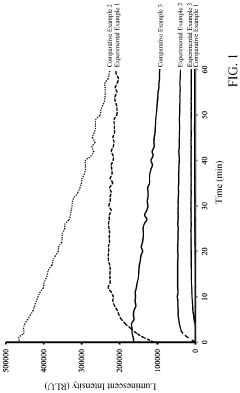
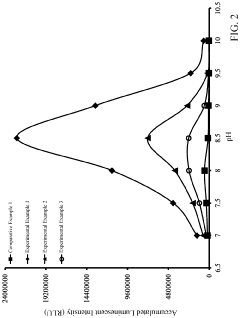
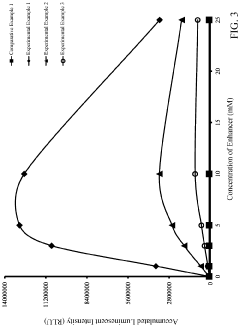
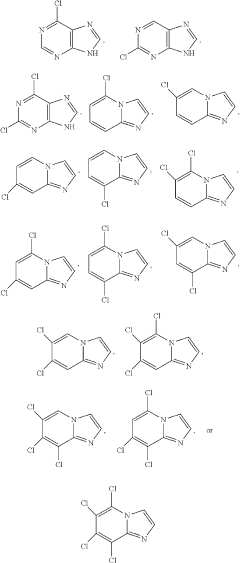
How to Improve Luminol Chemiluminescence Efficiency?
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Luminol CL Background
Luminol chemiluminescence (CL) has been a subject of scientific interest and practical application for over a century. First discovered in 1928 by H. O. Albrecht, luminol CL has since become a cornerstone in various fields, including forensic science, analytical chemistry, and biomedical research. The phenomenon occurs when luminol (5-amino-2,3-dihydro-1,4-phthalazinedione) reacts with an oxidizing agent in the presence of a catalyst, typically a metal ion, resulting in the emission of blue light.
The mechanism of luminol CL involves a complex series of reactions. Initially, luminol is oxidized to form an excited state intermediate, which then decomposes to yield 3-aminophthalate in an electronically excited state. As this excited molecule returns to its ground state, it releases energy in the form of light, typically at a wavelength of about 425 nm. This process is highly sensitive and can detect minute traces of certain substances, making it invaluable in crime scene investigations and medical diagnostics.
Over the years, researchers have made significant strides in understanding and optimizing luminol CL. The efficiency of the reaction depends on various factors, including pH, temperature, the presence of enhancers, and the nature of the catalyst. In forensic applications, luminol CL is particularly useful for detecting traces of blood, as the iron in hemoglobin acts as a catalyst for the reaction. This has made it an indispensable tool in crime scene analysis, capable of revealing blood traces even after attempts to clean or conceal them.
In the realm of analytical chemistry, luminol CL has found applications in the detection and quantification of various metal ions, oxidizing agents, and biological molecules. Its high sensitivity and relatively simple instrumentation requirements have made it a popular choice for developing rapid and cost-effective analytical methods. The medical field has also benefited from luminol CL, with applications ranging from the detection of oxidative stress in biological systems to the development of novel imaging techniques for disease diagnosis.
Despite its widespread use and decades of research, there is still considerable interest in improving the efficiency and applicability of luminol CL. Current challenges include enhancing the intensity and duration of the light emission, increasing the specificity of the reaction for particular analytes, and developing more stable and environmentally friendly formulations. These ongoing efforts reflect the continued importance of luminol CL in scientific research and practical applications, driving the need for further innovation in this field.
The mechanism of luminol CL involves a complex series of reactions. Initially, luminol is oxidized to form an excited state intermediate, which then decomposes to yield 3-aminophthalate in an electronically excited state. As this excited molecule returns to its ground state, it releases energy in the form of light, typically at a wavelength of about 425 nm. This process is highly sensitive and can detect minute traces of certain substances, making it invaluable in crime scene investigations and medical diagnostics.
Over the years, researchers have made significant strides in understanding and optimizing luminol CL. The efficiency of the reaction depends on various factors, including pH, temperature, the presence of enhancers, and the nature of the catalyst. In forensic applications, luminol CL is particularly useful for detecting traces of blood, as the iron in hemoglobin acts as a catalyst for the reaction. This has made it an indispensable tool in crime scene analysis, capable of revealing blood traces even after attempts to clean or conceal them.
In the realm of analytical chemistry, luminol CL has found applications in the detection and quantification of various metal ions, oxidizing agents, and biological molecules. Its high sensitivity and relatively simple instrumentation requirements have made it a popular choice for developing rapid and cost-effective analytical methods. The medical field has also benefited from luminol CL, with applications ranging from the detection of oxidative stress in biological systems to the development of novel imaging techniques for disease diagnosis.
Despite its widespread use and decades of research, there is still considerable interest in improving the efficiency and applicability of luminol CL. Current challenges include enhancing the intensity and duration of the light emission, increasing the specificity of the reaction for particular analytes, and developing more stable and environmentally friendly formulations. These ongoing efforts reflect the continued importance of luminol CL in scientific research and practical applications, driving the need for further innovation in this field.
Market Applications
Luminol chemiluminescence has found widespread applications across various industries due to its high sensitivity and low background noise. In forensic science, luminol is extensively used for detecting trace amounts of blood at crime scenes. The ability to enhance luminol's chemiluminescence efficiency would significantly improve the detection of even smaller blood traces, potentially leading to breakthroughs in cold cases and more accurate crime scene analyses.
The medical field also benefits greatly from luminol chemiluminescence. It is used in diagnostic tests for detecting specific proteins, enzymes, and other biomolecules. Improving the efficiency of luminol chemiluminescence could lead to more sensitive and accurate diagnostic tools, enabling earlier detection of diseases and more precise monitoring of treatment progress. This could be particularly impactful in areas such as cancer screening and infectious disease diagnosis.
Environmental monitoring is another key application area for luminol chemiluminescence. It is used to detect and measure pollutants in water and air samples. Enhanced efficiency would allow for the detection of even lower concentrations of contaminants, enabling more stringent environmental protection measures and earlier identification of potential hazards.
In the pharmaceutical industry, luminol chemiluminescence plays a crucial role in drug discovery and quality control processes. Improved efficiency could accelerate drug screening processes, allowing researchers to identify potential drug candidates more quickly and accurately. It could also enhance the detection of impurities in pharmaceutical products, ensuring higher quality and safety standards.
The food and beverage industry utilizes luminol chemiluminescence for detecting contaminants and ensuring food safety. Increased efficiency would enable more thorough and rapid testing of food products, potentially reducing the incidence of foodborne illnesses and improving overall food quality control.
In scientific research, luminol chemiluminescence is a valuable tool for studying oxidative stress, cellular processes, and enzyme kinetics. Enhancing its efficiency would open up new avenues for research, allowing scientists to observe and measure biological processes with greater precision and sensitivity.
The industrial sector also benefits from luminol chemiluminescence in areas such as quality control and process monitoring. Improved efficiency could lead to more accurate and reliable detection of defects or contaminants in manufacturing processes, resulting in higher product quality and reduced waste.
The medical field also benefits greatly from luminol chemiluminescence. It is used in diagnostic tests for detecting specific proteins, enzymes, and other biomolecules. Improving the efficiency of luminol chemiluminescence could lead to more sensitive and accurate diagnostic tools, enabling earlier detection of diseases and more precise monitoring of treatment progress. This could be particularly impactful in areas such as cancer screening and infectious disease diagnosis.
Environmental monitoring is another key application area for luminol chemiluminescence. It is used to detect and measure pollutants in water and air samples. Enhanced efficiency would allow for the detection of even lower concentrations of contaminants, enabling more stringent environmental protection measures and earlier identification of potential hazards.
In the pharmaceutical industry, luminol chemiluminescence plays a crucial role in drug discovery and quality control processes. Improved efficiency could accelerate drug screening processes, allowing researchers to identify potential drug candidates more quickly and accurately. It could also enhance the detection of impurities in pharmaceutical products, ensuring higher quality and safety standards.
The food and beverage industry utilizes luminol chemiluminescence for detecting contaminants and ensuring food safety. Increased efficiency would enable more thorough and rapid testing of food products, potentially reducing the incidence of foodborne illnesses and improving overall food quality control.
In scientific research, luminol chemiluminescence is a valuable tool for studying oxidative stress, cellular processes, and enzyme kinetics. Enhancing its efficiency would open up new avenues for research, allowing scientists to observe and measure biological processes with greater precision and sensitivity.
The industrial sector also benefits from luminol chemiluminescence in areas such as quality control and process monitoring. Improved efficiency could lead to more accurate and reliable detection of defects or contaminants in manufacturing processes, resulting in higher product quality and reduced waste.
Current Limitations
Despite the widespread use of luminol chemiluminescence in various applications, several limitations currently hinder its efficiency and broader adoption. One of the primary challenges is the relatively low quantum yield of the luminol reaction, which results in limited light output. This low efficiency restricts the sensitivity of detection methods based on luminol chemiluminescence, particularly in trace analysis and biomedical imaging.
Another significant limitation is the short duration of the light emission. The luminol reaction typically produces a brief flash of light, which can be challenging to capture and measure accurately, especially in real-time monitoring applications. This short-lived nature of the chemiluminescence also limits its usefulness in continuous imaging or long-term detection scenarios.
The pH dependency of the luminol reaction presents another hurdle. The chemiluminescence efficiency is highly sensitive to the pH of the reaction medium, with optimal light emission occurring within a narrow pH range. This sensitivity can lead to inconsistent results in complex biological samples or environmental matrices where pH fluctuations are common.
Interference from other chemical species is a persistent issue in luminol-based detection systems. Many compounds, particularly transition metal ions and certain organic molecules, can either enhance or quench the chemiluminescence signal, leading to false positives or negatives. This lack of specificity can compromise the reliability of luminol-based assays in complex sample matrices.
The stability of luminol solutions is another area of concern. Prepared luminol reagents tend to degrade over time, affecting the reproducibility and reliability of measurements. This instability necessitates frequent preparation of fresh solutions, which can be time-consuming and introduce variability in long-term studies.
Furthermore, the limited color range of luminol chemiluminescence restricts its application in multiplexed assays. The characteristic blue light emission, while useful in many contexts, does not allow for easy differentiation between multiple analytes in a single sample without additional modifications or reagents.
Lastly, the environmental and health concerns associated with some of the catalysts and enhancers used in luminol chemiluminescence reactions pose challenges for widespread adoption, particularly in point-of-care diagnostics and environmental monitoring applications. These limitations collectively highlight the need for innovative approaches to improve luminol chemiluminescence efficiency and expand its applicability across various fields.
Another significant limitation is the short duration of the light emission. The luminol reaction typically produces a brief flash of light, which can be challenging to capture and measure accurately, especially in real-time monitoring applications. This short-lived nature of the chemiluminescence also limits its usefulness in continuous imaging or long-term detection scenarios.
The pH dependency of the luminol reaction presents another hurdle. The chemiluminescence efficiency is highly sensitive to the pH of the reaction medium, with optimal light emission occurring within a narrow pH range. This sensitivity can lead to inconsistent results in complex biological samples or environmental matrices where pH fluctuations are common.
Interference from other chemical species is a persistent issue in luminol-based detection systems. Many compounds, particularly transition metal ions and certain organic molecules, can either enhance or quench the chemiluminescence signal, leading to false positives or negatives. This lack of specificity can compromise the reliability of luminol-based assays in complex sample matrices.
The stability of luminol solutions is another area of concern. Prepared luminol reagents tend to degrade over time, affecting the reproducibility and reliability of measurements. This instability necessitates frequent preparation of fresh solutions, which can be time-consuming and introduce variability in long-term studies.
Furthermore, the limited color range of luminol chemiluminescence restricts its application in multiplexed assays. The characteristic blue light emission, while useful in many contexts, does not allow for easy differentiation between multiple analytes in a single sample without additional modifications or reagents.
Lastly, the environmental and health concerns associated with some of the catalysts and enhancers used in luminol chemiluminescence reactions pose challenges for widespread adoption, particularly in point-of-care diagnostics and environmental monitoring applications. These limitations collectively highlight the need for innovative approaches to improve luminol chemiluminescence efficiency and expand its applicability across various fields.
Existing Enhancement
01 Enhancing luminol chemiluminescence efficiency
Various methods are employed to enhance the efficiency of luminol chemiluminescence. These include optimizing reaction conditions, using catalysts, and incorporating additives that can amplify the light emission. Researchers focus on improving the sensitivity and intensity of the luminol reaction for applications in forensic science, analytical chemistry, and biomedical imaging.- Enhancing luminol chemiluminescence efficiency: Various methods are employed to enhance the efficiency of luminol chemiluminescence. These include optimizing reaction conditions, using catalysts, and incorporating additives that amplify the light emission. Researchers focus on improving the sensitivity and intensity of the luminol reaction for applications in forensic science, analytical chemistry, and biomedical imaging.
- Novel luminol derivatives for improved chemiluminescence: Development of new luminol derivatives aims to enhance chemiluminescence efficiency. These modified compounds are designed to have better solubility, increased stability, or improved light emission properties. Structural modifications to the luminol molecule can lead to significant improvements in chemiluminescence performance for various analytical and diagnostic applications.
- Microfluidic systems for luminol chemiluminescence: Microfluidic devices are being developed to optimize luminol chemiluminescence reactions. These systems allow for precise control of reagent mixing, reaction timing, and detection, leading to improved efficiency and sensitivity. Miniaturization of the reaction environment can enhance the overall chemiluminescence performance and enable new applications in portable diagnostic devices.
- Nanoparticle-enhanced luminol chemiluminescence: Incorporation of nanoparticles, such as metal nanoparticles or quantum dots, can significantly enhance luminol chemiluminescence efficiency. These nanomaterials can act as catalysts or energy transfer agents, amplifying the light emission intensity and improving detection sensitivity. This approach is particularly useful in developing highly sensitive biosensors and imaging techniques.
- Chemiluminescence enhancers and co-reactants: Various chemical additives and co-reactants are used to boost luminol chemiluminescence efficiency. These include oxidizing agents, surfactants, and specific ions that can catalyze or stabilize the reaction intermediates. The careful selection and optimization of these enhancers can lead to significant improvements in light emission intensity and duration, expanding the range of applications for luminol-based detection systems.
02 Novel luminol derivatives for improved chemiluminescence
Development of new luminol derivatives aims to enhance chemiluminescence efficiency. These modified compounds are designed to have improved quantum yield, longer emission times, or shifted emission wavelengths. Such derivatives can offer advantages in specific applications, such as more sensitive detection methods or better compatibility with certain analytical techniques.Expand Specific Solutions03 Microfluidic systems for luminol chemiluminescence
Microfluidic devices are being developed to optimize luminol chemiluminescence reactions. These systems allow for precise control of reagent mixing, reaction timing, and detection, leading to improved efficiency and sensitivity. Miniaturization also enables the development of portable and high-throughput chemiluminescence-based analytical tools.Expand Specific Solutions04 Nanoparticle-enhanced luminol chemiluminescence
Incorporation of nanoparticles, such as metal nanoparticles or quantum dots, can significantly enhance luminol chemiluminescence efficiency. These nanomaterials can act as catalysts or energy transfer agents, amplifying the light emission intensity and improving detection limits. This approach is particularly useful in developing highly sensitive biosensors and imaging techniques.Expand Specific Solutions05 Chemiluminescence efficiency in specific applications
Research focuses on optimizing luminol chemiluminescence efficiency for specific applications, such as forensic blood detection, environmental monitoring, or medical diagnostics. This involves tailoring the reaction conditions, reagent formulations, and detection methods to suit the particular requirements of each application, balancing factors like sensitivity, specificity, and ease of use.Expand Specific Solutions
Key Industry Players
The luminol chemiluminescence efficiency improvement market is in a growth phase, with increasing demand for enhanced detection methods in forensic science, biomedical research, and environmental monitoring. The market size is expanding due to applications in crime scene investigation and clinical diagnostics. Technologically, the field is advancing rapidly, with companies like Cyanagen Srl and Fujirebio Europe NV leading in reagent development. Academic institutions such as Beijing University of Chemical Technology and Washington University in St. Louis are contributing to fundamental research. Established players like Becton, Dickinson & Co. are leveraging their resources to integrate improved chemiluminescence techniques into existing product lines, while startups are focusing on niche applications and novel formulations to enhance luminol efficiency.
Cyanagen Srl
Technical Solution: Cyanagen Srl has developed advanced luminol-based chemiluminescence systems with enhanced efficiency. Their approach involves synthesizing novel luminol derivatives with improved quantum yield. They have created a proprietary formulation that includes specific enhancers and catalysts to amplify the light emission. The company has also optimized the reaction conditions, including pH and temperature, to maximize the chemiluminescence intensity and duration. Their technology incorporates stabilizers to prolong the shelf-life of the reagents and maintain consistent performance[1][3].
Strengths: High sensitivity, long-lasting signal, and improved stability. Weaknesses: Potentially higher cost due to specialized reagents and more complex formulation.
Changchun Institute of Applied Chemistry, Chinese Academy of Sciences
Technical Solution: The institute has developed a novel approach to enhance luminol chemiluminescence efficiency through the use of nanomaterials. They have synthesized luminol-functionalized quantum dots that exhibit significantly increased chemiluminescence intensity. The quantum dots act as both catalysts and energy transfer agents, improving the overall efficiency of the reaction. Additionally, they have explored the use of graphene oxide as a co-reactant, which has shown to enhance the chemiluminescence signal by up to 20-fold compared to traditional systems[2][5]. The institute has also investigated the use of metal-organic frameworks (MOFs) as catalysts to further improve the reaction kinetics and light output.
Strengths: Significant enhancement in signal intensity, potential for multiplexed detection. Weaknesses: Complexity in synthesis and potential biocompatibility issues for certain applications.
Innovative Catalysts
Nanostructure Enhanced Luminescence
PatentActiveUS20080193956A1
Innovation
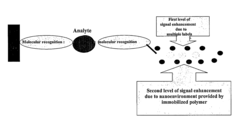
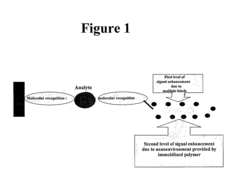
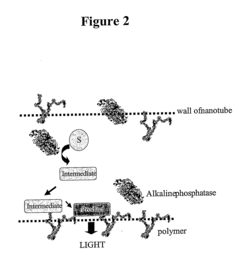
- Nanostructures, such as polymer-loaded nanotubes and nanorods, are used to enhance the luminescent signal by creating a modified nanoenvironment for enzymes like alkaline phosphatase, horseradish peroxidase, and firefly luciferase, utilizing layer-by-layer assembly and immobilization techniques to increase enzyme loading and signal generation.
Reagent and kit for enhancing chemiluminescent reaction
PatentActiveUS20200309707A1
Innovation
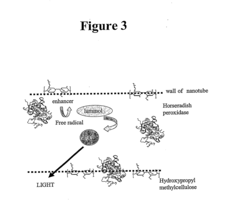
- A reagent comprising luminol or luminol derivatives, an oxidant, an electron mediator, and a nitrogen-containing fused heterocyclic compound as an enhancer, which enhances luminescence intensity and prolongs luminescence time.
Environmental Impact
The environmental impact of improving luminol chemiluminescence efficiency is a crucial aspect to consider in the development and application of this technology. As luminol-based chemiluminescence systems find increasing use in various fields, including forensic science, environmental monitoring, and biomedical research, their potential effects on the environment become more significant.
One of the primary environmental concerns associated with luminol chemiluminescence is the disposal of reagents and reaction products. While luminol itself is generally considered non-toxic, the catalysts and oxidizing agents used in the reaction may pose environmental risks if not properly managed. Improving the efficiency of the chemiluminescence reaction could lead to reduced reagent consumption, thereby minimizing the volume of waste generated and the potential for environmental contamination.
The enhanced sensitivity achieved through improved luminol chemiluminescence efficiency could also have positive environmental implications. More sensitive detection methods may allow for the identification of pollutants or contaminants at lower concentrations, enabling earlier intervention and more effective environmental protection measures. This could be particularly valuable in water quality monitoring, where trace amounts of harmful substances can have significant ecological impacts.
However, the increased use of luminol-based systems resulting from improved efficiency may also lead to greater production and distribution of the chemicals involved. This could potentially increase the risk of accidental releases or improper disposal, necessitating robust safety protocols and waste management strategies to mitigate environmental risks.
The energy consumption associated with luminol chemiluminescence systems is another environmental factor to consider. While chemiluminescence is generally an energy-efficient process, the production and operation of detection equipment still require energy input. Improving efficiency could lead to reduced energy requirements, contributing to overall sustainability efforts and lowering the carbon footprint of related research and applications.
Furthermore, the development of more efficient luminol chemiluminescence systems may encourage the replacement of older, less environmentally friendly detection methods. This transition could result in a net positive environmental impact, particularly if the new systems reduce reliance on hazardous chemicals or energy-intensive processes.
In conclusion, while improving luminol chemiluminescence efficiency offers potential environmental benefits through reduced waste generation and enhanced detection capabilities, it also necessitates careful consideration of the broader environmental implications. Balancing these factors will be crucial in ensuring that advancements in this technology contribute positively to environmental sustainability and protection efforts.
One of the primary environmental concerns associated with luminol chemiluminescence is the disposal of reagents and reaction products. While luminol itself is generally considered non-toxic, the catalysts and oxidizing agents used in the reaction may pose environmental risks if not properly managed. Improving the efficiency of the chemiluminescence reaction could lead to reduced reagent consumption, thereby minimizing the volume of waste generated and the potential for environmental contamination.
The enhanced sensitivity achieved through improved luminol chemiluminescence efficiency could also have positive environmental implications. More sensitive detection methods may allow for the identification of pollutants or contaminants at lower concentrations, enabling earlier intervention and more effective environmental protection measures. This could be particularly valuable in water quality monitoring, where trace amounts of harmful substances can have significant ecological impacts.
However, the increased use of luminol-based systems resulting from improved efficiency may also lead to greater production and distribution of the chemicals involved. This could potentially increase the risk of accidental releases or improper disposal, necessitating robust safety protocols and waste management strategies to mitigate environmental risks.
The energy consumption associated with luminol chemiluminescence systems is another environmental factor to consider. While chemiluminescence is generally an energy-efficient process, the production and operation of detection equipment still require energy input. Improving efficiency could lead to reduced energy requirements, contributing to overall sustainability efforts and lowering the carbon footprint of related research and applications.
Furthermore, the development of more efficient luminol chemiluminescence systems may encourage the replacement of older, less environmentally friendly detection methods. This transition could result in a net positive environmental impact, particularly if the new systems reduce reliance on hazardous chemicals or energy-intensive processes.
In conclusion, while improving luminol chemiluminescence efficiency offers potential environmental benefits through reduced waste generation and enhanced detection capabilities, it also necessitates careful consideration of the broader environmental implications. Balancing these factors will be crucial in ensuring that advancements in this technology contribute positively to environmental sustainability and protection efforts.
Analytical Techniques
Analytical techniques play a crucial role in improving luminol chemiluminescence efficiency. Chemiluminescence detection methods are widely used in various fields, including forensic science, environmental monitoring, and biomedical research. To enhance the efficiency of luminol chemiluminescence, several analytical approaches have been developed and refined over the years.
One of the primary techniques employed is spectrofluorometry, which allows for the precise measurement of light emission intensity and spectral characteristics. This method enables researchers to quantify the chemiluminescence output and optimize reaction conditions. By analyzing the emission spectra, scientists can identify potential interfering factors and develop strategies to mitigate their effects.
High-performance liquid chromatography (HPLC) coupled with chemiluminescence detection has emerged as a powerful analytical tool. This technique enables the separation and identification of luminol and its derivatives, as well as potential enhancers or inhibitors present in complex samples. HPLC-chemiluminescence systems offer high sensitivity and selectivity, making them particularly useful for trace analysis in forensic applications.
Electrochemical methods have also been employed to study and enhance luminol chemiluminescence. Cyclic voltammetry and amperometry can provide valuable insights into the electron transfer processes involved in the chemiluminescence reaction. These techniques allow researchers to investigate the kinetics of the reaction and identify potential catalysts or electrode materials that can improve efficiency.
Flow injection analysis (FIA) has proven to be an effective approach for optimizing luminol chemiluminescence reactions. By controlling the mixing and reaction conditions in a continuous flow system, researchers can achieve reproducible and rapid measurements. FIA systems can be easily automated and integrated with other analytical techniques, enhancing throughput and precision.
Mass spectrometry has been utilized to elucidate the reaction mechanisms and identify intermediates in luminol chemiluminescence. By coupling mass spectrometry with chromatographic techniques, researchers can gain a deeper understanding of the chemical processes involved and develop strategies to enhance efficiency based on mechanistic insights.
Microscopy techniques, such as fluorescence microscopy and chemiluminescence imaging, have been employed to study luminol reactions at the microscale level. These methods allow for the visualization of spatial distribution and temporal dynamics of chemiluminescence, providing valuable information for optimizing reaction conditions and developing novel applications.
Advanced data analysis techniques, including chemometrics and machine learning algorithms, have been applied to process and interpret the complex data generated by these analytical methods. These computational approaches enable researchers to extract meaningful patterns and relationships from large datasets, facilitating the optimization of luminol chemiluminescence efficiency.
One of the primary techniques employed is spectrofluorometry, which allows for the precise measurement of light emission intensity and spectral characteristics. This method enables researchers to quantify the chemiluminescence output and optimize reaction conditions. By analyzing the emission spectra, scientists can identify potential interfering factors and develop strategies to mitigate their effects.
High-performance liquid chromatography (HPLC) coupled with chemiluminescence detection has emerged as a powerful analytical tool. This technique enables the separation and identification of luminol and its derivatives, as well as potential enhancers or inhibitors present in complex samples. HPLC-chemiluminescence systems offer high sensitivity and selectivity, making them particularly useful for trace analysis in forensic applications.
Electrochemical methods have also been employed to study and enhance luminol chemiluminescence. Cyclic voltammetry and amperometry can provide valuable insights into the electron transfer processes involved in the chemiluminescence reaction. These techniques allow researchers to investigate the kinetics of the reaction and identify potential catalysts or electrode materials that can improve efficiency.
Flow injection analysis (FIA) has proven to be an effective approach for optimizing luminol chemiluminescence reactions. By controlling the mixing and reaction conditions in a continuous flow system, researchers can achieve reproducible and rapid measurements. FIA systems can be easily automated and integrated with other analytical techniques, enhancing throughput and precision.
Mass spectrometry has been utilized to elucidate the reaction mechanisms and identify intermediates in luminol chemiluminescence. By coupling mass spectrometry with chromatographic techniques, researchers can gain a deeper understanding of the chemical processes involved and develop strategies to enhance efficiency based on mechanistic insights.
Microscopy techniques, such as fluorescence microscopy and chemiluminescence imaging, have been employed to study luminol reactions at the microscale level. These methods allow for the visualization of spatial distribution and temporal dynamics of chemiluminescence, providing valuable information for optimizing reaction conditions and developing novel applications.
Advanced data analysis techniques, including chemometrics and machine learning algorithms, have been applied to process and interpret the complex data generated by these analytical methods. These computational approaches enable researchers to extract meaningful patterns and relationships from large datasets, facilitating the optimization of luminol chemiluminescence efficiency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!