How to Measure Phase Stability in HE Ceramics under Cyclic Thermal Loads in the Lab
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HE Ceramics Phase Stability Background & Objectives
High-entropy (HE) ceramics represent a revolutionary class of materials that have emerged from the concept of high-entropy alloys, extending the compositional complexity paradigm to ceramic systems. These materials, typically comprising five or more principal elements in near-equimolar ratios, have garnered significant attention due to their exceptional properties including high hardness, thermal stability, and oxidation resistance, making them promising candidates for extreme environment applications.
The evolution of HE ceramics has followed a trajectory from theoretical conceptualization in the early 2010s to experimental validation and property characterization in recent years. Initial research focused primarily on establishing synthesis methodologies, while current efforts are increasingly directed toward understanding structure-property relationships and performance under operational conditions.
A critical aspect of HE ceramics that remains insufficiently understood is their phase stability under cyclic thermal loading conditions. Unlike conventional ceramics, the complex compositional nature of HE ceramics introduces unique entropy-stabilization mechanisms that can significantly influence phase transformations, microstructural evolution, and ultimately, material performance during thermal cycling.
The primary objective of this technical investigation is to develop robust methodologies for measuring and characterizing phase stability in HE ceramics when subjected to cyclic thermal loads in laboratory settings. This includes establishing standardized testing protocols, identifying appropriate analytical techniques, and developing predictive models that can accurately capture phase transformation behaviors.
Specifically, we aim to address several key technical questions: How do entropy-stabilization mechanisms in HE ceramics respond to repeated thermal cycling? What are the critical temperature thresholds and cycling frequencies that trigger phase transformations? How do compositional variations influence phase stability under thermal cycling conditions? What analytical techniques provide the most accurate and comprehensive assessment of phase evolution?
By addressing these questions, we seek to establish a fundamental understanding of the thermodynamic and kinetic factors governing phase stability in HE ceramics, which will directly inform material design strategies and processing parameters. This knowledge is essential for advancing HE ceramics from laboratory curiosities to engineered materials capable of withstanding the demanding conditions encountered in aerospace, energy, and defense applications.
The outcomes of this investigation will contribute significantly to the broader field of advanced ceramics by establishing methodological frameworks that can be adapted for other complex ceramic systems, ultimately accelerating the development and deployment of next-generation structural ceramics.
The evolution of HE ceramics has followed a trajectory from theoretical conceptualization in the early 2010s to experimental validation and property characterization in recent years. Initial research focused primarily on establishing synthesis methodologies, while current efforts are increasingly directed toward understanding structure-property relationships and performance under operational conditions.
A critical aspect of HE ceramics that remains insufficiently understood is their phase stability under cyclic thermal loading conditions. Unlike conventional ceramics, the complex compositional nature of HE ceramics introduces unique entropy-stabilization mechanisms that can significantly influence phase transformations, microstructural evolution, and ultimately, material performance during thermal cycling.
The primary objective of this technical investigation is to develop robust methodologies for measuring and characterizing phase stability in HE ceramics when subjected to cyclic thermal loads in laboratory settings. This includes establishing standardized testing protocols, identifying appropriate analytical techniques, and developing predictive models that can accurately capture phase transformation behaviors.
Specifically, we aim to address several key technical questions: How do entropy-stabilization mechanisms in HE ceramics respond to repeated thermal cycling? What are the critical temperature thresholds and cycling frequencies that trigger phase transformations? How do compositional variations influence phase stability under thermal cycling conditions? What analytical techniques provide the most accurate and comprehensive assessment of phase evolution?
By addressing these questions, we seek to establish a fundamental understanding of the thermodynamic and kinetic factors governing phase stability in HE ceramics, which will directly inform material design strategies and processing parameters. This knowledge is essential for advancing HE ceramics from laboratory curiosities to engineered materials capable of withstanding the demanding conditions encountered in aerospace, energy, and defense applications.
The outcomes of this investigation will contribute significantly to the broader field of advanced ceramics by establishing methodological frameworks that can be adapted for other complex ceramic systems, ultimately accelerating the development and deployment of next-generation structural ceramics.
Market Applications for Thermally Stable HE Ceramics
High-entropy ceramics with superior thermal stability represent a significant advancement in materials science, opening new avenues for applications in extreme environments. The aerospace industry stands as a primary beneficiary, where thermally stable HE ceramics can be utilized in thermal protection systems for spacecraft, hypersonic vehicles, and rocket propulsion components. These materials offer exceptional resistance to thermal cycling, making them ideal for reusable launch vehicles where components must withstand repeated atmospheric re-entry conditions.
The energy sector presents another substantial market opportunity. Gas turbines for power generation operate under extreme thermal conditions and cyclic loading. HE ceramics with demonstrated phase stability can significantly extend turbine blade lifespans, improve operational efficiency, and reduce maintenance costs. Similarly, concentrated solar power systems require materials that maintain structural integrity under daily thermal cycling, where these advanced ceramics could revolutionize receiver designs.
Advanced manufacturing, particularly in metal processing, demands materials that can withstand repeated heating and cooling cycles. Crucibles, molds, and other tooling made from thermally stable HE ceramics could dramatically extend service life in foundries and metal forming operations, reducing production downtime and replacement costs. The semiconductor industry also requires materials with exceptional thermal stability for wafer processing equipment, where temperature uniformity and dimensional stability are critical.
Defense applications represent a high-value market segment, with thermal protection for missile systems, radar-absorbing materials, and armor systems all benefiting from the unique properties of HE ceramics. The ability to maintain phase stability under rapid thermal cycling is particularly valuable for ballistic protection systems that may experience extreme heat pulses.
Emerging technologies like fusion energy present long-term market opportunities. Fusion reactor components must withstand extreme thermal gradients and neutron bombardment, conditions where conventional materials fail rapidly. HE ceramics with demonstrated phase stability could become enabling materials for commercial fusion power.
The automotive sector, particularly for high-performance and electric vehicles, represents a growing market. Brake systems, turbocharger components, and battery thermal management systems could all benefit from thermally stable ceramic materials that maintain consistent performance across thousands of thermal cycles.
Medical device manufacturing, particularly for implantable devices requiring repeated sterilization cycles, represents a specialized but high-value application area where the biocompatibility and thermal stability of certain HE ceramic compositions could provide unique advantages.
The energy sector presents another substantial market opportunity. Gas turbines for power generation operate under extreme thermal conditions and cyclic loading. HE ceramics with demonstrated phase stability can significantly extend turbine blade lifespans, improve operational efficiency, and reduce maintenance costs. Similarly, concentrated solar power systems require materials that maintain structural integrity under daily thermal cycling, where these advanced ceramics could revolutionize receiver designs.
Advanced manufacturing, particularly in metal processing, demands materials that can withstand repeated heating and cooling cycles. Crucibles, molds, and other tooling made from thermally stable HE ceramics could dramatically extend service life in foundries and metal forming operations, reducing production downtime and replacement costs. The semiconductor industry also requires materials with exceptional thermal stability for wafer processing equipment, where temperature uniformity and dimensional stability are critical.
Defense applications represent a high-value market segment, with thermal protection for missile systems, radar-absorbing materials, and armor systems all benefiting from the unique properties of HE ceramics. The ability to maintain phase stability under rapid thermal cycling is particularly valuable for ballistic protection systems that may experience extreme heat pulses.
Emerging technologies like fusion energy present long-term market opportunities. Fusion reactor components must withstand extreme thermal gradients and neutron bombardment, conditions where conventional materials fail rapidly. HE ceramics with demonstrated phase stability could become enabling materials for commercial fusion power.
The automotive sector, particularly for high-performance and electric vehicles, represents a growing market. Brake systems, turbocharger components, and battery thermal management systems could all benefit from thermally stable ceramic materials that maintain consistent performance across thousands of thermal cycles.
Medical device manufacturing, particularly for implantable devices requiring repeated sterilization cycles, represents a specialized but high-value application area where the biocompatibility and thermal stability of certain HE ceramic compositions could provide unique advantages.
Current Challenges in HE Ceramics Phase Characterization
The characterization of phase stability in High-Entropy (HE) ceramics under cyclic thermal loading presents significant technical challenges that impede comprehensive understanding of these advanced materials. Current X-ray diffraction (XRD) techniques, while fundamental for phase identification, struggle with the complex overlapping diffraction patterns characteristic of multi-element HE ceramics. This complexity often leads to ambiguous phase identification, particularly when dealing with minor phases or those with similar crystal structures.
In-situ high-temperature XRD measurements face substantial technical limitations when attempting to replicate the rapid thermal cycling conditions that HE ceramics experience in real applications. The temporal resolution of conventional XRD systems is insufficient to capture transient phase transformations occurring during rapid thermal cycles, creating a significant gap between laboratory characterization and actual application conditions.
Electron microscopy techniques, including TEM and SEM, encounter difficulties in maintaining consistent sample conditions during thermal cycling experiments. Sample preparation for these techniques can inadvertently alter the microstructure or introduce artifacts that compromise data integrity. Additionally, the limited field of view in high-resolution imaging makes it challenging to obtain statistically significant data representative of bulk material behavior.
Neutron diffraction, while valuable for analyzing light elements often present in HE ceramics, suffers from limited accessibility due to the scarcity of neutron source facilities. This restricts the widespread application of this technique for routine phase stability assessments, despite its potential advantages over X-ray based methods for certain material compositions.
Thermal analysis techniques such as DSC and TGA provide valuable information about phase transitions but lack spatial resolution. These methods cannot directly correlate thermal events with specific microstructural features or localized phase changes, limiting their utility for comprehensive phase stability characterization in heterogeneous HE ceramic systems.
Computational modeling approaches face validation challenges due to the scarcity of experimental data on phase stability under cyclic thermal conditions. The complex interactions between multiple elements in HE ceramics create enormous computational demands that current simulation capabilities struggle to address adequately, particularly for predicting behavior over numerous thermal cycles.
Standardization issues further complicate phase characterization efforts, with inconsistent testing protocols across research groups making direct comparison of results difficult. The lack of reference materials specifically designed for HE ceramic systems hampers calibration efforts and introduces additional uncertainty in phase identification and quantification.
In-situ high-temperature XRD measurements face substantial technical limitations when attempting to replicate the rapid thermal cycling conditions that HE ceramics experience in real applications. The temporal resolution of conventional XRD systems is insufficient to capture transient phase transformations occurring during rapid thermal cycles, creating a significant gap between laboratory characterization and actual application conditions.
Electron microscopy techniques, including TEM and SEM, encounter difficulties in maintaining consistent sample conditions during thermal cycling experiments. Sample preparation for these techniques can inadvertently alter the microstructure or introduce artifacts that compromise data integrity. Additionally, the limited field of view in high-resolution imaging makes it challenging to obtain statistically significant data representative of bulk material behavior.
Neutron diffraction, while valuable for analyzing light elements often present in HE ceramics, suffers from limited accessibility due to the scarcity of neutron source facilities. This restricts the widespread application of this technique for routine phase stability assessments, despite its potential advantages over X-ray based methods for certain material compositions.
Thermal analysis techniques such as DSC and TGA provide valuable information about phase transitions but lack spatial resolution. These methods cannot directly correlate thermal events with specific microstructural features or localized phase changes, limiting their utility for comprehensive phase stability characterization in heterogeneous HE ceramic systems.
Computational modeling approaches face validation challenges due to the scarcity of experimental data on phase stability under cyclic thermal conditions. The complex interactions between multiple elements in HE ceramics create enormous computational demands that current simulation capabilities struggle to address adequately, particularly for predicting behavior over numerous thermal cycles.
Standardization issues further complicate phase characterization efforts, with inconsistent testing protocols across research groups making direct comparison of results difficult. The lack of reference materials specifically designed for HE ceramic systems hampers calibration efforts and introduces additional uncertainty in phase identification and quantification.
Established Protocols for Phase Stability Measurement
01 Compositional design for phase stability in HE ceramics
High-entropy ceramics can achieve enhanced phase stability through careful compositional design. By selecting elements with appropriate atomic size differences, electronegativity, and valence electron concentration, researchers can control the formation of single-phase structures. The balance between configurational entropy and enthalpy plays a crucial role in determining phase stability. Multi-principal element compositions with equimolar or near-equimolar ratios often demonstrate superior stability due to the high configurational entropy that minimizes Gibbs free energy.- Compositional design for phase stability in HE ceramics: The phase stability of high-entropy ceramics can be enhanced through careful compositional design. By selecting elements with similar atomic radii, electronegativity, and valence electron concentration, the formation of single-phase solid solutions can be promoted. This approach minimizes lattice distortion and reduces the driving force for phase separation, resulting in improved thermodynamic stability at elevated temperatures. The entropy contribution from multiple elements helps to overcome the enthalpy barriers to phase separation.
- Processing techniques to enhance phase stability: Various processing techniques can be employed to enhance the phase stability of high-entropy ceramics. These include rapid solidification methods, high-energy ball milling, spark plasma sintering, and hot isostatic pressing. These techniques can create non-equilibrium conditions that favor the formation of single-phase structures by kinetically trapping metastable phases. Post-processing heat treatments can also be used to relieve internal stresses and promote homogenization of the microstructure, further improving phase stability.
- Role of entropy in stabilizing high-entropy ceramic phases: The configurational entropy plays a crucial role in stabilizing high-entropy ceramic phases. As the number of constituent elements increases, the configurational entropy contribution to the Gibbs free energy becomes more significant, potentially overcoming the enthalpy penalties associated with atomic size mismatches and chemical interactions. This entropy-driven stabilization mechanism is particularly effective at elevated temperatures where the -TΔS term in the Gibbs free energy equation becomes more dominant, leading to enhanced phase stability in multi-component ceramic systems.
- Influence of defects and microstructure on phase stability: Defects and microstructural features significantly impact the phase stability of high-entropy ceramics. Point defects, such as vacancies and interstitials, can either enhance or deteriorate phase stability depending on their concentration and distribution. Grain boundaries and interfaces can serve as nucleation sites for secondary phases but can also act as sinks for defects, potentially improving overall stability. Controlling the defect chemistry and microstructure through dopants and processing parameters is essential for optimizing the phase stability of high-entropy ceramic systems.
- Environmental factors affecting phase stability: Environmental factors such as temperature, pressure, and atmosphere significantly influence the phase stability of high-entropy ceramics. Extreme temperatures can trigger phase transformations, while oxidizing or reducing atmospheres can alter the valence states of constituent elements, potentially leading to phase decomposition. Thermal cycling and thermal shock can induce microstructural changes that affect long-term stability. Understanding these environmental effects is crucial for designing high-entropy ceramics with reliable performance under specific service conditions.
02 Processing techniques affecting phase stability
Various processing techniques significantly impact the phase stability of high-entropy ceramics. Methods such as spark plasma sintering, hot pressing, and reactive spark plasma sintering can be optimized to achieve desired phase compositions. Processing parameters including temperature, pressure, holding time, and cooling rate directly influence grain growth, densification, and phase transformations. Advanced synthesis routes like solution-based methods and mechanochemical processing can produce more homogeneous elemental distributions, enhancing phase stability at both ambient and elevated temperatures.Expand Specific Solutions03 Thermodynamic factors influencing stability
Thermodynamic factors play a critical role in determining the phase stability of high-entropy ceramics. The competition between configurational entropy and mixing enthalpy governs phase formation and stability. Higher configurational entropy tends to stabilize single-phase structures by reducing Gibbs free energy. The enthalpy of mixing, influenced by atomic size differences and bond energies between constituent elements, can either promote or hinder phase stability. Understanding these thermodynamic relationships enables the design of high-entropy ceramic systems with enhanced stability across wider temperature ranges.Expand Specific Solutions04 Microstructural engineering for enhanced stability
Microstructural engineering approaches can significantly enhance the phase stability of high-entropy ceramics. Controlling grain size, grain boundary characteristics, and defect concentrations helps maintain stable phases under various conditions. The introduction of specific dopants or secondary phases can pin grain boundaries and prevent unwanted phase transformations. Gradient structures and core-shell architectures provide additional stability mechanisms by creating energy barriers against phase separation. These microstructural features can be tailored to maintain phase stability even under extreme environmental conditions.Expand Specific Solutions05 Environmental factors affecting phase stability
Environmental factors significantly impact the phase stability of high-entropy ceramics during both processing and application. Temperature fluctuations, oxidizing/reducing atmospheres, radiation exposure, and mechanical stresses can all trigger phase transformations. Understanding these environmental influences is crucial for designing high-entropy ceramics with robust phase stability. Certain compositional designs demonstrate superior resistance to phase decomposition under specific environmental conditions. Testing protocols that simulate actual service environments are essential for accurately predicting long-term phase stability in applications such as nuclear materials, high-temperature structural components, and protective coatings.Expand Specific Solutions
Leading Research Groups in HE Ceramics Development
The high-entropy ceramics market under cyclic thermal loads is in an early growth phase, characterized by increasing research activity but limited commercial applications. The market size is expanding as industries seek materials with enhanced thermal stability for extreme environments. Technologically, research institutions like Shanghai Institute of Ceramics and Zhengzhou University are leading fundamental investigations, while established materials companies including Corning, SCHOTT AG, and NGK Insulators are developing practical applications. Multinational corporations such as Panasonic and Toyota Motor Europe represent potential end-users exploring high-entropy ceramics for thermal management solutions. The technology remains in early maturity, with academic-industrial partnerships forming to bridge the gap between laboratory research and commercial implementation of phase-stable high-entropy ceramic systems.
Corning, Inc.
Technical Solution: Corning has developed a proprietary thermal cycling system specifically designed for evaluating phase stability in high-entropy ceramics. Their approach utilizes laser-heated thermal cycling chambers that can achieve extremely rapid heating rates (>500°C/min) while maintaining precise temperature control through advanced pyrometry systems[2]. They employ synchrotron X-ray diffraction during thermal cycling using specially designed sample holders that allow for in-situ measurements without compromising temperature uniformity. Corning's methodology incorporates Raman spectroscopy with temperature-controlled stages to detect subtle changes in local bonding environments that may precede visible phase transformations[4]. Their protocol includes proprietary image analysis algorithms that quantify microstructural evolution across multiple thermal cycles using automated SEM/EDS mapping. They've developed specialized sample preparation techniques that allow for direct comparison of the same microstructural regions before and after thermal cycling using fiducial marking systems[7]. Additionally, Corning utilizes machine learning approaches to analyze the multimodal data collected during thermal cycling tests to identify early indicators of phase instability before conventional detection methods.
Strengths: Their laser heating approach enables extremely rapid thermal cycling that can accelerate testing timelines while still providing high-quality data. Their integrated multi-technique characterization provides comprehensive insights into phase stability mechanisms. Weaknesses: Their proprietary systems and methodologies are not widely available outside the company, limiting broader adoption. Their approach requires significant capital investment in specialized equipment and software systems.
Shanghai Institute of Ceramics, Chinese Academy of Sciences
Technical Solution: Shanghai Institute of Ceramics has developed a comprehensive approach for measuring phase stability in high-entropy ceramics under cyclic thermal loads. Their methodology combines in-situ high-temperature X-ray diffraction (HT-XRD) with thermal cycling chambers capable of reaching temperatures up to 1600°C while simultaneously collecting diffraction patterns[1]. They employ differential scanning calorimetry (DSC) coupled with thermogravimetric analysis (TGA) to detect phase transitions and weight changes during thermal cycling. The institute has pioneered the use of advanced electron microscopy techniques, including high-resolution transmission electron microscopy (HRTEM) with heating stages to directly observe microstructural evolution during thermal cycling at the atomic scale[3]. Their approach includes quantitative phase analysis using Rietveld refinement to track phase fraction changes across multiple thermal cycles, and they've developed specialized sample holders that minimize thermal gradients during rapid heating/cooling cycles to ensure uniform temperature distribution[5].
Strengths: Their integrated multi-technique approach provides comprehensive phase stability data across different length scales. The institute's advanced in-situ characterization capabilities allow real-time observation of phase transformations. Weaknesses: Their methods require sophisticated and expensive equipment that may not be accessible to all laboratories, and sample preparation for their advanced electron microscopy techniques can be time-consuming and requires specialized expertise.
Key Analytical Techniques for Phase Transformation Detection
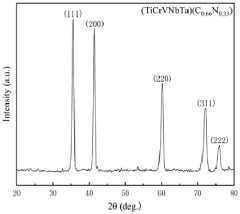
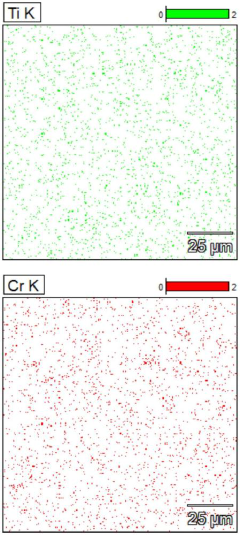
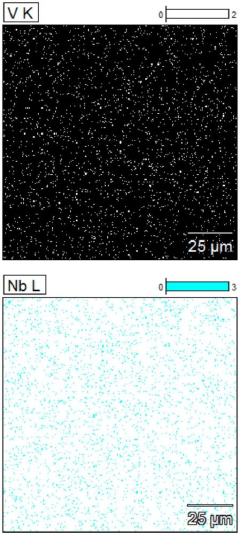
(TiCrVNbTa) (C0. 66N0. 33) high-entropy ceramic and preparation method thereof
PatentPendingCN117383944A
Innovation
- Through high-energy ball milling technology, TiC, VC, TaC, NbN and Cr2N powders are initially solid-solubilized, Cr2N is transformed into CrN containing vacancies, the sintering temperature is lowered, and the sintering temperature is within the range of 1200 to 1600°C to prepare single-phase face-centered cubic Structure of (TiCrVNbTa) (C0.66N0.33) high-entropy ceramics.
Safety Standards for High-Temperature Laboratory Testing
Safety standards for high-temperature laboratory testing of high-entropy ceramics under cyclic thermal loads require comprehensive protocols to ensure researcher protection and experimental integrity. These standards must address the extreme conditions where temperatures can exceed 1500°C during phase stability measurements.
Personal protective equipment requirements include heat-resistant gloves, face shields with appropriate thermal ratings, and flame-resistant laboratory coats. Researchers must wear appropriate eyewear that provides protection against both infrared and ultraviolet radiation emitted during high-temperature testing. Respiratory protection may be necessary when working with ceramic powders or when toxic gases might be released during thermal cycling.
Laboratory infrastructure must incorporate proper ventilation systems with fume hoods specifically designed for high-temperature operations. Emergency shutdown protocols for furnaces and other heating equipment should be clearly documented and accessible. Thermal insulation of testing equipment must meet or exceed international standards to prevent accidental burns and minimize heat transfer to the surrounding environment.
Specific safety measures for cyclic thermal load testing include continuous monitoring systems that track temperature fluctuations and automatically shut down equipment if parameters exceed safe thresholds. Thermal barriers must be installed around testing equipment, and clear demarcation of hot zones is essential. Cooling systems should be in place to manage equipment temperature between cycles.
Material handling protocols must address the potential for thermal shock when manipulating high-entropy ceramic samples. Standard operating procedures should detail safe methods for sample insertion and removal from high-temperature environments, including appropriate cooling periods before handling.
Emergency response planning must include procedures for dealing with equipment malfunctions, power failures during testing cycles, and potential fire hazards. First aid protocols specific to thermal burns should be prominently displayed, and emergency eyewash and shower stations must be readily accessible.
Training requirements stipulate that all personnel involved in high-temperature testing must complete specialized safety training, including hands-on demonstrations of emergency procedures. Regular safety drills should be conducted to ensure preparedness for potential incidents. Documentation of training completion and regular refresher courses are mandatory for all laboratory personnel.
Personal protective equipment requirements include heat-resistant gloves, face shields with appropriate thermal ratings, and flame-resistant laboratory coats. Researchers must wear appropriate eyewear that provides protection against both infrared and ultraviolet radiation emitted during high-temperature testing. Respiratory protection may be necessary when working with ceramic powders or when toxic gases might be released during thermal cycling.
Laboratory infrastructure must incorporate proper ventilation systems with fume hoods specifically designed for high-temperature operations. Emergency shutdown protocols for furnaces and other heating equipment should be clearly documented and accessible. Thermal insulation of testing equipment must meet or exceed international standards to prevent accidental burns and minimize heat transfer to the surrounding environment.
Specific safety measures for cyclic thermal load testing include continuous monitoring systems that track temperature fluctuations and automatically shut down equipment if parameters exceed safe thresholds. Thermal barriers must be installed around testing equipment, and clear demarcation of hot zones is essential. Cooling systems should be in place to manage equipment temperature between cycles.
Material handling protocols must address the potential for thermal shock when manipulating high-entropy ceramic samples. Standard operating procedures should detail safe methods for sample insertion and removal from high-temperature environments, including appropriate cooling periods before handling.
Emergency response planning must include procedures for dealing with equipment malfunctions, power failures during testing cycles, and potential fire hazards. First aid protocols specific to thermal burns should be prominently displayed, and emergency eyewash and shower stations must be readily accessible.
Training requirements stipulate that all personnel involved in high-temperature testing must complete specialized safety training, including hands-on demonstrations of emergency procedures. Regular safety drills should be conducted to ensure preparedness for potential incidents. Documentation of training completion and regular refresher courses are mandatory for all laboratory personnel.
Data Analysis Methods for Phase Stability Quantification
Quantitative analysis of phase stability in high-entropy ceramics requires sophisticated data processing techniques to extract meaningful insights from experimental measurements. X-ray diffraction (XRD) pattern analysis serves as the cornerstone method, employing Rietveld refinement to precisely quantify phase compositions and lattice parameters before and after thermal cycling. This technique allows researchers to detect subtle structural changes that may indicate phase instability.
Differential scanning calorimetry (DSC) data analysis complements XRD by identifying phase transitions through enthalpy changes. The integration of DSC peaks provides quantitative measures of transformation energetics, while peak shift analysis across multiple thermal cycles reveals degradation patterns in phase stability. Modern software packages enable deconvolution of overlapping thermal events, critical for complex high-entropy ceramic systems.
Microstructural evolution quantification employs image analysis algorithms applied to scanning electron microscopy (SEM) and transmission electron microscopy (TEM) micrographs. These algorithms measure grain size distributions, phase boundary characteristics, and precipitate formation across thermal cycles. Machine learning approaches increasingly enhance this analysis by automatically classifying microstructural features and tracking their evolution with minimal human intervention.
Statistical methods play a vital role in establishing reliability metrics for phase stability. Principal component analysis (PCA) reduces the dimensionality of multivariate datasets from various characterization techniques, while cluster analysis identifies patterns in phase behavior across different compositions and processing conditions. These methods enable researchers to develop predictive models for phase stability under varied thermal conditions.
Time-series analysis techniques applied to in-situ characterization data provide dynamic insights into phase evolution. Fourier transform and wavelet analysis extract periodic components from continuous measurement data, revealing cyclic degradation mechanisms. Autocorrelation functions quantify the "memory effect" in materials—how previous thermal cycles influence subsequent phase stability behavior.
Uncertainty quantification represents an essential component of data analysis, particularly for high-entropy ceramics where compositional complexity introduces significant variability. Monte Carlo simulations combined with sensitivity analysis identify which experimental parameters most significantly impact phase stability measurements, guiding more efficient experimental design and ensuring reproducible results across different laboratory settings.
Differential scanning calorimetry (DSC) data analysis complements XRD by identifying phase transitions through enthalpy changes. The integration of DSC peaks provides quantitative measures of transformation energetics, while peak shift analysis across multiple thermal cycles reveals degradation patterns in phase stability. Modern software packages enable deconvolution of overlapping thermal events, critical for complex high-entropy ceramic systems.
Microstructural evolution quantification employs image analysis algorithms applied to scanning electron microscopy (SEM) and transmission electron microscopy (TEM) micrographs. These algorithms measure grain size distributions, phase boundary characteristics, and precipitate formation across thermal cycles. Machine learning approaches increasingly enhance this analysis by automatically classifying microstructural features and tracking their evolution with minimal human intervention.
Statistical methods play a vital role in establishing reliability metrics for phase stability. Principal component analysis (PCA) reduces the dimensionality of multivariate datasets from various characterization techniques, while cluster analysis identifies patterns in phase behavior across different compositions and processing conditions. These methods enable researchers to develop predictive models for phase stability under varied thermal conditions.
Time-series analysis techniques applied to in-situ characterization data provide dynamic insights into phase evolution. Fourier transform and wavelet analysis extract periodic components from continuous measurement data, revealing cyclic degradation mechanisms. Autocorrelation functions quantify the "memory effect" in materials—how previous thermal cycles influence subsequent phase stability behavior.
Uncertainty quantification represents an essential component of data analysis, particularly for high-entropy ceramics where compositional complexity introduces significant variability. Monte Carlo simulations combined with sensitivity analysis identify which experimental parameters most significantly impact phase stability measurements, guiding more efficient experimental design and ensuring reproducible results across different laboratory settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!