How to Innovate Luminol Utilization in Research Labs?
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Luminol Research Evolution
Luminol, a chemiluminescent compound, has been a cornerstone in forensic science and biochemical research since its discovery in the early 20th century. The evolution of luminol research has been marked by significant milestones and continuous refinement of its applications. Initially used primarily for blood detection in crime scene investigations, luminol's potential has expanded far beyond its original scope.
In the 1930s, Walter Specht first applied luminol to forensic science, revolutionizing the field of crime scene analysis. This breakthrough laid the foundation for decades of research into improving luminol's sensitivity and specificity. The 1960s and 1970s saw a surge in luminol-based studies, with researchers focusing on enhancing its formulation and developing more sophisticated detection methods.
The advent of advanced imaging technologies in the 1980s and 1990s marked a new era in luminol research. Digital cameras and specialized light sources allowed for more precise documentation of luminol reactions, significantly improving the accuracy and reliability of forensic investigations. This period also witnessed the expansion of luminol applications into biochemistry and molecular biology, where it became a valuable tool for detecting various biological molecules and cellular processes.
The turn of the millennium brought about a renewed interest in luminol's potential beyond traditional applications. Researchers began exploring its use in environmental monitoring, food safety testing, and even medical diagnostics. The development of luminol-based nanoparticles and biosensors opened up new avenues for ultra-sensitive detection of trace substances in complex matrices.
Recent years have seen a focus on addressing some of luminol's limitations, such as its potential to interfere with DNA analysis and its sensitivity to environmental factors. Scientists have been working on developing more stable luminol formulations and alternative chemiluminescent compounds that offer improved selectivity and reduced interference.
The integration of luminol research with cutting-edge technologies like artificial intelligence and machine learning represents the latest frontier. These advancements are enabling more sophisticated pattern recognition in luminol-based imaging and enhancing the interpretation of complex chemiluminescent signals.
As research continues, the future of luminol utilization in laboratories looks promising. Efforts are underway to develop "smart" luminol systems that can autonomously detect and analyze specific molecular targets. Additionally, there is growing interest in combining luminol with other detection methods to create multi-modal analytical platforms, further expanding its utility in research settings.
In the 1930s, Walter Specht first applied luminol to forensic science, revolutionizing the field of crime scene analysis. This breakthrough laid the foundation for decades of research into improving luminol's sensitivity and specificity. The 1960s and 1970s saw a surge in luminol-based studies, with researchers focusing on enhancing its formulation and developing more sophisticated detection methods.
The advent of advanced imaging technologies in the 1980s and 1990s marked a new era in luminol research. Digital cameras and specialized light sources allowed for more precise documentation of luminol reactions, significantly improving the accuracy and reliability of forensic investigations. This period also witnessed the expansion of luminol applications into biochemistry and molecular biology, where it became a valuable tool for detecting various biological molecules and cellular processes.
The turn of the millennium brought about a renewed interest in luminol's potential beyond traditional applications. Researchers began exploring its use in environmental monitoring, food safety testing, and even medical diagnostics. The development of luminol-based nanoparticles and biosensors opened up new avenues for ultra-sensitive detection of trace substances in complex matrices.
Recent years have seen a focus on addressing some of luminol's limitations, such as its potential to interfere with DNA analysis and its sensitivity to environmental factors. Scientists have been working on developing more stable luminol formulations and alternative chemiluminescent compounds that offer improved selectivity and reduced interference.
The integration of luminol research with cutting-edge technologies like artificial intelligence and machine learning represents the latest frontier. These advancements are enabling more sophisticated pattern recognition in luminol-based imaging and enhancing the interpretation of complex chemiluminescent signals.
As research continues, the future of luminol utilization in laboratories looks promising. Efforts are underway to develop "smart" luminol systems that can autonomously detect and analyze specific molecular targets. Additionally, there is growing interest in combining luminol with other detection methods to create multi-modal analytical platforms, further expanding its utility in research settings.
Market Analysis for Luminol
The global market for luminol has been experiencing steady growth, driven by its widespread applications in forensic science, biochemistry research, and medical diagnostics. As a key chemiluminescent compound, luminol's unique properties have made it indispensable in various scientific and investigative fields.
In the forensic science sector, luminol remains a crucial tool for crime scene investigations, particularly in detecting trace amounts of blood. The increasing focus on advanced forensic techniques and the rising number of criminal investigations worldwide have contributed to the growing demand for luminol in this sector. Law enforcement agencies and forensic laboratories continue to be significant consumers of luminol-based products.
The biochemistry research market has also shown a strong appetite for luminol. Its ability to produce light when oxidized makes it valuable in studying cellular processes, enzyme kinetics, and oxidative stress. The expanding field of bioluminescence imaging in life sciences research has further boosted the demand for luminol and its derivatives.
In the medical diagnostics sector, luminol-based assays are gaining traction for their high sensitivity and specificity. These assays are used in detecting various biomarkers, hormones, and pathogens, contributing to more accurate and rapid diagnostic procedures. The growing emphasis on early disease detection and personalized medicine is expected to drive further adoption of luminol-based diagnostic tools.
The pharmaceutical industry represents another significant market for luminol, particularly in drug discovery and development processes. Luminol-based assays are employed in high-throughput screening of potential drug candidates, offering a cost-effective and efficient method for identifying promising compounds.
Geographically, North America and Europe lead the luminol market, owing to their advanced research infrastructure and stringent forensic practices. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing investments in life sciences research and forensic capabilities.
The market is characterized by a mix of established chemical companies and specialized biochemical suppliers. Key players are focusing on developing enhanced luminol formulations and expanding their product portfolios to cater to diverse research needs. Collaborations between industry and academic institutions are also becoming more common, aimed at exploring novel applications of luminol in various scientific disciplines.
Despite its established presence, the luminol market faces challenges such as the development of alternative chemiluminescent compounds and the need for more environmentally friendly formulations. However, ongoing research into improving luminol's stability, sensitivity, and specificity is expected to open new avenues for its application, potentially expanding its market reach.
In the forensic science sector, luminol remains a crucial tool for crime scene investigations, particularly in detecting trace amounts of blood. The increasing focus on advanced forensic techniques and the rising number of criminal investigations worldwide have contributed to the growing demand for luminol in this sector. Law enforcement agencies and forensic laboratories continue to be significant consumers of luminol-based products.
The biochemistry research market has also shown a strong appetite for luminol. Its ability to produce light when oxidized makes it valuable in studying cellular processes, enzyme kinetics, and oxidative stress. The expanding field of bioluminescence imaging in life sciences research has further boosted the demand for luminol and its derivatives.
In the medical diagnostics sector, luminol-based assays are gaining traction for their high sensitivity and specificity. These assays are used in detecting various biomarkers, hormones, and pathogens, contributing to more accurate and rapid diagnostic procedures. The growing emphasis on early disease detection and personalized medicine is expected to drive further adoption of luminol-based diagnostic tools.
The pharmaceutical industry represents another significant market for luminol, particularly in drug discovery and development processes. Luminol-based assays are employed in high-throughput screening of potential drug candidates, offering a cost-effective and efficient method for identifying promising compounds.
Geographically, North America and Europe lead the luminol market, owing to their advanced research infrastructure and stringent forensic practices. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing investments in life sciences research and forensic capabilities.
The market is characterized by a mix of established chemical companies and specialized biochemical suppliers. Key players are focusing on developing enhanced luminol formulations and expanding their product portfolios to cater to diverse research needs. Collaborations between industry and academic institutions are also becoming more common, aimed at exploring novel applications of luminol in various scientific disciplines.
Despite its established presence, the luminol market faces challenges such as the development of alternative chemiluminescent compounds and the need for more environmentally friendly formulations. However, ongoing research into improving luminol's stability, sensitivity, and specificity is expected to open new avenues for its application, potentially expanding its market reach.
Current Challenges in Luminol Usage
Despite its widespread use in forensic science and research laboratories, luminol faces several significant challenges that limit its effectiveness and broader application. One of the primary issues is its lack of specificity. While luminol is highly sensitive to blood, it also reacts with other substances such as copper, bleach, and certain plant materials, leading to false positives. This non-specificity can compromise the reliability of forensic investigations and research results, necessitating additional confirmatory tests.
Another challenge is the potential for luminol to interfere with subsequent DNA analysis. The chemical reaction that produces the characteristic blue luminescence can potentially degrade or alter DNA evidence, making it more difficult to obtain accurate genetic profiles. This limitation is particularly problematic in forensic contexts where preserving DNA evidence is crucial for identifying suspects or victims.
The short-lived nature of the luminol reaction poses another significant challenge. The luminescence typically lasts only for a few seconds, making it difficult to document and analyze the results thoroughly. This brevity can be especially problematic in large crime scenes or complex research setups where multiple areas need to be examined simultaneously.
Environmental factors also present challenges in luminol usage. Temperature, humidity, and pH levels can all affect the intensity and duration of the luminol reaction. These variables can lead to inconsistent results across different environments, making it difficult to standardize protocols and compare findings between different laboratories or crime scenes.
The preparation and application of luminol solutions also present practical challenges. The solution must be freshly prepared and used within a short time frame to maintain its effectiveness. This requirement can be logistically challenging, especially in field applications or resource-limited settings. Additionally, the need for complete darkness during luminol application and observation can be impractical in many real-world scenarios.
Health and safety concerns associated with luminol usage are another significant challenge. While luminol itself is not highly toxic, the chemicals used in its preparation and the potential exposure to blood or other biological materials pose risks to users. Proper safety protocols and personal protective equipment are essential but can be cumbersome and may limit the ease of use in some settings.
Lastly, the qualitative nature of luminol testing presents challenges in quantitative analysis. While luminol can detect the presence of blood, it does not provide information about the amount of blood present or its age. This limitation restricts its usefulness in certain research applications and forensic investigations where quantitative data is crucial for drawing accurate conclusions.
Another challenge is the potential for luminol to interfere with subsequent DNA analysis. The chemical reaction that produces the characteristic blue luminescence can potentially degrade or alter DNA evidence, making it more difficult to obtain accurate genetic profiles. This limitation is particularly problematic in forensic contexts where preserving DNA evidence is crucial for identifying suspects or victims.
The short-lived nature of the luminol reaction poses another significant challenge. The luminescence typically lasts only for a few seconds, making it difficult to document and analyze the results thoroughly. This brevity can be especially problematic in large crime scenes or complex research setups where multiple areas need to be examined simultaneously.
Environmental factors also present challenges in luminol usage. Temperature, humidity, and pH levels can all affect the intensity and duration of the luminol reaction. These variables can lead to inconsistent results across different environments, making it difficult to standardize protocols and compare findings between different laboratories or crime scenes.
The preparation and application of luminol solutions also present practical challenges. The solution must be freshly prepared and used within a short time frame to maintain its effectiveness. This requirement can be logistically challenging, especially in field applications or resource-limited settings. Additionally, the need for complete darkness during luminol application and observation can be impractical in many real-world scenarios.
Health and safety concerns associated with luminol usage are another significant challenge. While luminol itself is not highly toxic, the chemicals used in its preparation and the potential exposure to blood or other biological materials pose risks to users. Proper safety protocols and personal protective equipment are essential but can be cumbersome and may limit the ease of use in some settings.
Lastly, the qualitative nature of luminol testing presents challenges in quantitative analysis. While luminol can detect the presence of blood, it does not provide information about the amount of blood present or its age. This limitation restricts its usefulness in certain research applications and forensic investigations where quantitative data is crucial for drawing accurate conclusions.
Existing Luminol Applications
01 Luminol in forensic applications
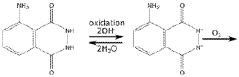
Luminol is widely used in forensic science for detecting trace amounts of blood at crime scenes. When luminol comes into contact with the iron in hemoglobin, it produces a bright blue chemiluminescence. This reaction can reveal blood traces that are invisible to the naked eye, even if the area has been cleaned. Forensic investigators use luminol-based solutions to spray suspected areas and photograph the resulting luminescence.- Luminol-based chemiluminescence detection methods: Luminol is widely used in chemiluminescence detection methods for various applications. These methods utilize the light-emitting properties of luminol when it reacts with specific substances, allowing for sensitive and selective detection of target analytes. The techniques are applied in fields such as forensic science, environmental monitoring, and biomedical research.
- Luminol derivatives and modifications: Research focuses on developing luminol derivatives and modifications to enhance its properties for specific applications. These modifications aim to improve sensitivity, selectivity, or stability of luminol-based detection systems. Some approaches include synthesizing new luminol analogues or incorporating luminol into more complex molecular structures.
- Luminol in forensic applications: Luminol plays a crucial role in forensic science, particularly in the detection of blood traces at crime scenes. The compound reacts with the iron in hemoglobin, producing a blue luminescence that can reveal blood stains not visible to the naked eye. Forensic investigators use luminol-based solutions to identify and document potential evidence in criminal investigations.
- Luminol in biomedical and clinical diagnostics: Luminol-based assays are employed in biomedical research and clinical diagnostics. These applications include detecting specific biomolecules, studying cellular processes, and developing diagnostic tests for various diseases. The high sensitivity of luminol chemiluminescence allows for the detection of low concentrations of target analytes in biological samples.
- Luminol in environmental monitoring: Luminol-based detection methods are utilized in environmental monitoring applications. These techniques can detect and quantify various pollutants, heavy metals, or other substances of interest in water, soil, or air samples. The high sensitivity and relatively simple instrumentation make luminol-based methods attractive for on-site environmental testing and monitoring.
02 Luminol in medical diagnostics
Luminol has applications in medical diagnostics, particularly in detecting certain enzymes and cellular components. It can be used to measure the activity of myeloperoxidase, an enzyme found in white blood cells, which is useful in diagnosing inflammatory conditions. Additionally, luminol-based assays are employed in detecting reactive oxygen species in biological samples, aiding in the study of oxidative stress and cellular processes.Expand Specific Solutions03 Enhanced luminol formulations
Researchers have developed enhanced luminol formulations to improve its sensitivity and specificity. These formulations may include additives that intensify the chemiluminescence, extend the duration of the light emission, or reduce interference from other substances. Some enhancements focus on improving the stability of luminol solutions, allowing for longer shelf life and more consistent results in various applications.Expand Specific Solutions04 Luminol in environmental monitoring
Luminol is utilized in environmental monitoring applications, particularly for detecting pollutants and contaminants in water and soil samples. It can be used to measure the presence of certain heavy metals, oxidizing agents, and other environmental toxins. Luminol-based sensors and test kits have been developed for rapid on-site analysis of environmental samples, providing a cost-effective and sensitive method for pollution detection and monitoring.Expand Specific Solutions05 Novel applications of luminol
Researchers are exploring novel applications of luminol beyond its traditional uses. These include incorporating luminol into biosensors for detecting specific biological molecules, using it in the development of new imaging techniques for cellular studies, and applying it in the field of nanotechnology. Some studies are investigating the potential of luminol-based systems in drug delivery and cancer treatment, leveraging its chemiluminescent properties for targeted therapies and diagnostics.Expand Specific Solutions
Key Players in Luminol Research
The innovation of luminol utilization in research labs is in a mature stage, with a well-established market and diverse applications. The global market for chemiluminescence reagents, including luminol, is projected to grow steadily due to increasing demand in forensic science, biomedical research, and clinical diagnostics. Technologically, luminol-based assays are well-developed, with companies like Cyanagen Srl and DiaSorin Italia SpA leading in reagent development. Academic institutions such as Washington University in St. Louis and Fuzhou University are actively researching novel applications and improvements in luminol-based techniques, indicating ongoing innovation in this field despite its maturity.
Alverix, Inc.
Technical Solution: Alverix has developed innovative point-of-care diagnostic platforms that utilize luminol-based chemiluminescence for rapid and sensitive detection of various analytes. Their proprietary CMOS-based photon counting technology enables the detection of extremely low levels of light produced by luminol reactions, allowing for highly sensitive assays in a compact, portable format[10]. The company has also created specialized microfluidic cartridges that integrate luminol reagents with sample processing steps, enabling complex assays to be performed with minimal user intervention. Alverix's approach focuses on miniaturizing and simplifying luminol-based detection systems for use outside of traditional laboratory settings, potentially expanding the applications of this technology in field research and resource-limited environments.
Strengths: Portable and user-friendly systems, high sensitivity in a compact format, potential for field applications. Weaknesses: Limited range of available assays, may not be suitable for all research applications.
Cyanagen Srl
Technical Solution: Cyanagen Srl has developed innovative luminol-based chemiluminescence substrates for enhanced sensitivity in research applications. Their Westar chemiluminescent HRP substrates utilize optimized luminol derivatives to achieve up to 10-fold higher light output compared to standard ECL reagents[1]. The company has also introduced Lumit-Ω, a novel luminol-based substrate that produces a long-lasting glow signal for over 24 hours, enabling extended imaging times for Western blots and other assays[2]. Additionally, Cyanagen has formulated specialized luminol reagents for specific research needs, such as their SuperNova substrate for ultrasensitive protein detection with femtogram-level sensitivity[3].
Strengths: High sensitivity, long-lasting signal, versatility for various research applications. Weaknesses: May require specialized imaging equipment, potentially higher cost compared to traditional substrates.
Breakthrough Luminol Studies
Reagent and kit for performing chemiluminescent reaction
PatentActiveUS20200017764A1
Innovation
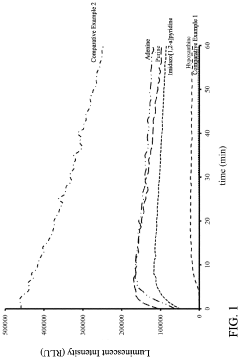
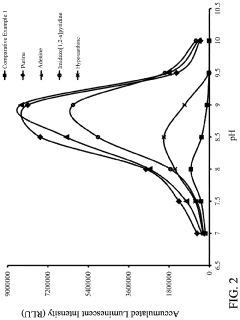
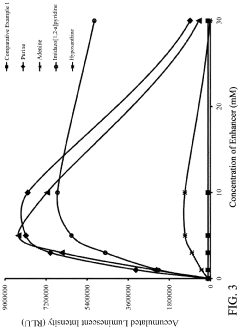
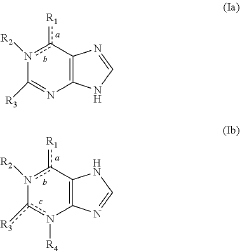
- A reagent comprising luminol or luminol derivatives, an oxidant, and a nitrogen-containing fused heterocyclic enhancer such as imidazo[1,2-a]pyridine, which enhances luminescent intensity and prolongs luminescence time.
Method and device for chemiluminescence-based analysis
PatentWO2017221258A1
Innovation
- Employing noble metal nanoparticles with an average diameter greater than 25 nm, specifically gold or silver nanospheres, in a microfluidic device with a curved channel design to enhance chemiluminescence intensity and facilitate detection of analytes, such as metal ions or oxidizers, by optimizing particle size distribution and channel geometry for improved mixing and signal emission.
Safety Protocols for Luminol
Luminol, a versatile chemiluminescent compound, requires careful handling and strict safety protocols in research laboratories to ensure the well-being of personnel and the integrity of experiments. Implementing comprehensive safety measures is crucial for maximizing the benefits of luminol while minimizing potential risks.
Personal protective equipment (PPE) is the first line of defense when working with luminol. Researchers must wear appropriate gloves, lab coats, and safety goggles to prevent skin contact and eye exposure. Nitrile gloves are recommended due to their chemical resistance. Additionally, working in a well-ventilated area or under a fume hood is essential to minimize inhalation of luminol particles or vapors.
Proper storage of luminol is critical to maintain its stability and prevent accidental exposure. It should be kept in a cool, dry place, away from direct sunlight and heat sources. Luminol should be stored in airtight, opaque containers to protect it from light and moisture, which can degrade its effectiveness. Clearly labeling containers with the compound name, concentration, and any relevant hazard warnings is essential for safe handling and inventory management.
Handling procedures for luminol should be standardized and communicated to all laboratory personnel. This includes guidelines for weighing, dissolving, and transferring the compound. Using designated equipment and work areas for luminol preparation can help prevent cross-contamination and reduce the risk of accidental exposure. Implementing a buddy system for high-risk procedures can provide an additional layer of safety.
Waste management is a crucial aspect of luminol safety protocols. Proper disposal methods for unused luminol solutions and contaminated materials must be established and followed. This may include neutralization procedures or specialized waste collection systems. Educating researchers on the importance of proper waste disposal and providing clear guidelines can help prevent environmental contamination and potential health hazards.
Emergency response procedures specific to luminol incidents should be developed and regularly reviewed. This includes protocols for spill cleanup, first aid measures for accidental exposure, and evacuation procedures if necessary. Ensuring that all laboratory personnel are trained in these procedures and that emergency equipment, such as eyewash stations and safety showers, is readily accessible and in good working order is essential.
Regular safety audits and training sessions should be conducted to maintain a high level of awareness and compliance with luminol safety protocols. These sessions can cover topics such as proper handling techniques, updates to safety procedures, and reviews of any incidents or near-misses. Encouraging a culture of safety and open communication about potential hazards can significantly enhance the overall safety environment in research laboratories utilizing luminol.
Personal protective equipment (PPE) is the first line of defense when working with luminol. Researchers must wear appropriate gloves, lab coats, and safety goggles to prevent skin contact and eye exposure. Nitrile gloves are recommended due to their chemical resistance. Additionally, working in a well-ventilated area or under a fume hood is essential to minimize inhalation of luminol particles or vapors.
Proper storage of luminol is critical to maintain its stability and prevent accidental exposure. It should be kept in a cool, dry place, away from direct sunlight and heat sources. Luminol should be stored in airtight, opaque containers to protect it from light and moisture, which can degrade its effectiveness. Clearly labeling containers with the compound name, concentration, and any relevant hazard warnings is essential for safe handling and inventory management.
Handling procedures for luminol should be standardized and communicated to all laboratory personnel. This includes guidelines for weighing, dissolving, and transferring the compound. Using designated equipment and work areas for luminol preparation can help prevent cross-contamination and reduce the risk of accidental exposure. Implementing a buddy system for high-risk procedures can provide an additional layer of safety.
Waste management is a crucial aspect of luminol safety protocols. Proper disposal methods for unused luminol solutions and contaminated materials must be established and followed. This may include neutralization procedures or specialized waste collection systems. Educating researchers on the importance of proper waste disposal and providing clear guidelines can help prevent environmental contamination and potential health hazards.
Emergency response procedures specific to luminol incidents should be developed and regularly reviewed. This includes protocols for spill cleanup, first aid measures for accidental exposure, and evacuation procedures if necessary. Ensuring that all laboratory personnel are trained in these procedures and that emergency equipment, such as eyewash stations and safety showers, is readily accessible and in good working order is essential.
Regular safety audits and training sessions should be conducted to maintain a high level of awareness and compliance with luminol safety protocols. These sessions can cover topics such as proper handling techniques, updates to safety procedures, and reviews of any incidents or near-misses. Encouraging a culture of safety and open communication about potential hazards can significantly enhance the overall safety environment in research laboratories utilizing luminol.
Luminol Disposal Methods
Luminol disposal methods in research labs have become increasingly important due to environmental concerns and safety regulations. The traditional approach of simply discarding luminol down the drain is no longer acceptable, as it can lead to water pollution and potential harm to aquatic ecosystems. To address this issue, several innovative disposal methods have been developed and implemented in research facilities worldwide.
One of the most effective methods for luminol disposal is chemical degradation. This process involves treating luminol with oxidizing agents such as hydrogen peroxide or sodium hypochlorite. These chemicals break down the luminol molecules into simpler, less harmful compounds that can be safely disposed of through conventional waste management systems. The degradation process is typically carried out in a controlled environment, such as a fume hood, to ensure proper ventilation and minimize exposure risks.
Another promising approach is the use of advanced filtration systems. These systems employ activated carbon filters or specialized membranes to remove luminol from waste solutions. The filtered luminol can then be collected and properly disposed of as hazardous waste, while the treated water can be safely released into the sewage system. This method is particularly useful for labs that generate large volumes of luminol-containing waste.
Photocatalytic degradation has also emerged as an innovative luminol disposal technique. This method utilizes light-activated catalysts, such as titanium dioxide, to break down luminol molecules. When exposed to ultraviolet light, these catalysts generate highly reactive species that can rapidly degrade luminol into harmless byproducts. Photocatalytic systems can be integrated into existing waste treatment processes, offering a sustainable and energy-efficient solution for luminol disposal.
For smaller quantities of luminol waste, solidification and encapsulation methods have proven effective. These techniques involve mixing luminol with inert materials such as cement or polymers to create a solid matrix that immobilizes the compound. The resulting solid waste can then be disposed of in accordance with local hazardous waste regulations. This approach is particularly useful for labs with limited access to advanced treatment facilities.
Biological treatment systems have also shown promise in luminol disposal. These systems utilize specially selected microorganisms capable of metabolizing luminol and converting it into less harmful substances. Bioreactors containing these microorganisms can be integrated into existing wastewater treatment processes, offering a natural and sustainable approach to luminol degradation.
As research labs continue to innovate in luminol utilization, it is crucial to develop and implement responsible disposal methods. By adopting these advanced techniques, laboratories can minimize their environmental impact while ensuring compliance with regulatory requirements. Furthermore, ongoing research into novel disposal methods, such as electrochemical degradation and advanced oxidation processes, holds promise for even more efficient and sustainable luminol management in the future.
One of the most effective methods for luminol disposal is chemical degradation. This process involves treating luminol with oxidizing agents such as hydrogen peroxide or sodium hypochlorite. These chemicals break down the luminol molecules into simpler, less harmful compounds that can be safely disposed of through conventional waste management systems. The degradation process is typically carried out in a controlled environment, such as a fume hood, to ensure proper ventilation and minimize exposure risks.
Another promising approach is the use of advanced filtration systems. These systems employ activated carbon filters or specialized membranes to remove luminol from waste solutions. The filtered luminol can then be collected and properly disposed of as hazardous waste, while the treated water can be safely released into the sewage system. This method is particularly useful for labs that generate large volumes of luminol-containing waste.
Photocatalytic degradation has also emerged as an innovative luminol disposal technique. This method utilizes light-activated catalysts, such as titanium dioxide, to break down luminol molecules. When exposed to ultraviolet light, these catalysts generate highly reactive species that can rapidly degrade luminol into harmless byproducts. Photocatalytic systems can be integrated into existing waste treatment processes, offering a sustainable and energy-efficient solution for luminol disposal.
For smaller quantities of luminol waste, solidification and encapsulation methods have proven effective. These techniques involve mixing luminol with inert materials such as cement or polymers to create a solid matrix that immobilizes the compound. The resulting solid waste can then be disposed of in accordance with local hazardous waste regulations. This approach is particularly useful for labs with limited access to advanced treatment facilities.
Biological treatment systems have also shown promise in luminol disposal. These systems utilize specially selected microorganisms capable of metabolizing luminol and converting it into less harmful substances. Bioreactors containing these microorganisms can be integrated into existing wastewater treatment processes, offering a natural and sustainable approach to luminol degradation.
As research labs continue to innovate in luminol utilization, it is crucial to develop and implement responsible disposal methods. By adopting these advanced techniques, laboratories can minimize their environmental impact while ensuring compliance with regulatory requirements. Furthermore, ongoing research into novel disposal methods, such as electrochemical degradation and advanced oxidation processes, holds promise for even more efficient and sustainable luminol management in the future.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!