatibility and reduced immune recognition.
02 Immune system modulation for nanorobot compatibility
Methods to temporarily suppress or modulate specific aspects of the immune system to improve the compatibility of medical nanorobots. This may involve the use of immunosuppressive agents or targeted approaches to reduce immune responses against the nanorobots while maintaining overall immune function.Expand Specific Solutions03 Biocompatible materials for nanorobot construction
Utilizing biocompatible and biodegradable materials in the construction of medical nanorobots to reduce immune reactions. These materials are designed to be recognized as 'self' by the immune system or to degrade harmlessly in the body after completing their tasks.Expand Specific Solutions04 Nanorobot-based immunotherapy
Developing nanorobots that can interact with the immune system in beneficial ways, such as enhancing immune responses against cancer cells or pathogens. These nanorobots are designed to work in harmony with the immune system rather than triggering adverse reactions.Expand Specific Solutions05 Real-time monitoring and control of immune responses
Implementing systems for real-time monitoring of immune reactions to medical nanorobots and developing mechanisms for rapid intervention or control. This approach allows for immediate adjustments to nanorobot function or deployment based on detected immune responses.Expand Specific Solutions
Key Players in Medical Nanorobotics and Immunology
The field of mitigating immune reactions to medical nanorobots is in an early developmental stage, with significant potential for growth. The market size is expanding as nanorobotics advances, but remains relatively small. Technical maturity is low, with ongoing research to overcome immunological challenges. Key players like Selecta Biosciences and Rigel Pharmaceuticals are developing nanoparticle-based immunomodulatory approaches. Academic institutions such as MIT, Johns Hopkins, and Tianjin University are contributing fundamental research. Collaborations between industry and academia, exemplified by partnerships involving companies like Dynavax Technologies, are driving progress in this emerging field.
Selecta Biosciences, Inc.
Technical Solution: Selecta Biosciences has developed a proprietary immune tolerance platform called ImmTOR™ to mitigate immune reactions to medical nanorobots and other therapeutic agents. The ImmTOR platform uses biodegradable nanoparticles encapsulating rapamycin, an immunomodulatory drug, to induce antigen-specific immune tolerance[2]. When co-administered with medical nanorobots, ImmTOR nanoparticles selectively target antigen-presenting cells in the liver and spleen, promoting the generation of regulatory T cells that suppress unwanted immune responses. This approach has shown promise in preventing anti-drug antibodies (ADAs) and reducing pre-existing ADAs in various preclinical and clinical studies[4]. Selecta has also explored combining ImmTOR with AAV gene therapy vectors, which could be applied to nanorobot-based gene delivery systems to enhance their efficacy and safety profile[6].
Strengths: Proprietary ImmTOR platform for inducing antigen-specific tolerance; Demonstrated efficacy in preventing and reducing anti-drug antibodies; Potential for combination with gene therapy vectors. Weaknesses: Reliance on a single immunomodulatory drug (rapamycin); May require repeated administrations for long-term effectiveness.
National Center for Nanoscience & Technology
Technical Solution: The National Center for Nanoscience & Technology (NCNST) in China has developed innovative approaches to mitigate immune reactions to medical nanorobots. One of their key strategies involves the design of "stealth" nanoparticles using a combination of PEGylation and zwitterionic surface modifications[10]. This dual-modification approach creates a highly hydrated shell around the nanorobots, effectively reducing protein adsorption and complement activation. NCNST researchers have also explored the use of cell membrane-coated nanorobots, which leverage the natural immune-evading properties of cell membranes to camouflage the synthetic nanostructures[11]. Additionally, the center has investigated the incorporation of immunosuppressive drugs, such as rapamycin and dexamethasone, into the nanorobot structure for localized immune modulation. NCNST has further developed stimuli-responsive nanorobots that can alter their surface properties in response to specific physiological triggers, allowing for dynamic immune evasion strategies[12].
Strengths: Combination of PEGylation and zwitterionic modifications for enhanced stealth properties; Use of cell membrane-coated nanorobots for natural camouflage; Development of stimuli-responsive nanorobots. Weaknesses: Potential challenges in scaling up production of complex nanorobot designs; May require further in vivo studies to validate long-term safety and efficacy.
Core Innovations in Nanorobot Immune Evasion
Compositions and methods for reducing antigen-specific immunogenicity
PatentPendingUS20220016241A1
Innovation
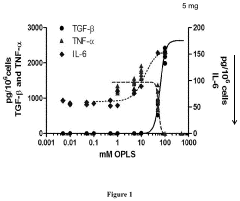
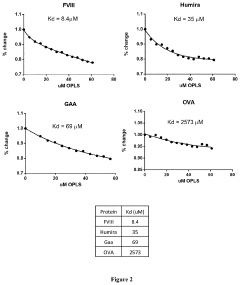
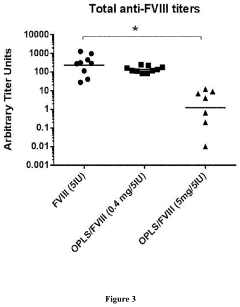
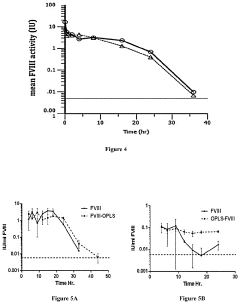
- The use of lipidic particles, specifically O-Phospho-L-Serine (OPLS) or lipidic compositions, in conjunction with antigens, either concomitantly or sequentially, to reduce immunogenicity and improve pharmacokinetic properties by inducing immune tolerance through specific immunological mechanisms, including increased T regulatory cells and decreased antigen-specific antibody titers, without affecting general immune competence.
Composition for immune tolerance induction and use in gene therapy
PatentWO2022159603A1
Innovation
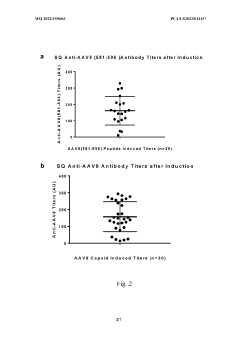
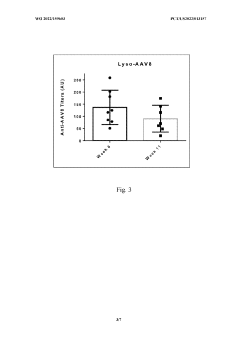
- The use of lyso-PS liposomes complexed to viral vector proteins to induce immune tolerance by down-regulating immune responses, specifically targeting antigenic peptides like AAV8 581-596, thereby reducing pre-existing antibody titers and enhancing gene therapy delivery.
Regulatory Framework for Medical Nanorobots
The regulatory framework for medical nanorobots is a critical aspect of their development and implementation. As these devices operate at the nanoscale and interact directly with biological systems, stringent regulations are necessary to ensure safety and efficacy. Currently, no specific regulatory framework exists for medical nanorobots, but they fall under the broader category of nanotechnology-based medical devices.
Regulatory bodies such as the FDA in the United States and the EMA in Europe are working to adapt existing regulations to accommodate the unique challenges posed by nanorobots. These agencies are focusing on developing guidelines for the evaluation of safety, efficacy, and quality control of nanorobotic systems. Key areas of regulatory concern include the potential for unintended interactions with biological systems, long-term effects, and the ability to control and monitor nanorobots in vivo.
One of the primary regulatory challenges is the classification of medical nanorobots. Depending on their specific function and mode of action, they may be categorized as drugs, devices, or combination products. This classification determines the regulatory pathway and requirements for approval. For instance, nanorobots designed to deliver drugs might be regulated as combination products, requiring compliance with both drug and device regulations.
Safety assessment is a crucial component of the regulatory framework. Regulators are particularly concerned with the potential for nanorobots to trigger immune responses or cause unintended damage to tissues. As such, manufacturers are required to conduct extensive preclinical and clinical studies to demonstrate the safety profile of their nanorobotic systems. This includes evaluating the biodistribution, clearance mechanisms, and potential for accumulation in organs.
The regulatory framework also addresses manufacturing and quality control standards. Given the complexity and precision required in nanorobot production, regulators are developing guidelines for Good Manufacturing Practices (GMP) specific to nanorobotic systems. These guidelines cover aspects such as material purity, consistency in nanorobot size and structure, and sterility assurance.
Ethical considerations form another important aspect of the regulatory framework. Issues such as patient privacy, data security, and the potential for nanorobots to be used for non-medical purposes are being addressed. Regulators are working with ethicists and policy makers to develop guidelines that balance the potential benefits of medical nanorobots with ethical concerns and patient rights.
As the field of medical nanorobotics continues to advance, regulatory frameworks will need to evolve to keep pace with technological developments. This will likely involve ongoing collaboration between regulatory agencies, researchers, and industry stakeholders to ensure that regulations are both effective in protecting public health and conducive to innovation in this promising field.
Regulatory bodies such as the FDA in the United States and the EMA in Europe are working to adapt existing regulations to accommodate the unique challenges posed by nanorobots. These agencies are focusing on developing guidelines for the evaluation of safety, efficacy, and quality control of nanorobotic systems. Key areas of regulatory concern include the potential for unintended interactions with biological systems, long-term effects, and the ability to control and monitor nanorobots in vivo.
One of the primary regulatory challenges is the classification of medical nanorobots. Depending on their specific function and mode of action, they may be categorized as drugs, devices, or combination products. This classification determines the regulatory pathway and requirements for approval. For instance, nanorobots designed to deliver drugs might be regulated as combination products, requiring compliance with both drug and device regulations.
Safety assessment is a crucial component of the regulatory framework. Regulators are particularly concerned with the potential for nanorobots to trigger immune responses or cause unintended damage to tissues. As such, manufacturers are required to conduct extensive preclinical and clinical studies to demonstrate the safety profile of their nanorobotic systems. This includes evaluating the biodistribution, clearance mechanisms, and potential for accumulation in organs.
The regulatory framework also addresses manufacturing and quality control standards. Given the complexity and precision required in nanorobot production, regulators are developing guidelines for Good Manufacturing Practices (GMP) specific to nanorobotic systems. These guidelines cover aspects such as material purity, consistency in nanorobot size and structure, and sterility assurance.
Ethical considerations form another important aspect of the regulatory framework. Issues such as patient privacy, data security, and the potential for nanorobots to be used for non-medical purposes are being addressed. Regulators are working with ethicists and policy makers to develop guidelines that balance the potential benefits of medical nanorobots with ethical concerns and patient rights.
As the field of medical nanorobotics continues to advance, regulatory frameworks will need to evolve to keep pace with technological developments. This will likely involve ongoing collaboration between regulatory agencies, researchers, and industry stakeholders to ensure that regulations are both effective in protecting public health and conducive to innovation in this promising field.
Ethical Implications of Nanorobot Immune Modulation
The ethical implications of nanorobot immune modulation are complex and multifaceted, requiring careful consideration as this technology advances. One primary concern is the potential for unintended consequences on the human immune system. Altering immune responses, even temporarily, could leave patients vulnerable to opportunistic infections or disrupt the delicate balance of immune function. There are also questions about long-term effects and whether repeated nanorobot interventions could lead to permanent changes in immune regulation.
Privacy and autonomy issues arise when considering the use of nanorobots to modulate immune responses. The ability to manipulate the immune system at a nanoscale level raises concerns about bodily integrity and the right of individuals to maintain control over their own biological processes. There is a need to establish clear protocols for informed consent and ensure patients fully understand the potential risks and benefits of such interventions.
The equitable distribution of nanorobot-based immune therapies is another ethical consideration. As with many advanced medical technologies, there is a risk that these treatments could exacerbate existing healthcare disparities if access is limited to wealthy individuals or developed nations. Ensuring fair access to such potentially life-saving technologies will be crucial to avoid deepening societal inequalities.
The dual-use potential of immune-modulating nanorobots also presents ethical challenges. While designed for medical purposes, this technology could potentially be weaponized to create targeted biological agents or manipulate immune systems for nefarious purposes. Establishing robust safeguards and international regulations will be essential to prevent misuse.
Lastly, there are broader philosophical questions about the nature of human identity and the boundaries of medical intervention. As nanorobots become capable of fine-tuning our immune responses, we must grapple with how much technological control over our biological processes is desirable or ethically acceptable. This touches on fundamental debates about human enhancement and the line between treatment and augmentation.
Privacy and autonomy issues arise when considering the use of nanorobots to modulate immune responses. The ability to manipulate the immune system at a nanoscale level raises concerns about bodily integrity and the right of individuals to maintain control over their own biological processes. There is a need to establish clear protocols for informed consent and ensure patients fully understand the potential risks and benefits of such interventions.
The equitable distribution of nanorobot-based immune therapies is another ethical consideration. As with many advanced medical technologies, there is a risk that these treatments could exacerbate existing healthcare disparities if access is limited to wealthy individuals or developed nations. Ensuring fair access to such potentially life-saving technologies will be crucial to avoid deepening societal inequalities.
The dual-use potential of immune-modulating nanorobots also presents ethical challenges. While designed for medical purposes, this technology could potentially be weaponized to create targeted biological agents or manipulate immune systems for nefarious purposes. Establishing robust safeguards and international regulations will be essential to prevent misuse.
Lastly, there are broader philosophical questions about the nature of human identity and the boundaries of medical intervention. As nanorobots become capable of fine-tuning our immune responses, we must grapple with how much technological control over our biological processes is desirable or ethically acceptable. This touches on fundamental debates about human enhancement and the line between treatment and augmentation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!