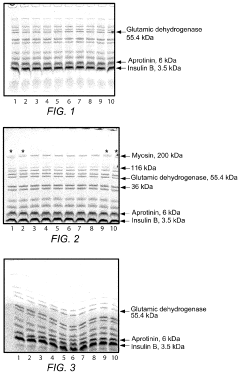
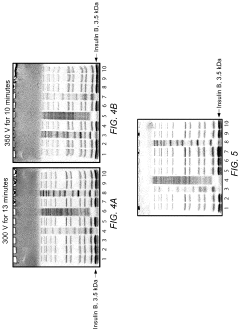
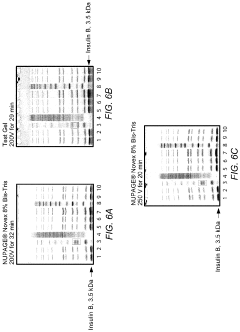
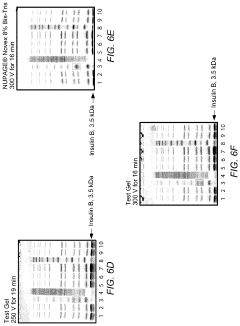
How to Minimize Errors in Gel Electrophoresis?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gel Electrophoresis Background and Objectives
Gel electrophoresis is a fundamental technique in molecular biology, used for separating and analyzing DNA, RNA, and proteins based on their size and electrical charge. Developed in the 1960s, this method has become an indispensable tool in various fields, including genetics, forensics, and medical diagnostics. The technique's evolution has been marked by continuous improvements in accuracy, resolution, and efficiency.
The primary objective of gel electrophoresis is to achieve precise separation of biomolecules for subsequent analysis or purification. However, the technique is susceptible to various errors that can compromise the reliability and reproducibility of results. These errors can stem from multiple sources, including sample preparation, gel composition, buffer conditions, and electrophoresis parameters.
As the demand for more accurate and sensitive molecular analysis grows, minimizing errors in gel electrophoresis has become a critical focus for researchers and laboratory technicians. The need for error reduction is particularly crucial in applications such as genetic testing, where even minor inaccuracies can lead to misdiagnosis or misinterpretation of results.
Recent technological advancements have aimed to address these challenges. Innovations in gel matrix materials, buffer systems, and electrophoresis equipment have contributed to enhanced resolution and reduced error rates. Additionally, the integration of digital imaging and analysis software has improved the accuracy of band detection and quantification.
The ongoing trend in gel electrophoresis technology is moving towards automation and standardization. These developments aim to minimize human error, increase throughput, and ensure consistency across different laboratories. Furthermore, there is a growing interest in miniaturization and microfluidic-based electrophoresis systems, which promise to reduce sample and reagent consumption while maintaining or improving accuracy.
As we look to the future, the field of gel electrophoresis is likely to see further innovations aimed at error minimization. This may include the development of smart, self-calibrating systems, advanced error-detection algorithms, and novel gel materials with superior separation properties. The ultimate goal is to achieve a level of precision and reliability that can meet the increasingly stringent requirements of modern molecular biology and clinical diagnostics.
The primary objective of gel electrophoresis is to achieve precise separation of biomolecules for subsequent analysis or purification. However, the technique is susceptible to various errors that can compromise the reliability and reproducibility of results. These errors can stem from multiple sources, including sample preparation, gel composition, buffer conditions, and electrophoresis parameters.
As the demand for more accurate and sensitive molecular analysis grows, minimizing errors in gel electrophoresis has become a critical focus for researchers and laboratory technicians. The need for error reduction is particularly crucial in applications such as genetic testing, where even minor inaccuracies can lead to misdiagnosis or misinterpretation of results.
Recent technological advancements have aimed to address these challenges. Innovations in gel matrix materials, buffer systems, and electrophoresis equipment have contributed to enhanced resolution and reduced error rates. Additionally, the integration of digital imaging and analysis software has improved the accuracy of band detection and quantification.
The ongoing trend in gel electrophoresis technology is moving towards automation and standardization. These developments aim to minimize human error, increase throughput, and ensure consistency across different laboratories. Furthermore, there is a growing interest in miniaturization and microfluidic-based electrophoresis systems, which promise to reduce sample and reagent consumption while maintaining or improving accuracy.
As we look to the future, the field of gel electrophoresis is likely to see further innovations aimed at error minimization. This may include the development of smart, self-calibrating systems, advanced error-detection algorithms, and novel gel materials with superior separation properties. The ultimate goal is to achieve a level of precision and reliability that can meet the increasingly stringent requirements of modern molecular biology and clinical diagnostics.
Market Demand for Accurate Gel Electrophoresis
The market demand for accurate gel electrophoresis has been steadily increasing due to its critical role in various scientific and medical applications. This technique is widely used in molecular biology, genetics, forensics, and clinical diagnostics, driving the need for more precise and reliable results.
In the research sector, there is a growing emphasis on reproducibility and data integrity. Laboratories and academic institutions are increasingly seeking advanced gel electrophoresis systems that can minimize errors and provide consistent results. This demand is fueled by the need to publish high-quality research and secure competitive funding.
The pharmaceutical and biotechnology industries represent a significant portion of the market demand. These sectors rely heavily on gel electrophoresis for drug development, quality control, and protein analysis. As the complexity of biopharmaceuticals increases, so does the need for more accurate separation and analysis techniques.
Clinical diagnostics is another key driver of market demand. Hospitals and diagnostic laboratories require precise gel electrophoresis results for various tests, including genetic disorder screening, cancer diagnostics, and infectious disease detection. The increasing prevalence of personalized medicine has further amplified this demand, as accurate molecular profiling becomes essential for tailored treatment strategies.
The forensic science field also contributes to the market demand for accurate gel electrophoresis. Law enforcement agencies and forensic laboratories require highly reliable DNA analysis techniques for criminal investigations and identity verification. Any errors in this process can have severe legal implications, thus driving the need for error-minimizing technologies.
Environmental and food safety testing sectors are emerging markets for accurate gel electrophoresis. These industries use the technique for detecting contaminants, identifying genetically modified organisms, and ensuring food quality. Stringent regulations in these areas necessitate highly accurate and reproducible results.
The global market for gel electrophoresis equipment and consumables reflects this growing demand for accuracy. Market reports indicate a steady growth rate, with particular emphasis on advanced systems that offer improved resolution, sensitivity, and reproducibility. Manufacturers are responding to this demand by developing innovative products that incorporate automation, digital imaging, and software-assisted analysis to minimize human error and enhance result accuracy.
As research in genomics and proteomics continues to expand, the demand for high-throughput and high-precision gel electrophoresis systems is expected to rise. This trend is particularly evident in large-scale projects such as population-wide genetic studies and biomarker discovery initiatives, where the volume and accuracy of data are paramount.
In the research sector, there is a growing emphasis on reproducibility and data integrity. Laboratories and academic institutions are increasingly seeking advanced gel electrophoresis systems that can minimize errors and provide consistent results. This demand is fueled by the need to publish high-quality research and secure competitive funding.
The pharmaceutical and biotechnology industries represent a significant portion of the market demand. These sectors rely heavily on gel electrophoresis for drug development, quality control, and protein analysis. As the complexity of biopharmaceuticals increases, so does the need for more accurate separation and analysis techniques.
Clinical diagnostics is another key driver of market demand. Hospitals and diagnostic laboratories require precise gel electrophoresis results for various tests, including genetic disorder screening, cancer diagnostics, and infectious disease detection. The increasing prevalence of personalized medicine has further amplified this demand, as accurate molecular profiling becomes essential for tailored treatment strategies.
The forensic science field also contributes to the market demand for accurate gel electrophoresis. Law enforcement agencies and forensic laboratories require highly reliable DNA analysis techniques for criminal investigations and identity verification. Any errors in this process can have severe legal implications, thus driving the need for error-minimizing technologies.
Environmental and food safety testing sectors are emerging markets for accurate gel electrophoresis. These industries use the technique for detecting contaminants, identifying genetically modified organisms, and ensuring food quality. Stringent regulations in these areas necessitate highly accurate and reproducible results.
The global market for gel electrophoresis equipment and consumables reflects this growing demand for accuracy. Market reports indicate a steady growth rate, with particular emphasis on advanced systems that offer improved resolution, sensitivity, and reproducibility. Manufacturers are responding to this demand by developing innovative products that incorporate automation, digital imaging, and software-assisted analysis to minimize human error and enhance result accuracy.
As research in genomics and proteomics continues to expand, the demand for high-throughput and high-precision gel electrophoresis systems is expected to rise. This trend is particularly evident in large-scale projects such as population-wide genetic studies and biomarker discovery initiatives, where the volume and accuracy of data are paramount.
Current Challenges in Gel Electrophoresis
Gel electrophoresis remains a fundamental technique in molecular biology, yet it faces several challenges that can lead to errors and inconsistencies in results. One of the primary issues is sample preparation, where improper handling or contamination can significantly affect the quality of the results. Inconsistent sample loading, both in terms of volume and concentration, can lead to uneven band patterns and difficulties in interpretation.
The gel preparation process itself presents challenges, with variations in agarose concentration, buffer composition, and gel thickness potentially impacting the separation and resolution of DNA or protein fragments. Inconsistencies in gel polymerization can result in non-uniform pore sizes, affecting the migration of molecules and leading to distorted band patterns.
Electrophoresis conditions pose another set of challenges. Fluctuations in voltage or current during the run can cause irregular migration patterns, while overheating of the gel can lead to band smearing or even gel melting. The choice of buffer and its concentration also play crucial roles, as improper buffer conditions can affect the pH and ionic strength, leading to suboptimal separation.
Staining and visualization techniques present their own set of difficulties. Overstaining or understaining can result in either excessive background noise or faint, hard-to-detect bands. The choice of staining method (pre-staining, post-staining, or in-gel staining) can affect sensitivity and may introduce artifacts if not properly optimized.
Size determination and quantification of bands remain challenging, particularly when dealing with complex mixtures or when precise measurements are required. The limitations of traditional DNA ladders and protein markers in providing accurate size estimations for all sample types can lead to errors in molecular weight determination.
Cross-contamination between samples is an ongoing concern, especially in high-throughput environments. This can occur during sample loading or through the reuse of equipment without proper cleaning, leading to false positive results or misinterpretation of band patterns.
The interpretation of results presents its own set of challenges. Anomalous band patterns, such as ghost bands or unexpected migrations, can complicate analysis. Additionally, the subjective nature of gel image interpretation can lead to inconsistencies between different researchers or laboratories.
Reproducibility remains a significant challenge in gel electrophoresis. Variations in environmental conditions, reagent quality, and operator technique can all contribute to inconsistencies between runs, making it difficult to compare results across different experiments or laboratories.
The gel preparation process itself presents challenges, with variations in agarose concentration, buffer composition, and gel thickness potentially impacting the separation and resolution of DNA or protein fragments. Inconsistencies in gel polymerization can result in non-uniform pore sizes, affecting the migration of molecules and leading to distorted band patterns.
Electrophoresis conditions pose another set of challenges. Fluctuations in voltage or current during the run can cause irregular migration patterns, while overheating of the gel can lead to band smearing or even gel melting. The choice of buffer and its concentration also play crucial roles, as improper buffer conditions can affect the pH and ionic strength, leading to suboptimal separation.
Staining and visualization techniques present their own set of difficulties. Overstaining or understaining can result in either excessive background noise or faint, hard-to-detect bands. The choice of staining method (pre-staining, post-staining, or in-gel staining) can affect sensitivity and may introduce artifacts if not properly optimized.
Size determination and quantification of bands remain challenging, particularly when dealing with complex mixtures or when precise measurements are required. The limitations of traditional DNA ladders and protein markers in providing accurate size estimations for all sample types can lead to errors in molecular weight determination.
Cross-contamination between samples is an ongoing concern, especially in high-throughput environments. This can occur during sample loading or through the reuse of equipment without proper cleaning, leading to false positive results or misinterpretation of band patterns.
The interpretation of results presents its own set of challenges. Anomalous band patterns, such as ghost bands or unexpected migrations, can complicate analysis. Additionally, the subjective nature of gel image interpretation can lead to inconsistencies between different researchers or laboratories.
Reproducibility remains a significant challenge in gel electrophoresis. Variations in environmental conditions, reagent quality, and operator technique can all contribute to inconsistencies between runs, making it difficult to compare results across different experiments or laboratories.
Existing Error Minimization Strategies
01 Gel composition and preparation errors
Errors in gel electrophoresis can arise from improper gel composition or preparation. This includes issues with gel concentration, polymerization, and uniformity. Incorrect gel composition can lead to inconsistent separation of molecules and affect the resolution of bands. Proper gel preparation techniques and quality control measures are essential to minimize these errors.- Gel composition and preparation errors: Errors in gel electrophoresis can arise from improper gel composition or preparation. This includes issues with gel concentration, polymerization, and uniformity. Incorrect gel composition can lead to inconsistent separation of molecules and affect the resolution of bands. Proper gel preparation techniques and quality control measures are essential to minimize these errors.
- Sample loading and running buffer errors: Errors can occur during sample loading and due to issues with running buffer composition. Uneven sample loading, overloading, or using incorrect buffer concentrations can lead to distorted band patterns and poor resolution. Maintaining consistent sample volumes, proper buffer preparation, and careful loading techniques are crucial for accurate results.
- Voltage and current control errors: Inconsistent or improper voltage and current settings during electrophoresis can cause errors. Fluctuations in electrical parameters can lead to uneven migration of molecules and affect band resolution. Maintaining stable power supply and using appropriate voltage settings for different gel types and sizes are important for reliable results.
- Temperature control and heat dissipation errors: Errors can arise from inadequate temperature control and heat dissipation during electrophoresis. Overheating can cause gel deformation, band distortion, and even sample degradation. Proper cooling systems, appropriate running conditions, and monitoring of gel temperature are essential to prevent heat-related errors.
- Detection and imaging errors: Errors in gel electrophoresis can occur during the detection and imaging stages. These may include issues with staining techniques, improper imaging equipment settings, or incorrect interpretation of results. Using appropriate staining methods, calibrated imaging systems, and standardized analysis protocols are crucial for accurate detection and quantification of separated molecules.
02 Sample loading and running buffer errors
Errors can occur during sample loading and with the running buffer. Uneven sample loading, overloading, or using incorrect buffer compositions can lead to distorted band patterns or poor resolution. Ensuring proper sample preparation, consistent loading techniques, and using the correct buffer composition and concentration are crucial for accurate results.Expand Specific Solutions03 Voltage and current control errors
Inconsistent or improper voltage and current control during electrophoresis can lead to errors. Fluctuations in power supply, incorrect voltage settings, or uneven current distribution across the gel can cause band distortion or inconsistent migration. Maintaining stable electrical conditions throughout the run is essential for reproducible results.Expand Specific Solutions04 Temperature and heat dissipation issues
Temperature fluctuations and inadequate heat dissipation during electrophoresis can cause errors. Overheating can lead to gel deformation, band smearing, or DNA denaturation. Proper temperature control systems and buffer circulation are important to maintain consistent running conditions and prevent heat-related artifacts.Expand Specific Solutions05 Detection and imaging errors
Errors can occur during the detection and imaging of electrophoresis results. These may include issues with staining techniques, improper UV exposure, or inadequate image capture settings. Optimizing staining protocols, using appropriate imaging equipment, and implementing standardized image analysis procedures are crucial for accurate interpretation of results.Expand Specific Solutions
Key Players in Gel Electrophoresis Industry
The gel electrophoresis market is in a mature stage, with a global size estimated at over $1 billion. The technology is well-established and widely used in research and clinical laboratories. Key players like Life Technologies, Bio-Rad Laboratories, and Cytiva dominate the market with advanced systems and consumables. These companies continually innovate to improve accuracy and efficiency, addressing the challenge of minimizing errors. Emerging players such as Sage Science and Monad Biotech are also contributing to technological advancements. The competitive landscape is characterized by a focus on automation, reproducibility, and integration with downstream applications to enhance overall workflow and reduce human error in gel electrophoresis procedures.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad has developed advanced gel electrophoresis systems to minimize errors. Their approach includes the use of precast gels with consistent pore sizes and buffer compositions, reducing variability in gel preparation. They have also introduced automated gel documentation systems with high-resolution imaging capabilities, allowing for precise band detection and quantification[1]. Bio-Rad's electrophoresis power supplies feature microprocessor-controlled voltage regulation, ensuring stable electrical conditions throughout the run[2]. Additionally, they have developed specialized buffer systems that maintain pH stability during electrophoresis, preventing band distortion[3].
Strengths: Comprehensive solution from gel preparation to imaging, high reproducibility, and advanced automation. Weaknesses: Higher cost compared to traditional methods, may require specialized training for optimal use.
Cytiva Sweden AB
Technical Solution: Cytiva (formerly GE Healthcare Life Sciences) has innovated in gel electrophoresis error minimization through their Amersham ECL Gel system. This system utilizes a horizontal gel format with precast agarose gels, reducing handling errors and improving consistency[4]. They have developed a unique buffer circulation system that maintains uniform pH and ion concentration across the gel, preventing "smile effect" distortions[5]. Cytiva's approach also includes the use of fluorescent protein ladders for more accurate size determination and quantification. Their imaging systems incorporate advanced algorithms for automated lane and band detection, minimizing human error in data analysis[6].
Strengths: User-friendly systems, high reproducibility, and advanced imaging capabilities. Weaknesses: Limited flexibility in gel composition compared to custom-cast gels, potentially higher running costs.
Innovative Approaches to Error Reduction
System for rapid high-resolution GEL electrophoresis
PatentActiveUS20190391113A1
Innovation
- The development of electrophoretic systems and formulations that allow for higher field strengths up to 50% more than conventional systems, using a discontinuous buffer system with specific gel amine and ampholyte buffers, and a pH range of 5.5 to 7.5, enabling faster separation of proteins within 30 minutes or less, even at higher voltages.
Method and device for gel electrophoresis
PatentWO2000077510A2
Innovation
- A gel electrophoresis system with at least two pairs of electrodes extending parallel to the rows or columns of the sample matrix, creating an electric field direction that deviates from the sample arrangement, allowing for direct sample transfer and extended migration distances without enlarging the buffer chamber, using a gel comb with angled teeth to form sample pockets aligned with the electric field direction.
Quality Control in Gel Electrophoresis
Quality control is a critical aspect of gel electrophoresis that ensures accurate and reproducible results. Implementing robust quality control measures can significantly minimize errors and enhance the reliability of experimental outcomes. A comprehensive quality control strategy for gel electrophoresis encompasses several key elements.
Firstly, it is essential to maintain strict control over the preparation and storage of reagents and buffers. All solutions should be prepared using high-quality, molecular biology-grade chemicals and ultrapure water. Proper labeling, storage conditions, and expiration date tracking are crucial to prevent contamination and degradation of reagents. Regular quality checks of buffers, including pH measurements and conductivity tests, help ensure consistency across experiments.
The quality of the gel itself is paramount in achieving accurate results. Careful attention must be paid to the gel preparation process, including the precise measurement of agarose concentration and the complete dissolution of agarose powder. Uniform gel casting without air bubbles or inconsistencies is vital for even migration of samples. Implementing standardized protocols for gel preparation and using calibrated equipment can significantly reduce variability between experiments.
Sample preparation is another critical area for quality control. Ensuring consistent sample concentrations, volumes, and loading techniques is essential for accurate comparison of results. The use of internal controls and standardized markers in each gel run provides reference points for data interpretation and allows for the detection of any anomalies in sample migration or gel performance.
Electrophoresis conditions must be carefully controlled and documented. This includes maintaining consistent voltage, current, and run time across experiments. The use of temperature-controlled electrophoresis systems can prevent overheating and gel distortion, particularly during extended runs. Regular calibration and maintenance of power supplies and electrophoresis equipment are necessary to ensure consistent performance.
Post-electrophoresis processes, such as staining and imaging, also require quality control measures. Standardized staining protocols, including precise timing and destaining procedures, help achieve consistent band visualization. The use of calibrated imaging systems with appropriate controls for exposure and contrast settings ensures accurate and reproducible documentation of results.
Implementing a system of thorough documentation and record-keeping is crucial for maintaining quality control in gel electrophoresis. This includes detailed logging of all experimental parameters, reagent batches, and equipment settings. Regular analysis of these records can help identify trends or inconsistencies that may affect experimental outcomes.
Finally, ongoing training and proficiency testing of laboratory personnel are essential components of a robust quality control program. This ensures that all staff members are familiar with best practices and can consistently perform gel electrophoresis techniques to the required standards.
Firstly, it is essential to maintain strict control over the preparation and storage of reagents and buffers. All solutions should be prepared using high-quality, molecular biology-grade chemicals and ultrapure water. Proper labeling, storage conditions, and expiration date tracking are crucial to prevent contamination and degradation of reagents. Regular quality checks of buffers, including pH measurements and conductivity tests, help ensure consistency across experiments.
The quality of the gel itself is paramount in achieving accurate results. Careful attention must be paid to the gel preparation process, including the precise measurement of agarose concentration and the complete dissolution of agarose powder. Uniform gel casting without air bubbles or inconsistencies is vital for even migration of samples. Implementing standardized protocols for gel preparation and using calibrated equipment can significantly reduce variability between experiments.
Sample preparation is another critical area for quality control. Ensuring consistent sample concentrations, volumes, and loading techniques is essential for accurate comparison of results. The use of internal controls and standardized markers in each gel run provides reference points for data interpretation and allows for the detection of any anomalies in sample migration or gel performance.
Electrophoresis conditions must be carefully controlled and documented. This includes maintaining consistent voltage, current, and run time across experiments. The use of temperature-controlled electrophoresis systems can prevent overheating and gel distortion, particularly during extended runs. Regular calibration and maintenance of power supplies and electrophoresis equipment are necessary to ensure consistent performance.
Post-electrophoresis processes, such as staining and imaging, also require quality control measures. Standardized staining protocols, including precise timing and destaining procedures, help achieve consistent band visualization. The use of calibrated imaging systems with appropriate controls for exposure and contrast settings ensures accurate and reproducible documentation of results.
Implementing a system of thorough documentation and record-keeping is crucial for maintaining quality control in gel electrophoresis. This includes detailed logging of all experimental parameters, reagent batches, and equipment settings. Regular analysis of these records can help identify trends or inconsistencies that may affect experimental outcomes.
Finally, ongoing training and proficiency testing of laboratory personnel are essential components of a robust quality control program. This ensures that all staff members are familiar with best practices and can consistently perform gel electrophoresis techniques to the required standards.
Automation in Gel Electrophoresis
Automation in gel electrophoresis represents a significant advancement in minimizing errors and improving the efficiency of this crucial laboratory technique. The integration of robotics and computer-controlled systems has revolutionized the way gel electrophoresis is performed, reducing human intervention and the associated risks of manual errors.
One of the key areas where automation has made a substantial impact is in sample preparation and loading. Automated liquid handling systems can precisely measure and dispense samples into gel wells, ensuring consistency and eliminating variations that often occur with manual pipetting. These systems can handle multiple samples simultaneously, increasing throughput and reducing the time required for sample loading.
Gel casting has also benefited from automation. Automated gel casting systems can produce gels with uniform thickness and composition, eliminating inconsistencies that can affect band migration and resolution. These systems often include temperature control mechanisms to ensure optimal polymerization conditions, resulting in gels of consistent quality.
The electrophoresis process itself has been enhanced through automation. Computerized power supplies can maintain precise voltage and current settings throughout the run, preventing fluctuations that can lead to uneven band migration. Some advanced systems even incorporate real-time monitoring of the electrophoresis progress, allowing for automatic termination of the run when optimal separation is achieved.
Imaging and analysis of gels have also been significantly improved through automation. Automated gel documentation systems can capture high-resolution images of gels under standardized conditions, ensuring consistent and reproducible results. These systems often come with integrated software for band detection, quantification, and analysis, reducing the subjectivity associated with manual interpretation.
Furthermore, automation has enabled the development of high-throughput gel electrophoresis systems. These platforms can run multiple gels simultaneously, dramatically increasing the number of samples that can be processed in a given time. Such systems are particularly valuable in large-scale genomics and proteomics studies, where hundreds or thousands of samples need to be analyzed efficiently.
The integration of automation with other analytical techniques has also expanded the capabilities of gel electrophoresis. For instance, automated systems that combine gel electrophoresis with mass spectrometry or Western blotting have been developed, allowing for seamless transition between different analytical steps and reducing the risk of sample loss or contamination during manual transfers.
While automation offers numerous advantages in minimizing errors in gel electrophoresis, it is important to note that proper calibration, maintenance, and quality control of automated systems are crucial for reliable results. Regular validation of automated processes against manual methods is essential to ensure the accuracy and reliability of the automated systems.
One of the key areas where automation has made a substantial impact is in sample preparation and loading. Automated liquid handling systems can precisely measure and dispense samples into gel wells, ensuring consistency and eliminating variations that often occur with manual pipetting. These systems can handle multiple samples simultaneously, increasing throughput and reducing the time required for sample loading.
Gel casting has also benefited from automation. Automated gel casting systems can produce gels with uniform thickness and composition, eliminating inconsistencies that can affect band migration and resolution. These systems often include temperature control mechanisms to ensure optimal polymerization conditions, resulting in gels of consistent quality.
The electrophoresis process itself has been enhanced through automation. Computerized power supplies can maintain precise voltage and current settings throughout the run, preventing fluctuations that can lead to uneven band migration. Some advanced systems even incorporate real-time monitoring of the electrophoresis progress, allowing for automatic termination of the run when optimal separation is achieved.
Imaging and analysis of gels have also been significantly improved through automation. Automated gel documentation systems can capture high-resolution images of gels under standardized conditions, ensuring consistent and reproducible results. These systems often come with integrated software for band detection, quantification, and analysis, reducing the subjectivity associated with manual interpretation.
Furthermore, automation has enabled the development of high-throughput gel electrophoresis systems. These platforms can run multiple gels simultaneously, dramatically increasing the number of samples that can be processed in a given time. Such systems are particularly valuable in large-scale genomics and proteomics studies, where hundreds or thousands of samples need to be analyzed efficiently.
The integration of automation with other analytical techniques has also expanded the capabilities of gel electrophoresis. For instance, automated systems that combine gel electrophoresis with mass spectrometry or Western blotting have been developed, allowing for seamless transition between different analytical steps and reducing the risk of sample loss or contamination during manual transfers.
While automation offers numerous advantages in minimizing errors in gel electrophoresis, it is important to note that proper calibration, maintenance, and quality control of automated systems are crucial for reliable results. Regular validation of automated processes against manual methods is essential to ensure the accuracy and reliability of the automated systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!