How to Improve Gel Electrophoresis Throughput?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gel Electrophoresis Evolution and Objectives
Gel electrophoresis has been a cornerstone technique in molecular biology since its inception in the 1960s. This method, which separates molecules based on their size and electrical charge, has undergone significant evolution over the decades. Initially developed for protein analysis, it quickly found applications in DNA and RNA separation, becoming an indispensable tool in genomics research and diagnostics.
The evolution of gel electrophoresis has been marked by several key advancements. The transition from slab gels to capillary electrophoresis in the 1980s dramatically improved resolution and speed. The introduction of pulsed-field gel electrophoresis (PFGE) in 1984 enabled the separation of much larger DNA fragments, expanding the technique's utility in genomic studies. More recently, the development of microfluidic devices has further miniaturized and accelerated the process.
Despite these improvements, the increasing demands of high-throughput genomics and proteomics have highlighted the need for even greater efficiency in gel electrophoresis. The primary objective in improving gel electrophoresis throughput is to increase the speed and volume of sample processing without compromising resolution or accuracy. This goal is driven by the exponential growth in genomic and proteomic data generation, particularly in fields such as personalized medicine, drug discovery, and large-scale genetic studies.
Key objectives for enhancing gel electrophoresis throughput include:
1. Reducing run times while maintaining or improving separation quality.
2. Increasing the number of samples that can be processed simultaneously.
3. Automating sample loading and result analysis to minimize manual intervention.
4. Developing more sensitive detection methods to work with smaller sample volumes.
5. Improving compatibility with downstream processes such as mass spectrometry.
These objectives are not only technical but also aim to address practical considerations such as cost-effectiveness, reproducibility, and ease of use in both research and clinical settings. The ultimate goal is to transform gel electrophoresis from a potentially rate-limiting step into a rapid, high-capacity technique that can keep pace with the ever-increasing demands of modern molecular biology and biotechnology.
As we look to the future, the evolution of gel electrophoresis is likely to continue along several paths. These include further miniaturization, integration with other analytical techniques, and the development of novel materials for gel matrices. The incorporation of artificial intelligence and machine learning for data analysis and process optimization is also expected to play a crucial role in achieving higher throughput and more sophisticated analytical capabilities.
The evolution of gel electrophoresis has been marked by several key advancements. The transition from slab gels to capillary electrophoresis in the 1980s dramatically improved resolution and speed. The introduction of pulsed-field gel electrophoresis (PFGE) in 1984 enabled the separation of much larger DNA fragments, expanding the technique's utility in genomic studies. More recently, the development of microfluidic devices has further miniaturized and accelerated the process.
Despite these improvements, the increasing demands of high-throughput genomics and proteomics have highlighted the need for even greater efficiency in gel electrophoresis. The primary objective in improving gel electrophoresis throughput is to increase the speed and volume of sample processing without compromising resolution or accuracy. This goal is driven by the exponential growth in genomic and proteomic data generation, particularly in fields such as personalized medicine, drug discovery, and large-scale genetic studies.
Key objectives for enhancing gel electrophoresis throughput include:
1. Reducing run times while maintaining or improving separation quality.
2. Increasing the number of samples that can be processed simultaneously.
3. Automating sample loading and result analysis to minimize manual intervention.
4. Developing more sensitive detection methods to work with smaller sample volumes.
5. Improving compatibility with downstream processes such as mass spectrometry.
These objectives are not only technical but also aim to address practical considerations such as cost-effectiveness, reproducibility, and ease of use in both research and clinical settings. The ultimate goal is to transform gel electrophoresis from a potentially rate-limiting step into a rapid, high-capacity technique that can keep pace with the ever-increasing demands of modern molecular biology and biotechnology.
As we look to the future, the evolution of gel electrophoresis is likely to continue along several paths. These include further miniaturization, integration with other analytical techniques, and the development of novel materials for gel matrices. The incorporation of artificial intelligence and machine learning for data analysis and process optimization is also expected to play a crucial role in achieving higher throughput and more sophisticated analytical capabilities.
High-Throughput Demand Analysis
The demand for high-throughput gel electrophoresis has been steadily increasing across various fields of life sciences, particularly in genomics, proteomics, and molecular biology research. This surge in demand is primarily driven by the exponential growth in sample volumes and the need for faster, more efficient analysis methods in both academic and industrial settings.
In genomics research, the advent of next-generation sequencing technologies has led to a significant increase in the number of DNA samples that need to be processed and analyzed. Traditional gel electrophoresis methods, while reliable, have become a bottleneck in the workflow due to their time-consuming nature. Researchers and clinicians are seeking faster alternatives that can handle a larger number of samples simultaneously without compromising on resolution or accuracy.
The pharmaceutical industry has also been a major driver in the demand for high-throughput gel electrophoresis. Drug discovery and development processes require the screening of vast libraries of compounds, necessitating rapid and efficient separation techniques. High-throughput gel electrophoresis systems can significantly accelerate these processes, allowing for quicker identification of potential drug candidates and reducing the time-to-market for new therapeutics.
In the field of proteomics, the complexity of protein samples and the need for comprehensive analysis have pushed the limits of traditional gel electrophoresis techniques. Researchers are increasingly looking for high-throughput solutions that can handle complex protein mixtures and provide rapid, high-resolution separation. This demand is further amplified by the growing interest in personalized medicine and biomarker discovery, where large-scale protein profiling is essential.
The biotechnology sector, particularly in the production of recombinant proteins and enzymes, has also contributed to the rising demand for high-throughput gel electrophoresis. Quality control processes in biomanufacturing require frequent analysis of protein samples, and the ability to process multiple samples quickly is crucial for maintaining production efficiency and ensuring product consistency.
Academic research institutions are another significant source of demand for high-throughput gel electrophoresis. With increasing pressure to publish and the growing complexity of research projects, there is a need for tools that can accelerate experimental workflows without sacrificing data quality. High-throughput systems allow researchers to process more samples in less time, potentially leading to faster discoveries and more comprehensive studies.
The market for high-throughput gel electrophoresis solutions is expected to grow substantially in the coming years. This growth is not only driven by the increasing sample volumes but also by the integration of automation and digital technologies in laboratory processes. Researchers and industry professionals are looking for systems that offer not just increased throughput, but also improved data management, analysis, and integration with other laboratory information systems.
In genomics research, the advent of next-generation sequencing technologies has led to a significant increase in the number of DNA samples that need to be processed and analyzed. Traditional gel electrophoresis methods, while reliable, have become a bottleneck in the workflow due to their time-consuming nature. Researchers and clinicians are seeking faster alternatives that can handle a larger number of samples simultaneously without compromising on resolution or accuracy.
The pharmaceutical industry has also been a major driver in the demand for high-throughput gel electrophoresis. Drug discovery and development processes require the screening of vast libraries of compounds, necessitating rapid and efficient separation techniques. High-throughput gel electrophoresis systems can significantly accelerate these processes, allowing for quicker identification of potential drug candidates and reducing the time-to-market for new therapeutics.
In the field of proteomics, the complexity of protein samples and the need for comprehensive analysis have pushed the limits of traditional gel electrophoresis techniques. Researchers are increasingly looking for high-throughput solutions that can handle complex protein mixtures and provide rapid, high-resolution separation. This demand is further amplified by the growing interest in personalized medicine and biomarker discovery, where large-scale protein profiling is essential.
The biotechnology sector, particularly in the production of recombinant proteins and enzymes, has also contributed to the rising demand for high-throughput gel electrophoresis. Quality control processes in biomanufacturing require frequent analysis of protein samples, and the ability to process multiple samples quickly is crucial for maintaining production efficiency and ensuring product consistency.
Academic research institutions are another significant source of demand for high-throughput gel electrophoresis. With increasing pressure to publish and the growing complexity of research projects, there is a need for tools that can accelerate experimental workflows without sacrificing data quality. High-throughput systems allow researchers to process more samples in less time, potentially leading to faster discoveries and more comprehensive studies.
The market for high-throughput gel electrophoresis solutions is expected to grow substantially in the coming years. This growth is not only driven by the increasing sample volumes but also by the integration of automation and digital technologies in laboratory processes. Researchers and industry professionals are looking for systems that offer not just increased throughput, but also improved data management, analysis, and integration with other laboratory information systems.
Current Limitations in Gel Electrophoresis Speed
Gel electrophoresis is a widely used technique in molecular biology for separating and analyzing DNA, RNA, and proteins. However, its current speed limitations pose significant challenges to high-throughput applications. The primary factor affecting gel electrophoresis speed is the need to balance resolution with run time. Increasing voltage to accelerate the process often leads to band distortion and reduced separation quality.
One major limitation is the heat generation during electrophoresis. As electrical current passes through the gel, it produces heat, which can cause band smearing and gel melting if not properly managed. This thermal constraint restricts the maximum voltage that can be applied, thereby limiting the speed of separation.
Another critical factor is the gel matrix itself. Traditional agarose and polyacrylamide gels, while effective for separation, create significant resistance to DNA migration. This resistance increases with larger DNA fragments, leading to longer run times for comprehensive genomic analysis.
The loading and running process also contributes to throughput limitations. Manual gel preparation, sample loading, and post-run analysis are time-consuming steps that reduce overall efficiency. The need for careful sample preparation and precise loading to avoid cross-contamination further slows the process.
Additionally, the visualization and documentation of results often require separate staining and imaging steps, adding to the total analysis time. Real-time monitoring of band separation during electrophoresis is challenging with conventional setups, necessitating multiple runs for optimal separation.
The size limitations of standard gel electrophoresis apparatus also restrict throughput. Most systems can only accommodate a limited number of samples per run, requiring multiple runs for large-scale studies. This limitation becomes particularly apparent in high-volume genomic research and clinical diagnostics.
Furthermore, the resolution of gel electrophoresis decreases for very large DNA fragments, making it less effective for analyzing complex genomic structures. This limitation often necessitates the use of complementary techniques, further extending the overall analysis time.
Lastly, the interpretation of gel electrophoresis results can be subjective and time-consuming, especially when dealing with complex band patterns. The lack of automated, high-throughput analysis tools for gel images contributes to bottlenecks in data processing and interpretation.
Addressing these limitations is crucial for improving gel electrophoresis throughput. Innovations in gel materials, electrophoresis apparatus design, and automated analysis systems are needed to overcome these challenges and meet the growing demands of modern molecular biology and genomics research.
One major limitation is the heat generation during electrophoresis. As electrical current passes through the gel, it produces heat, which can cause band smearing and gel melting if not properly managed. This thermal constraint restricts the maximum voltage that can be applied, thereby limiting the speed of separation.
Another critical factor is the gel matrix itself. Traditional agarose and polyacrylamide gels, while effective for separation, create significant resistance to DNA migration. This resistance increases with larger DNA fragments, leading to longer run times for comprehensive genomic analysis.
The loading and running process also contributes to throughput limitations. Manual gel preparation, sample loading, and post-run analysis are time-consuming steps that reduce overall efficiency. The need for careful sample preparation and precise loading to avoid cross-contamination further slows the process.
Additionally, the visualization and documentation of results often require separate staining and imaging steps, adding to the total analysis time. Real-time monitoring of band separation during electrophoresis is challenging with conventional setups, necessitating multiple runs for optimal separation.
The size limitations of standard gel electrophoresis apparatus also restrict throughput. Most systems can only accommodate a limited number of samples per run, requiring multiple runs for large-scale studies. This limitation becomes particularly apparent in high-volume genomic research and clinical diagnostics.
Furthermore, the resolution of gel electrophoresis decreases for very large DNA fragments, making it less effective for analyzing complex genomic structures. This limitation often necessitates the use of complementary techniques, further extending the overall analysis time.
Lastly, the interpretation of gel electrophoresis results can be subjective and time-consuming, especially when dealing with complex band patterns. The lack of automated, high-throughput analysis tools for gel images contributes to bottlenecks in data processing and interpretation.
Addressing these limitations is crucial for improving gel electrophoresis throughput. Innovations in gel materials, electrophoresis apparatus design, and automated analysis systems are needed to overcome these challenges and meet the growing demands of modern molecular biology and genomics research.
Existing High-Throughput Gel Electrophoresis Methods
01 High-throughput gel electrophoresis systems
Advanced systems have been developed to increase the throughput of gel electrophoresis. These systems often incorporate multiple lanes, automated sample loading, and parallel processing capabilities. They may also include integrated imaging and analysis tools to speed up the overall process.- High-throughput gel electrophoresis systems: Advanced systems have been developed to increase the throughput of gel electrophoresis. These systems often incorporate multiple lanes, automated sample loading, and parallel processing capabilities. They may also include integrated imaging and analysis tools to speed up the overall process.
- Microfluidic devices for gel electrophoresis: Microfluidic devices have been designed to miniaturize and accelerate gel electrophoresis processes. These devices often use microchannels and integrated electrodes to perform electrophoresis on a smaller scale, allowing for faster separation and analysis of samples with reduced reagent consumption.
- Multi-dimensional gel electrophoresis techniques: Multi-dimensional gel electrophoresis techniques have been developed to improve separation and resolution of complex samples. These methods often combine different separation principles, such as isoelectric focusing and SDS-PAGE, to achieve higher throughput and better resolution of protein mixtures.
- Automated gel electrophoresis systems: Automated systems have been created to streamline the gel electrophoresis process. These systems may include robotic sample handling, automated gel casting, and integrated software for data analysis. Automation reduces manual intervention, increases reproducibility, and improves overall throughput.
- Capillary gel electrophoresis for high-throughput applications: Capillary gel electrophoresis techniques have been adapted for high-throughput applications. These methods use narrow capillaries filled with gel to perform rapid separations, often with multiple capillaries running in parallel. This approach allows for faster analysis times and increased sample throughput compared to traditional slab gel electrophoresis.
02 Microfluidic devices for gel electrophoresis
Microfluidic devices have been designed to miniaturize and accelerate gel electrophoresis processes. These devices often use microchannels and integrated electrodes to perform separations on a smaller scale, allowing for faster analysis times and reduced sample volumes.Expand Specific Solutions03 Multi-dimensional gel electrophoresis techniques
Multi-dimensional gel electrophoresis techniques have been developed to improve separation resolution and increase throughput. These methods often combine different separation principles, such as isoelectric focusing followed by size-based separation, to achieve better resolution of complex samples.Expand Specific Solutions04 Automated gel electrophoresis systems
Automated systems have been created to streamline the gel electrophoresis process. These systems may include robotic sample handling, automated gel casting, and integrated analysis software to reduce manual intervention and increase overall throughput.Expand Specific Solutions05 Novel gel materials and buffer systems
New gel materials and buffer systems have been developed to enhance separation speed and efficiency. These innovations may include specialized polymer matrices, gradient gels, or buffer additives that allow for faster migration of molecules while maintaining or improving resolution.Expand Specific Solutions
Key Industry Players and Competition
The gel electrophoresis market is in a mature stage, with a steady growth trajectory driven by increasing applications in genomics, proteomics, and diagnostics. The global market size is estimated to be in the billions, with a compound annual growth rate of around 5-7%. Technologically, the field is evolving towards higher throughput and automation, with companies like Life Technologies Corp., Bio-Rad Laboratories, and Agilent Technologies leading innovation. These firms, along with others such as Beckman Coulter and Applied Biosystems, are developing advanced systems that integrate sample preparation, separation, and analysis. The focus is on improving speed, resolution, and reproducibility while reducing manual intervention, addressing the growing demand for high-throughput solutions in research and clinical settings.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad has developed the Criterion™ and Mini-PROTEAN® gel electrophoresis systems to improve throughput. The Criterion™ system features precast gels that can accommodate up to 26 samples per gel, increasing sample capacity by 60% compared to traditional mini gels[3]. The Mini-PROTEAN® Tetra Vertical Electrophoresis Cell can run up to four gels simultaneously, effectively quadrupling throughput. Bio-Rad has also introduced the V3 Western Workflow™, which integrates stain-free technology with their gel documentation systems, allowing for rapid protein visualization without the need for staining or destaining steps[4]. This significantly reduces the overall time required for gel analysis.
Strengths: Increased sample capacity, simultaneous multi-gel runs, and integrated visualization technology. Weaknesses: Dependence on proprietary consumables and potential limitations in customization for specialized applications.
Beckman Coulter, Inc.
Technical Solution: Beckman Coulter has developed the ProteomeLab PA 800 plus Pharmaceutical Analysis System, which utilizes capillary electrophoresis (CE) to improve throughput in gel electrophoresis applications. This system can perform both gel-based and gel-free separations, offering flexibility in analytical approaches. The PA 800 plus uses an autosampler that can hold up to 50 vials, allowing for unattended operation and increased sample throughput[5]. Additionally, Beckman Coulter's MCE-202 MultiNA Microchip Electrophoresis System employs microfluidic technology to perform rapid DNA and RNA analysis. This system can process up to 120 samples per hour, significantly improving throughput compared to traditional slab gel electrophoresis[6].
Strengths: High-throughput capabilities, versatility in separation techniques, and automation. Weaknesses: Higher complexity and cost compared to traditional gel electrophoresis systems, and potential limitations in resolution for certain applications.
Innovative Approaches to Accelerate Separation
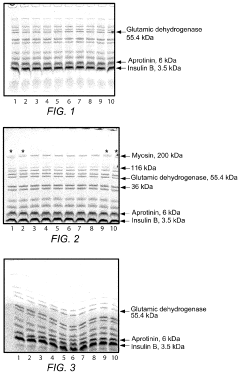
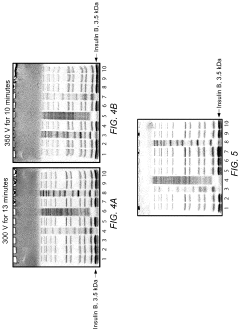
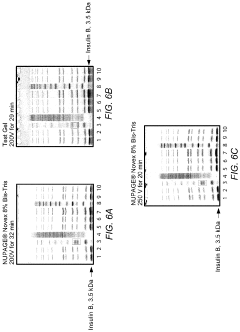
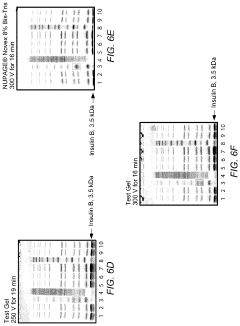
System for rapid high-resolution GEL electrophoresis
PatentActiveUS20190391113A1
Innovation
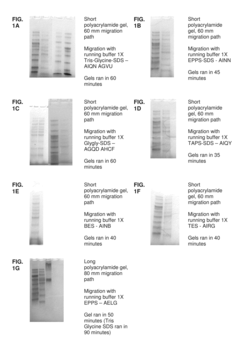
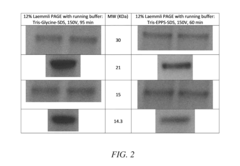
- The development of electrophoretic systems and formulations that allow for higher field strengths up to 50% more than conventional systems, using a discontinuous buffer system with specific gel amine and ampholyte buffers, and a pH range of 5.5 to 7.5, enabling faster separation of proteins within 30 minutes or less, even at higher voltages.
Electrophoresis buffer for faster migration, improved resolution and extended shelf-life
PatentInactiveUS20160216232A1
Innovation
- An electrolyte solution comprising Tris(hydroxymethyl)aminomethane (TRIS) and specific zwitterions such as 4-(2-hydroxyethyl)piperazine-1-propanesulfonic acid (EPPS or HEPPS) is used, which can be employed as a running buffer or incorporated into gel preparation, enhancing migration speed and resolution while extending the shelf life of gels.
Automation and Robotics Integration
Automation and robotics integration have emerged as key strategies to significantly improve gel electrophoresis throughput. By incorporating advanced robotic systems and automated processes, laboratories can streamline their workflows, reduce manual labor, and increase overall efficiency.
One of the primary areas where automation has made a substantial impact is in sample preparation and loading. Robotic liquid handling systems can precisely and consistently prepare samples, mix buffers, and load gels with minimal human intervention. These systems can handle multiple samples simultaneously, dramatically reducing the time required for sample preparation and minimizing the risk of human error.
Automated gel running systems have also been developed to enhance throughput. These systems can manage multiple gel runs concurrently, with programmable power supplies that can adjust voltage and current settings automatically. Some advanced systems even incorporate cooling mechanisms to maintain optimal running conditions, allowing for faster separation without compromising resolution.
Image capture and analysis have been revolutionized by automation as well. Automated gel documentation systems can capture high-resolution images of gels at predetermined intervals or at the end of a run. These systems often come with integrated software that can perform band detection, quantification, and data analysis, significantly reducing the time required for result interpretation.
Robotic systems have been designed to handle post-electrophoresis tasks such as gel staining, destaining, and extraction. These processes, which traditionally require multiple manual steps and extended waiting periods, can now be performed with minimal human intervention. Automated gel staining and destaining systems can process multiple gels simultaneously, ensuring consistent results and freeing up laboratory personnel for other tasks.
Integration of these automated components into a cohesive system represents the cutting edge of gel electrophoresis technology. Fully integrated platforms that combine sample preparation, gel running, imaging, and analysis are now available. These systems can be programmed to run complex, multi-step protocols with minimal user input, allowing for 24/7 operation and maximizing laboratory productivity.
The implementation of laboratory information management systems (LIMS) in conjunction with automated gel electrophoresis equipment further enhances throughput by streamlining data management and improving traceability. These systems can track samples, manage workflows, and generate comprehensive reports, facilitating better resource allocation and decision-making.
While the initial investment in automation and robotics can be substantial, the long-term benefits in terms of increased throughput, improved reproducibility, and reduced labor costs make it an attractive option for laboratories dealing with high sample volumes. As technology continues to advance, we can expect even more sophisticated and efficient automated solutions for gel electrophoresis, further pushing the boundaries of throughput and productivity in molecular biology research and diagnostics.
One of the primary areas where automation has made a substantial impact is in sample preparation and loading. Robotic liquid handling systems can precisely and consistently prepare samples, mix buffers, and load gels with minimal human intervention. These systems can handle multiple samples simultaneously, dramatically reducing the time required for sample preparation and minimizing the risk of human error.
Automated gel running systems have also been developed to enhance throughput. These systems can manage multiple gel runs concurrently, with programmable power supplies that can adjust voltage and current settings automatically. Some advanced systems even incorporate cooling mechanisms to maintain optimal running conditions, allowing for faster separation without compromising resolution.
Image capture and analysis have been revolutionized by automation as well. Automated gel documentation systems can capture high-resolution images of gels at predetermined intervals or at the end of a run. These systems often come with integrated software that can perform band detection, quantification, and data analysis, significantly reducing the time required for result interpretation.
Robotic systems have been designed to handle post-electrophoresis tasks such as gel staining, destaining, and extraction. These processes, which traditionally require multiple manual steps and extended waiting periods, can now be performed with minimal human intervention. Automated gel staining and destaining systems can process multiple gels simultaneously, ensuring consistent results and freeing up laboratory personnel for other tasks.
Integration of these automated components into a cohesive system represents the cutting edge of gel electrophoresis technology. Fully integrated platforms that combine sample preparation, gel running, imaging, and analysis are now available. These systems can be programmed to run complex, multi-step protocols with minimal user input, allowing for 24/7 operation and maximizing laboratory productivity.
The implementation of laboratory information management systems (LIMS) in conjunction with automated gel electrophoresis equipment further enhances throughput by streamlining data management and improving traceability. These systems can track samples, manage workflows, and generate comprehensive reports, facilitating better resource allocation and decision-making.
While the initial investment in automation and robotics can be substantial, the long-term benefits in terms of increased throughput, improved reproducibility, and reduced labor costs make it an attractive option for laboratories dealing with high sample volumes. As technology continues to advance, we can expect even more sophisticated and efficient automated solutions for gel electrophoresis, further pushing the boundaries of throughput and productivity in molecular biology research and diagnostics.
Miniaturization and Microfluidic Applications
Miniaturization and microfluidic applications represent a significant advancement in improving gel electrophoresis throughput. By scaling down the traditional gel electrophoresis process to microfluidic dimensions, researchers have achieved faster separation times, reduced sample and reagent consumption, and increased parallelization capabilities.
Microfluidic devices for gel electrophoresis typically consist of miniaturized channels etched or molded into materials such as glass, silicon, or polymers. These channels are filled with gel matrices, allowing for the separation of biomolecules under an applied electric field. The reduced dimensions of these channels result in higher electric field strengths, leading to more rapid separations compared to conventional gel electrophoresis systems.
One of the key advantages of miniaturized gel electrophoresis is the ability to integrate multiple analytical steps onto a single chip. This "lab-on-a-chip" approach allows for sample preparation, separation, and detection to be performed in a single, compact device. Such integration not only improves throughput but also reduces the risk of sample contamination and human error associated with manual handling steps.
Microfluidic gel electrophoresis systems have demonstrated impressive improvements in separation speed. Some devices can achieve complete DNA separations in less than a minute, compared to hours required for traditional slab gel electrophoresis. This dramatic reduction in analysis time significantly enhances overall throughput, especially when combined with automated sample loading and detection systems.
The miniaturization of gel electrophoresis also enables the development of portable and point-of-care diagnostic devices. These compact systems can perform rapid DNA or protein analysis in resource-limited settings, expanding the applicability of gel electrophoresis beyond traditional laboratory environments. Such portability is particularly valuable for on-site environmental monitoring, rapid medical diagnostics, and forensic analysis.
Another significant advantage of microfluidic gel electrophoresis is the reduced sample volume requirement. Traditional gel electrophoresis typically requires microliters to milliliters of sample, whereas microfluidic devices can operate with nanoliter or even picoliter volumes. This reduction in sample volume is particularly beneficial when working with limited or precious samples, such as in single-cell analysis or rare biomarker detection.
Researchers have also explored the use of alternative gel materials in microfluidic devices to further enhance separation performance. For example, nanoporous hydrogels and self-assembling DNA nanostructures have been employed to create highly uniform and precisely controlled separation matrices within microfluidic channels. These advanced materials can offer improved resolution and faster separation times compared to traditional polyacrylamide or agarose gels.
Microfluidic devices for gel electrophoresis typically consist of miniaturized channels etched or molded into materials such as glass, silicon, or polymers. These channels are filled with gel matrices, allowing for the separation of biomolecules under an applied electric field. The reduced dimensions of these channels result in higher electric field strengths, leading to more rapid separations compared to conventional gel electrophoresis systems.
One of the key advantages of miniaturized gel electrophoresis is the ability to integrate multiple analytical steps onto a single chip. This "lab-on-a-chip" approach allows for sample preparation, separation, and detection to be performed in a single, compact device. Such integration not only improves throughput but also reduces the risk of sample contamination and human error associated with manual handling steps.
Microfluidic gel electrophoresis systems have demonstrated impressive improvements in separation speed. Some devices can achieve complete DNA separations in less than a minute, compared to hours required for traditional slab gel electrophoresis. This dramatic reduction in analysis time significantly enhances overall throughput, especially when combined with automated sample loading and detection systems.
The miniaturization of gel electrophoresis also enables the development of portable and point-of-care diagnostic devices. These compact systems can perform rapid DNA or protein analysis in resource-limited settings, expanding the applicability of gel electrophoresis beyond traditional laboratory environments. Such portability is particularly valuable for on-site environmental monitoring, rapid medical diagnostics, and forensic analysis.
Another significant advantage of microfluidic gel electrophoresis is the reduced sample volume requirement. Traditional gel electrophoresis typically requires microliters to milliliters of sample, whereas microfluidic devices can operate with nanoliter or even picoliter volumes. This reduction in sample volume is particularly beneficial when working with limited or precious samples, such as in single-cell analysis or rare biomarker detection.
Researchers have also explored the use of alternative gel materials in microfluidic devices to further enhance separation performance. For example, nanoporous hydrogels and self-assembling DNA nanostructures have been employed to create highly uniform and precisely controlled separation matrices within microfluidic channels. These advanced materials can offer improved resolution and faster separation times compared to traditional polyacrylamide or agarose gels.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!