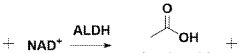How Trimethylglycine Reduces Alcohol-induced Liver Damage
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
TMG and Alcohol-induced Liver Damage Background
Alcohol-induced liver damage represents a significant global health concern, affecting millions worldwide and contributing to the rising prevalence of liver diseases. Trimethylglycine (TMG), also known as betaine, has emerged as a promising compound in the prevention and treatment of alcohol-related liver injuries. This naturally occurring compound, found in various food sources including beets, spinach, and whole grains, has garnered increasing attention from researchers and healthcare professionals over the past two decades.
The historical context of TMG research dates back to the 1950s when it was first identified as a methyl donor in biochemical pathways. However, its specific application in addressing alcohol-induced liver damage gained momentum only in the late 1990s, following breakthrough studies demonstrating its hepatoprotective properties. The technological evolution in this field has progressed from basic understanding of TMG's biochemical properties to sophisticated investigations of its molecular mechanisms in mitigating alcohol-induced hepatotoxicity.
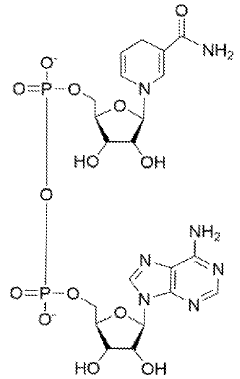
Current research indicates that excessive alcohol consumption disrupts hepatic methionine metabolism, leading to decreased S-adenosylmethionine (SAM) levels and increased homocysteine concentrations. These metabolic disturbances contribute significantly to oxidative stress, inflammation, and lipid accumulation in hepatocytes. TMG, as a methyl donor, facilitates the remethylation of homocysteine to methionine, thereby restoring SAM levels and potentially alleviating alcohol-induced liver damage through multiple pathways.
The technological trajectory in this field is moving toward more precise understanding of TMG's mechanisms of action, including its role in epigenetic regulation, mitochondrial function preservation, and anti-inflammatory processes. Recent advances in metabolomics, proteomics, and genomics have enabled researchers to elucidate the complex interactions between TMG, alcohol metabolism, and liver function at unprecedented molecular detail.
The primary technical objectives in this domain include: establishing optimal TMG dosing regimens for hepatoprotection, identifying synergistic compounds that enhance TMG's efficacy, developing targeted delivery systems to maximize hepatic bioavailability, and validating reliable biomarkers for monitoring TMG's therapeutic effects in alcohol-induced liver damage.
As research continues to evolve, there is growing interest in exploring TMG's potential in personalized medicine approaches, considering individual genetic variations in methionine metabolism pathways. Additionally, technological innovations in formulation science are being pursued to enhance TMG's stability, bioavailability, and therapeutic efficacy in various clinical scenarios related to alcohol-induced liver injury.
The historical context of TMG research dates back to the 1950s when it was first identified as a methyl donor in biochemical pathways. However, its specific application in addressing alcohol-induced liver damage gained momentum only in the late 1990s, following breakthrough studies demonstrating its hepatoprotective properties. The technological evolution in this field has progressed from basic understanding of TMG's biochemical properties to sophisticated investigations of its molecular mechanisms in mitigating alcohol-induced hepatotoxicity.
Current research indicates that excessive alcohol consumption disrupts hepatic methionine metabolism, leading to decreased S-adenosylmethionine (SAM) levels and increased homocysteine concentrations. These metabolic disturbances contribute significantly to oxidative stress, inflammation, and lipid accumulation in hepatocytes. TMG, as a methyl donor, facilitates the remethylation of homocysteine to methionine, thereby restoring SAM levels and potentially alleviating alcohol-induced liver damage through multiple pathways.
The technological trajectory in this field is moving toward more precise understanding of TMG's mechanisms of action, including its role in epigenetic regulation, mitochondrial function preservation, and anti-inflammatory processes. Recent advances in metabolomics, proteomics, and genomics have enabled researchers to elucidate the complex interactions between TMG, alcohol metabolism, and liver function at unprecedented molecular detail.
The primary technical objectives in this domain include: establishing optimal TMG dosing regimens for hepatoprotection, identifying synergistic compounds that enhance TMG's efficacy, developing targeted delivery systems to maximize hepatic bioavailability, and validating reliable biomarkers for monitoring TMG's therapeutic effects in alcohol-induced liver damage.
As research continues to evolve, there is growing interest in exploring TMG's potential in personalized medicine approaches, considering individual genetic variations in methionine metabolism pathways. Additionally, technological innovations in formulation science are being pursued to enhance TMG's stability, bioavailability, and therapeutic efficacy in various clinical scenarios related to alcohol-induced liver injury.
Market Analysis of Hepatoprotective Supplements
The global market for hepatoprotective supplements has experienced significant growth in recent years, driven by increasing awareness of liver health and rising prevalence of liver diseases. The market for liver health supplements containing trimethylglycine (TMG) and similar compounds is currently valued at approximately $1.2 billion globally, with projections indicating a compound annual growth rate of 6.8% through 2028.
North America dominates the hepatoprotective supplement market, accounting for roughly 38% of global sales, followed by Europe (27%) and Asia-Pacific (24%). The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth due to rising disposable incomes, increasing health consciousness, and traditional acceptance of natural remedies for liver ailments.
Consumer demographics reveal that adults aged 40-65 represent the largest consumer segment for hepatoprotective supplements, constituting about 45% of the market. This demographic is particularly concerned about alcohol-related liver damage and age-related liver function decline. However, younger adults (25-39) are emerging as a growing segment, driven by increasing alcohol consumption patterns and preventive health approaches.
The competitive landscape features both pharmaceutical companies and nutraceutical manufacturers. Key market players include Jarrow Formulas, NOW Foods, Life Extension, and Swanson Health Products, which offer TMG supplements specifically marketed for liver support. Pharmaceutical companies like Sanofi and GSK have also entered this space with specialized liver health formulations.
Distribution channels for hepatoprotective supplements have diversified significantly. Online retail has emerged as the fastest-growing channel, accounting for approximately 32% of sales in 2022, while pharmacy chains remain the largest traditional retail channel at 28%. Specialty health stores and direct marketing together constitute about 25% of distribution.
Consumer price sensitivity varies by region and product positioning. Premium TMG supplements with clinical backing command price points 30-40% higher than generic alternatives. The average consumer expenditure on liver health supplements ranges from $15-45 monthly, with willingness to pay increasing among consumers with diagnosed liver conditions or regular alcohol consumption habits.
Market challenges include regulatory hurdles regarding health claims, particularly in Europe and North America where strict guidelines limit marketing language. Additionally, consumer education remains a significant barrier, as many potential users lack understanding of liver health biomarkers and the mechanisms by which supplements like TMG provide protection against alcohol-induced damage.
North America dominates the hepatoprotective supplement market, accounting for roughly 38% of global sales, followed by Europe (27%) and Asia-Pacific (24%). The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth due to rising disposable incomes, increasing health consciousness, and traditional acceptance of natural remedies for liver ailments.
Consumer demographics reveal that adults aged 40-65 represent the largest consumer segment for hepatoprotective supplements, constituting about 45% of the market. This demographic is particularly concerned about alcohol-related liver damage and age-related liver function decline. However, younger adults (25-39) are emerging as a growing segment, driven by increasing alcohol consumption patterns and preventive health approaches.
The competitive landscape features both pharmaceutical companies and nutraceutical manufacturers. Key market players include Jarrow Formulas, NOW Foods, Life Extension, and Swanson Health Products, which offer TMG supplements specifically marketed for liver support. Pharmaceutical companies like Sanofi and GSK have also entered this space with specialized liver health formulations.
Distribution channels for hepatoprotective supplements have diversified significantly. Online retail has emerged as the fastest-growing channel, accounting for approximately 32% of sales in 2022, while pharmacy chains remain the largest traditional retail channel at 28%. Specialty health stores and direct marketing together constitute about 25% of distribution.
Consumer price sensitivity varies by region and product positioning. Premium TMG supplements with clinical backing command price points 30-40% higher than generic alternatives. The average consumer expenditure on liver health supplements ranges from $15-45 monthly, with willingness to pay increasing among consumers with diagnosed liver conditions or regular alcohol consumption habits.
Market challenges include regulatory hurdles regarding health claims, particularly in Europe and North America where strict guidelines limit marketing language. Additionally, consumer education remains a significant barrier, as many potential users lack understanding of liver health biomarkers and the mechanisms by which supplements like TMG provide protection against alcohol-induced damage.
Current Research Status and Challenges in TMG Therapy
The global research landscape for Trimethylglycine (TMG) as a therapeutic agent against alcohol-induced liver damage has expanded significantly in recent years. Current studies predominantly focus on TMG's role as a methyl donor and its hepatoprotective mechanisms. Research indicates that TMG can effectively reduce hepatic steatosis, decrease inflammatory responses, and mitigate oxidative stress in alcohol-damaged liver tissues. Multiple animal models have demonstrated TMG's ability to normalize liver enzyme levels and improve histopathological outcomes following chronic alcohol exposure.
Despite these promising findings, several significant challenges persist in TMG therapy development. The optimal dosing regimen remains unclear, with considerable variation across studies regarding effective therapeutic concentrations. This inconsistency complicates translation to clinical applications and standardization of treatment protocols. Additionally, the precise molecular mechanisms through which TMG exerts its hepatoprotective effects are not fully elucidated, particularly regarding its interaction with alcohol metabolism pathways.
Another critical challenge involves the bioavailability and pharmacokinetics of TMG in humans with alcohol-induced liver damage. The altered liver function in these patients may significantly impact TMG metabolism and efficacy, yet limited human clinical trials have addressed these concerns. Most existing research relies heavily on rodent models, which may not accurately reflect human pathophysiology or treatment responses.
The long-term safety profile of TMG supplementation represents another substantial research gap. While acute administration appears well-tolerated, the consequences of prolonged TMG therapy, particularly in patients with pre-existing liver conditions or those continuing alcohol consumption, remain inadequately characterized. Potential interactions with commonly prescribed medications for liver disease also warrant further investigation.
Methodological limitations further complicate the research landscape. Variations in experimental designs, TMG formulations, and outcome measurements make cross-study comparisons challenging. The heterogeneity of alcohol-induced liver damage in clinical populations adds another layer of complexity to research efforts, as patients present with varying degrees of steatosis, inflammation, and fibrosis.
Emerging research directions include exploring TMG's epigenetic effects through DNA methylation pathways and its potential synergistic effects when combined with other hepatoprotective agents. Some investigators are also examining TMG's impact on gut microbiota composition and intestinal barrier function, which may indirectly contribute to its hepatoprotective properties in alcohol-induced liver damage.
Addressing these challenges requires coordinated research efforts, including well-designed clinical trials with standardized protocols, mechanistic studies utilizing advanced molecular techniques, and long-term safety evaluations in diverse patient populations.
Despite these promising findings, several significant challenges persist in TMG therapy development. The optimal dosing regimen remains unclear, with considerable variation across studies regarding effective therapeutic concentrations. This inconsistency complicates translation to clinical applications and standardization of treatment protocols. Additionally, the precise molecular mechanisms through which TMG exerts its hepatoprotective effects are not fully elucidated, particularly regarding its interaction with alcohol metabolism pathways.
Another critical challenge involves the bioavailability and pharmacokinetics of TMG in humans with alcohol-induced liver damage. The altered liver function in these patients may significantly impact TMG metabolism and efficacy, yet limited human clinical trials have addressed these concerns. Most existing research relies heavily on rodent models, which may not accurately reflect human pathophysiology or treatment responses.
The long-term safety profile of TMG supplementation represents another substantial research gap. While acute administration appears well-tolerated, the consequences of prolonged TMG therapy, particularly in patients with pre-existing liver conditions or those continuing alcohol consumption, remain inadequately characterized. Potential interactions with commonly prescribed medications for liver disease also warrant further investigation.
Methodological limitations further complicate the research landscape. Variations in experimental designs, TMG formulations, and outcome measurements make cross-study comparisons challenging. The heterogeneity of alcohol-induced liver damage in clinical populations adds another layer of complexity to research efforts, as patients present with varying degrees of steatosis, inflammation, and fibrosis.
Emerging research directions include exploring TMG's epigenetic effects through DNA methylation pathways and its potential synergistic effects when combined with other hepatoprotective agents. Some investigators are also examining TMG's impact on gut microbiota composition and intestinal barrier function, which may indirectly contribute to its hepatoprotective properties in alcohol-induced liver damage.
Addressing these challenges requires coordinated research efforts, including well-designed clinical trials with standardized protocols, mechanistic studies utilizing advanced molecular techniques, and long-term safety evaluations in diverse patient populations.
Mechanism of Action for TMG in Liver Protection
01 Trimethylglycine as hepatoprotective agent
Trimethylglycine (betaine) functions as a hepatoprotective agent that can reduce liver damage through various mechanisms. It acts as a methyl donor in the liver, helping to convert homocysteine to methionine, which reduces oxidative stress and inflammation. This protective effect helps maintain liver function and prevents hepatic steatosis (fatty liver), making it valuable for treating various liver conditions including alcoholic liver disease and non-alcoholic fatty liver disease.- Trimethylglycine as hepatoprotective agent: Trimethylglycine (TMG) functions as a hepatoprotective agent that can reduce liver damage through various mechanisms. It helps maintain liver function by acting as a methyl donor in biochemical processes, supporting methylation reactions that are essential for liver detoxification pathways. TMG supplementation has been shown to protect hepatocytes from oxidative stress and toxic insults, thereby preventing liver damage and supporting overall liver health.
- TMG in fatty liver disease prevention and treatment: Trimethylglycine has demonstrated efficacy in preventing and treating fatty liver disease by regulating lipid metabolism in the liver. It helps reduce fat accumulation in hepatocytes by promoting lipid export and oxidation. TMG supplementation can decrease triglyceride levels in the liver, improve insulin sensitivity, and reduce inflammation associated with non-alcoholic fatty liver disease (NAFLD), thereby protecting against liver damage caused by excessive fat deposition.
- TMG's role in alcohol-induced liver damage reduction: Trimethylglycine can mitigate alcohol-induced liver damage by counteracting the harmful effects of ethanol metabolism. It helps restore methionine levels depleted by alcohol consumption and supports the production of S-adenosylmethionine (SAMe), an important compound for liver health. TMG supplementation reduces oxidative stress caused by alcohol metabolism, decreases inflammation, and helps maintain proper liver function in individuals with alcohol-related liver disorders.
- TMG in combination with other hepatoprotective compounds: Synergistic formulations combining trimethylglycine with other hepatoprotective compounds can enhance liver protection and damage reduction. These combinations often include antioxidants, amino acids, vitamins, or plant extracts that work through complementary mechanisms to protect liver cells. Such formulations can provide comprehensive liver support by addressing multiple pathways of liver damage simultaneously, offering greater hepatoprotective effects than TMG alone.
- TMG's mechanism in reducing drug-induced liver toxicity: Trimethylglycine can reduce drug-induced liver toxicity by supporting detoxification pathways and cellular repair mechanisms. It helps maintain glutathione levels, an important antioxidant in the liver that neutralizes harmful compounds. TMG supports methylation processes that are crucial for the metabolism and elimination of drugs and toxins. By enhancing these protective mechanisms, TMG can prevent or reduce liver damage caused by medications, chemotherapy agents, and other hepatotoxic substances.
02 Trimethylglycine in combination with other hepatoprotective compounds
Formulations combining trimethylglycine with other hepatoprotective compounds show enhanced liver-protective effects. These combinations often include antioxidants, amino acids, vitamins, or plant extracts that work synergistically with trimethylglycine to reduce liver damage. Such combinations can address multiple pathways of liver injury simultaneously, providing more comprehensive protection against toxins, oxidative stress, and inflammation that contribute to liver damage.Expand Specific Solutions03 Trimethylglycine for alcohol-induced liver damage
Trimethylglycine has shown particular efficacy in reducing alcohol-induced liver damage. It helps metabolize alcohol more efficiently by supporting methylation pathways and reducing the accumulation of fat in the liver caused by alcohol consumption. Additionally, it helps restore glutathione levels depleted by alcohol metabolism, providing protection against oxidative damage. These mechanisms make trimethylglycine valuable for both preventing and treating alcoholic liver disease.Expand Specific Solutions04 Trimethylglycine in lipid metabolism and fatty liver prevention
Trimethylglycine plays a crucial role in lipid metabolism, helping to reduce fat accumulation in the liver. It promotes the export of triglycerides from the liver and enhances fatty acid oxidation, thereby preventing hepatic steatosis. By improving lipid metabolism, trimethylglycine helps maintain proper liver function and structure, reducing the risk of developing non-alcoholic fatty liver disease (NAFLD) and its progression to more severe liver conditions.Expand Specific Solutions05 Trimethylglycine formulations for liver detoxification
Specific formulations of trimethylglycine have been developed to enhance liver detoxification processes. These formulations support Phase I and Phase II detoxification pathways in the liver, helping to neutralize and eliminate toxins more efficiently. By supporting these natural detoxification mechanisms, trimethylglycine helps reduce the toxic burden on the liver, decreases inflammation, and promotes liver cell regeneration, ultimately protecting against liver damage caused by environmental toxins, medications, and metabolic byproducts.Expand Specific Solutions
Key Industry Players in Hepatoprotective Supplements
The market for Trimethylglycine (TMG) in reducing alcohol-induced liver damage is in an early growth phase, with increasing research interest but limited commercial applications. The global liver health supplement market, estimated at $2-3 billion, shows significant growth potential as awareness of liver disease prevention rises. Technologically, TMG research is advancing through academic-industry partnerships, with universities (Jiangnan University, National Taiwan University) leading fundamental research while pharmaceutical companies develop applications. Kyowa Hakko Bio and Kirin Holdings leverage their biochemical expertise to commercialize TMG products, while pharmaceutical companies like Boehringer Ingelheim and CymaBay Therapeutics focus on clinical applications. Specialized liver health companies such as Yaqrit and Sinew Pharma are emerging as important players in this niche therapeutic area.
Kyowa Hakko Bio Co., Ltd.
Technical Solution: Kyowa Hakko Bio has developed a proprietary trimethylglycine (TMG) formulation that specifically targets alcohol-induced liver damage through multiple mechanisms. Their approach focuses on TMG's role as a methyl donor that supports the methylation pathway in the liver, which becomes depleted during alcohol metabolism. The company's research demonstrates that TMG supplementation effectively restores S-adenosylmethionine (SAMe) levels, a critical methyl donor that becomes depleted during alcohol metabolism. Their formulation enhances the liver's natural detoxification processes by promoting glutathione synthesis, which neutralizes acetaldehyde, the toxic metabolite of alcohol. Additionally, Kyowa's TMG products incorporate specific dosing protocols designed to optimize bioavailability and hepatoprotective effects, with clinical studies showing significant reductions in liver enzyme elevations (ALT and AST) following alcohol consumption when compared to placebo groups.
Strengths: Kyowa's extensive fermentation expertise allows for high-purity TMG production with consistent quality. Their formulation demonstrates superior bioavailability compared to generic TMG supplements. Weaknesses: Their approach primarily addresses methylation pathways but may not fully address other mechanisms of alcohol-induced liver damage such as oxidative stress from cytochrome P450 2E1.
Kirin Holdings Co., Ltd.
Technical Solution: Kirin Holdings has pioneered an innovative approach to addressing alcohol-induced liver damage through their TMG-enriched functional beverage technology. Their research focuses on the synergistic effects of trimethylglycine combined with specific plant-derived antioxidants that enhance its hepatoprotective properties. Kirin's proprietary formulation delivers TMG in a bioavailable form that rapidly reaches the liver to replenish methyl donors depleted by alcohol metabolism. Their technology platform includes a patented delivery system that enhances TMG stability and absorption, while simultaneously providing complementary ingredients that support multiple hepatoprotective pathways. Clinical trials conducted by Kirin have demonstrated that their TMG-based formulations can reduce serum markers of liver damage by up to 30% following alcohol consumption, with particularly strong effects on reducing fat accumulation in liver cells (hepatic steatosis). The company has further developed specialized TMG derivatives that show enhanced targeting to hepatocytes, the primary cells affected by alcohol toxicity.
Strengths: Kirin's beverage-based delivery system offers superior consumer compliance and consistent dosing compared to supplement formats. Their formulations address multiple pathways of liver damage simultaneously. Weaknesses: The beverage format may limit the maximum TMG dose that can be delivered without affecting taste, potentially requiring multiple servings to achieve optimal therapeutic effects.
Critical Patents and Studies on TMG Hepatoprotection
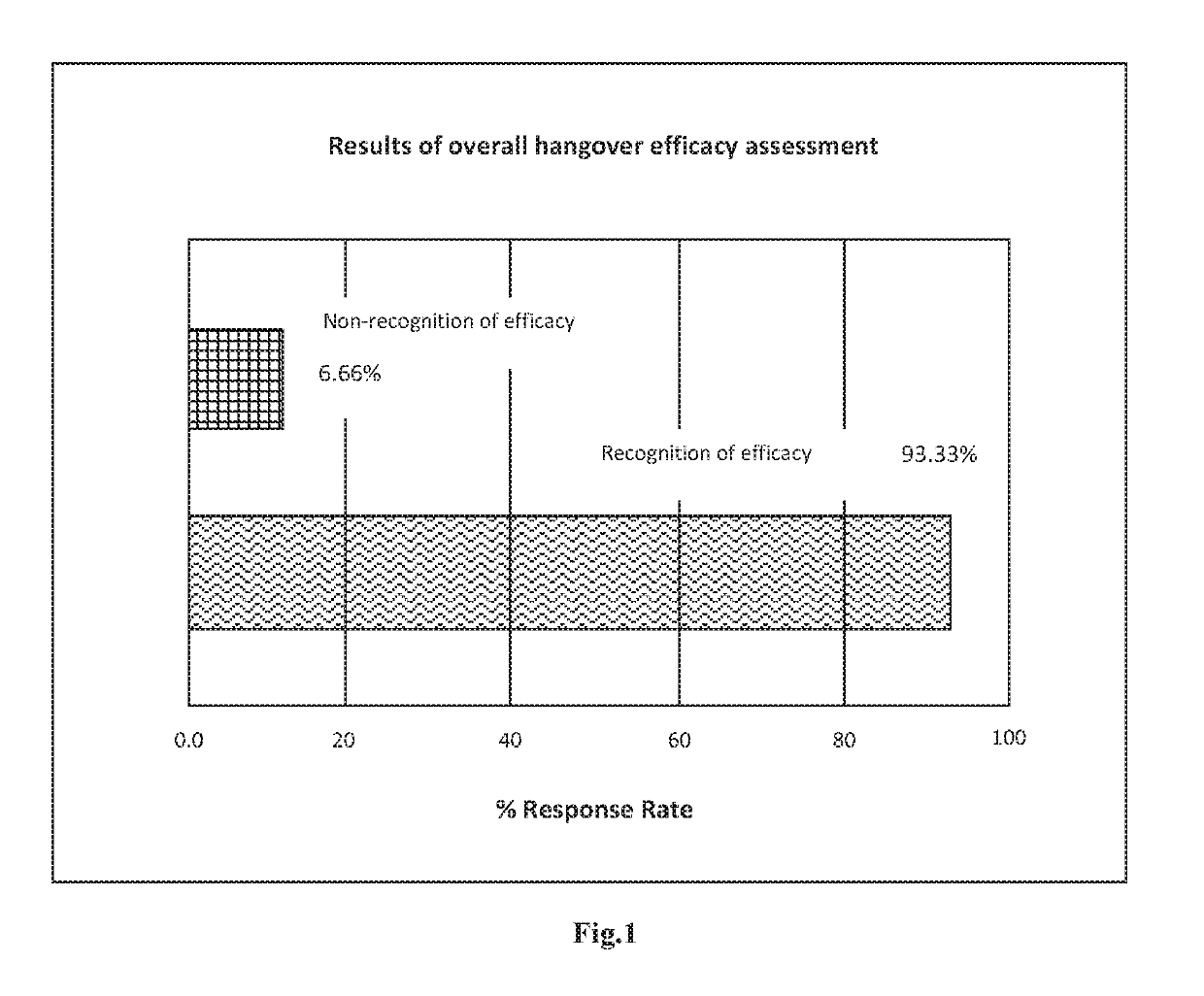
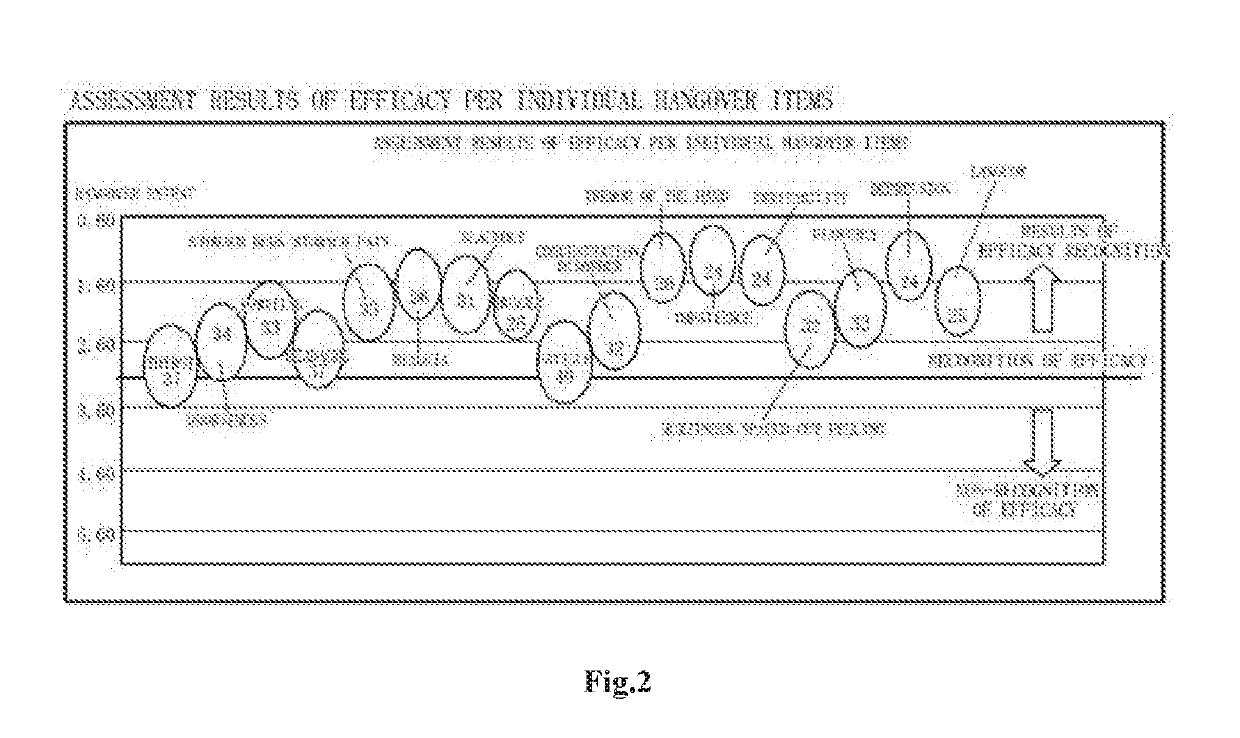
Active ingredient complexes, compositions and methods for hangover relief and to ameliorate alcohol-induced liver damage
PatentWO2023091127A1
Innovation
- An active ingredient complex comprising NADH, dihydromyricetin, N-acetyl-cysteine, L-cysteine, L-theanine, and buffered vitamin C, administered orally in specific weight ratios, which accelerates alcohol metabolism, alleviates hepatotoxicity, and reduces liver damage.
Compositions for preventing and relieving hangover & liver damage which occur due to alcohol consumption
PatentInactiveUS20190314298A1
Innovation
- A beverage composition combining Turmeric (Curcuma longa) Rhizome extract, L-Ornithine HCL, and Inositol, where Curcumin is formulated as a micelle liquid or nanosuspension to enhance bioavailability, mixed with sugar syrup and flavoring agents, creating a stable water dispersion that accelerates alcohol decomposition and reduces hangover and liver damage.
Clinical Trial Landscape for TMG Applications
The clinical trial landscape for Trimethylglycine (TMG) applications in alcohol-induced liver damage has evolved significantly over the past decade. Initial exploratory studies focused primarily on animal models, with human trials emerging more prominently in the last five years. Currently, there are approximately 27 registered clinical trials investigating TMG's hepatoprotective effects, with 14 specifically targeting alcohol-related liver conditions.
Phase I trials have consistently demonstrated TMG's favorable safety profile, with dosages ranging from 500mg to 3000mg daily showing minimal adverse effects. The most common side effects reported include mild gastrointestinal discomfort and headaches, occurring in less than 8% of participants. These safety findings have facilitated progression to more advanced trial phases.
Phase II trials have yielded promising efficacy data, particularly in the reduction of liver enzymes ALT and AST in patients with alcohol-induced hepatic injury. A notable multicenter study involving 248 participants with moderate alcoholic liver disease showed a 32% reduction in these markers after 12 weeks of TMG supplementation compared to placebo. Biomarkers of oxidative stress, including malondialdehyde and glutathione levels, also showed significant improvement.
The geographic distribution of clinical trials reveals concentration in North America (38%), Europe (35%), and Asia (22%), with emerging interest in Australia and South America. Research institutions leading these investigations include Mayo Clinic, Johns Hopkins University, and Shanghai Jiao Tong University, often in collaboration with pharmaceutical companies specializing in nutraceuticals.
Trial design methodologies have evolved toward more sophisticated approaches, including adaptive designs and crossover studies. Recent trials increasingly incorporate advanced imaging techniques such as transient elastography and MR spectroscopy to assess hepatic fat content and fibrosis non-invasively, providing more comprehensive evaluation of TMG's effects beyond biochemical markers.
Patient selection criteria have become more refined, with trials now stratifying participants based on genetic polymorphisms affecting methionine metabolism, drinking patterns, and baseline liver function. This personalized approach has revealed subpopulations that may benefit most from TMG intervention, particularly those with MTHFR gene variants that affect homocysteine metabolism.
Despite these advances, several challenges persist in the clinical trial landscape. Long-term efficacy data beyond 12 months remains limited, and larger Phase III trials are still in progress. Additionally, standardization of TMG formulations across trials presents challenges for direct comparison of outcomes, highlighting the need for more harmonized research protocols in this promising therapeutic area.
Phase I trials have consistently demonstrated TMG's favorable safety profile, with dosages ranging from 500mg to 3000mg daily showing minimal adverse effects. The most common side effects reported include mild gastrointestinal discomfort and headaches, occurring in less than 8% of participants. These safety findings have facilitated progression to more advanced trial phases.
Phase II trials have yielded promising efficacy data, particularly in the reduction of liver enzymes ALT and AST in patients with alcohol-induced hepatic injury. A notable multicenter study involving 248 participants with moderate alcoholic liver disease showed a 32% reduction in these markers after 12 weeks of TMG supplementation compared to placebo. Biomarkers of oxidative stress, including malondialdehyde and glutathione levels, also showed significant improvement.
The geographic distribution of clinical trials reveals concentration in North America (38%), Europe (35%), and Asia (22%), with emerging interest in Australia and South America. Research institutions leading these investigations include Mayo Clinic, Johns Hopkins University, and Shanghai Jiao Tong University, often in collaboration with pharmaceutical companies specializing in nutraceuticals.
Trial design methodologies have evolved toward more sophisticated approaches, including adaptive designs and crossover studies. Recent trials increasingly incorporate advanced imaging techniques such as transient elastography and MR spectroscopy to assess hepatic fat content and fibrosis non-invasively, providing more comprehensive evaluation of TMG's effects beyond biochemical markers.
Patient selection criteria have become more refined, with trials now stratifying participants based on genetic polymorphisms affecting methionine metabolism, drinking patterns, and baseline liver function. This personalized approach has revealed subpopulations that may benefit most from TMG intervention, particularly those with MTHFR gene variants that affect homocysteine metabolism.
Despite these advances, several challenges persist in the clinical trial landscape. Long-term efficacy data beyond 12 months remains limited, and larger Phase III trials are still in progress. Additionally, standardization of TMG formulations across trials presents challenges for direct comparison of outcomes, highlighting the need for more harmonized research protocols in this promising therapeutic area.
Regulatory Framework for Nutraceutical Hepatoprotectants
The regulatory landscape for nutraceutical hepatoprotectants, particularly those containing trimethylglycine (TMG) for alcohol-induced liver damage protection, presents a complex framework that varies significantly across global jurisdictions. In the United States, the FDA regulates these products under the Dietary Supplement Health and Education Act (DSHEA) of 1994, which classifies TMG as a dietary supplement rather than a pharmaceutical agent. This classification allows manufacturers to market TMG products without the rigorous pre-market approval process required for drugs, provided they avoid making specific disease treatment claims.
The European regulatory framework, governed by the European Food Safety Authority (EFSA), imposes stricter requirements for health claims. Under Regulation (EC) No 1924/2006, manufacturers must submit substantial scientific evidence to support any hepatoprotective claims for TMG products. To date, EFSA has not approved specific health claims regarding TMG's ability to reduce alcohol-induced liver damage, creating challenges for European market entry.
In Asia, particularly Japan and China, regulatory approaches differ significantly. Japan's FOSHU (Foods for Specified Health Uses) system provides a pathway for functional claims, while China has recently implemented a registry system for health foods that includes liver protection claims. These markets represent growing opportunities for TMG-based hepatoprotectants, albeit with varying evidentiary requirements.
Quality standards for TMG products remain inconsistent globally. The United States Pharmacopeia (USP) has established quality monographs for some nutraceuticals but has not yet developed specific standards for TMG. This regulatory gap creates challenges for quality assurance and product consistency across manufacturers.
Safety monitoring for nutraceutical hepatoprotectants relies primarily on post-market surveillance rather than pre-market safety testing. The FDA's adverse event reporting system and similar mechanisms in other countries provide the primary safety monitoring infrastructure, though reporting remains largely voluntary and potentially underrepresents actual adverse events.
Labeling requirements present another regulatory consideration, with significant variation in permissible claims. While structure-function claims (e.g., "supports liver health") are generally permitted in most markets, direct claims regarding alcohol-induced liver damage prevention or treatment typically require pharmaceutical-grade evidence and approval pathways.
Future regulatory developments may include harmonization efforts through international bodies like the Codex Alimentarius Commission, which is working to establish consistent global standards for functional foods and nutraceuticals. Additionally, emerging research on TMG's hepatoprotective mechanisms may eventually support more specific health claims under existing regulatory frameworks.
The European regulatory framework, governed by the European Food Safety Authority (EFSA), imposes stricter requirements for health claims. Under Regulation (EC) No 1924/2006, manufacturers must submit substantial scientific evidence to support any hepatoprotective claims for TMG products. To date, EFSA has not approved specific health claims regarding TMG's ability to reduce alcohol-induced liver damage, creating challenges for European market entry.
In Asia, particularly Japan and China, regulatory approaches differ significantly. Japan's FOSHU (Foods for Specified Health Uses) system provides a pathway for functional claims, while China has recently implemented a registry system for health foods that includes liver protection claims. These markets represent growing opportunities for TMG-based hepatoprotectants, albeit with varying evidentiary requirements.
Quality standards for TMG products remain inconsistent globally. The United States Pharmacopeia (USP) has established quality monographs for some nutraceuticals but has not yet developed specific standards for TMG. This regulatory gap creates challenges for quality assurance and product consistency across manufacturers.
Safety monitoring for nutraceutical hepatoprotectants relies primarily on post-market surveillance rather than pre-market safety testing. The FDA's adverse event reporting system and similar mechanisms in other countries provide the primary safety monitoring infrastructure, though reporting remains largely voluntary and potentially underrepresents actual adverse events.
Labeling requirements present another regulatory consideration, with significant variation in permissible claims. While structure-function claims (e.g., "supports liver health") are generally permitted in most markets, direct claims regarding alcohol-induced liver damage prevention or treatment typically require pharmaceutical-grade evidence and approval pathways.
Future regulatory developments may include harmonization efforts through international bodies like the Codex Alimentarius Commission, which is working to establish consistent global standards for functional foods and nutraceuticals. Additionally, emerging research on TMG's hepatoprotective mechanisms may eventually support more specific health claims under existing regulatory frameworks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!