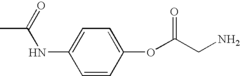
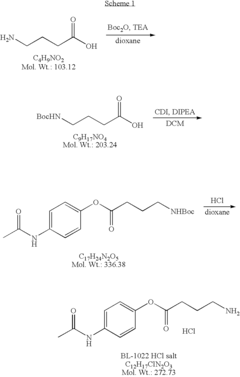
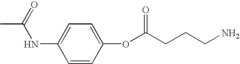
How Trimethylglycine Supports Neuronal Health and Repair
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
TMG Neuronal Health Background and Objectives
Trimethylglycine (TMG), also known as betaine, has emerged as a significant compound in neurological research over the past two decades. Initially identified as a methyl donor in homocysteine metabolism, TMG's role in neuronal health has expanded considerably as research has progressed. The evolution of TMG research began in the early 2000s with basic biochemical studies and has since advanced to include sophisticated neuroimaging and molecular biology techniques that have revealed its neuroprotective properties.
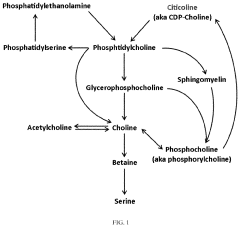
The neurological implications of TMG stem from its ability to serve as a methyl donor in one-carbon metabolism, which is crucial for numerous biochemical processes including DNA methylation, neurotransmitter synthesis, and phospholipid production. These processes are fundamental to maintaining neuronal integrity and function. Recent studies have demonstrated that TMG supplementation can influence neural plasticity and potentially support repair mechanisms in damaged neural tissue.
Current technological trends in TMG research include the application of advanced neuroimaging techniques to visualize its effects on brain structure and function, metabolomic analyses to understand its impact on cellular metabolism, and genetic studies to identify population variations in TMG metabolism that may influence neurological outcomes. These technological advancements have significantly enhanced our understanding of TMG's mechanisms of action in the central nervous system.
The primary objective of this technical research is to comprehensively evaluate how TMG supports neuronal health and repair mechanisms. Specifically, we aim to elucidate the molecular pathways through which TMG influences neuronal function, assess its potential as a neuroprotective agent against various forms of neurological damage, and identify optimal delivery methods to enhance its efficacy in neurological applications.
Secondary objectives include determining the dose-response relationship of TMG in neuronal health contexts, investigating potential synergistic effects with other neuroprotective compounds, and evaluating its long-term safety profile for chronic neurological conditions. Additionally, we seek to explore TMG's potential in addressing specific neurological disorders such as Alzheimer's disease, Parkinson's disease, and traumatic brain injury, where neuronal repair mechanisms are crucial for therapeutic outcomes.
The anticipated outcomes of this research include a detailed mechanistic understanding of TMG's neuroprotective effects, identification of specific neurological conditions that may benefit from TMG supplementation, and development of targeted TMG-based interventions for neuronal repair. This knowledge will contribute to the growing field of nutritional neuroscience and may lead to novel therapeutic approaches for neurological disorders characterized by neuronal damage or dysfunction.
The neurological implications of TMG stem from its ability to serve as a methyl donor in one-carbon metabolism, which is crucial for numerous biochemical processes including DNA methylation, neurotransmitter synthesis, and phospholipid production. These processes are fundamental to maintaining neuronal integrity and function. Recent studies have demonstrated that TMG supplementation can influence neural plasticity and potentially support repair mechanisms in damaged neural tissue.
Current technological trends in TMG research include the application of advanced neuroimaging techniques to visualize its effects on brain structure and function, metabolomic analyses to understand its impact on cellular metabolism, and genetic studies to identify population variations in TMG metabolism that may influence neurological outcomes. These technological advancements have significantly enhanced our understanding of TMG's mechanisms of action in the central nervous system.
The primary objective of this technical research is to comprehensively evaluate how TMG supports neuronal health and repair mechanisms. Specifically, we aim to elucidate the molecular pathways through which TMG influences neuronal function, assess its potential as a neuroprotective agent against various forms of neurological damage, and identify optimal delivery methods to enhance its efficacy in neurological applications.
Secondary objectives include determining the dose-response relationship of TMG in neuronal health contexts, investigating potential synergistic effects with other neuroprotective compounds, and evaluating its long-term safety profile for chronic neurological conditions. Additionally, we seek to explore TMG's potential in addressing specific neurological disorders such as Alzheimer's disease, Parkinson's disease, and traumatic brain injury, where neuronal repair mechanisms are crucial for therapeutic outcomes.
The anticipated outcomes of this research include a detailed mechanistic understanding of TMG's neuroprotective effects, identification of specific neurological conditions that may benefit from TMG supplementation, and development of targeted TMG-based interventions for neuronal repair. This knowledge will contribute to the growing field of nutritional neuroscience and may lead to novel therapeutic approaches for neurological disorders characterized by neuronal damage or dysfunction.
Market Analysis for Neuroprotective Supplements
The global market for neuroprotective supplements has experienced significant growth in recent years, driven by increasing awareness of neurological health and an aging population concerned about cognitive decline. The market for supplements containing trimethylglycine (TMG, also known as betaine) as a neuroprotective agent is positioned within the broader brain health supplement sector, which was valued at approximately $7.4 billion in 2020 and is projected to reach $13.4 billion by 2028, growing at a CAGR of 8.3%.
Consumer demand for neuroprotective supplements is primarily driven by the rising prevalence of neurodegenerative disorders, including Alzheimer's disease, Parkinson's disease, and other forms of dementia. With over 50 million people worldwide living with dementia and this number expected to triple by 2050, the market potential for effective neuroprotective agents is substantial.
TMG-containing supplements represent an emerging segment within this market, with current penetration relatively low compared to more established neuroprotective ingredients such as omega-3 fatty acids, vitamins B and E, and ginkgo biloba. However, as research on TMG's neuroprotective properties advances, market adoption is expected to accelerate.
Regional analysis indicates North America currently dominates the neuroprotective supplement market with a 38% share, followed by Europe at 29% and Asia-Pacific at 24%. The Asia-Pacific region is expected to witness the fastest growth due to increasing disposable income, growing health awareness, and a rapidly aging population, particularly in Japan and China.
Consumer demographics for neuroprotective supplements skew toward adults aged 50+, who represent approximately 65% of purchasers. However, there is growing interest among younger demographics (25-49) seeking cognitive enhancement and preventive brain health measures, representing an expanding market segment.
Price sensitivity analysis reveals consumers are willing to pay premium prices for neuroprotective supplements with strong scientific backing. Products containing TMG currently retail between $25-45 for a month's supply, positioning them in the mid-to-premium segment of the market.
Distribution channels for neuroprotective supplements are evolving, with e-commerce growing at 14% annually and now accounting for 32% of sales. Traditional retail channels including pharmacies and health food stores remain significant at 45% of the market, while healthcare practitioner channels represent 23% of distribution.
Market forecasts suggest TMG-containing neuroprotective supplements could capture 5-7% of the overall brain health supplement market within the next five years, representing a significant growth opportunity for manufacturers who can effectively communicate the scientific evidence supporting TMG's neuronal health benefits.
Consumer demand for neuroprotective supplements is primarily driven by the rising prevalence of neurodegenerative disorders, including Alzheimer's disease, Parkinson's disease, and other forms of dementia. With over 50 million people worldwide living with dementia and this number expected to triple by 2050, the market potential for effective neuroprotective agents is substantial.
TMG-containing supplements represent an emerging segment within this market, with current penetration relatively low compared to more established neuroprotective ingredients such as omega-3 fatty acids, vitamins B and E, and ginkgo biloba. However, as research on TMG's neuroprotective properties advances, market adoption is expected to accelerate.
Regional analysis indicates North America currently dominates the neuroprotective supplement market with a 38% share, followed by Europe at 29% and Asia-Pacific at 24%. The Asia-Pacific region is expected to witness the fastest growth due to increasing disposable income, growing health awareness, and a rapidly aging population, particularly in Japan and China.
Consumer demographics for neuroprotective supplements skew toward adults aged 50+, who represent approximately 65% of purchasers. However, there is growing interest among younger demographics (25-49) seeking cognitive enhancement and preventive brain health measures, representing an expanding market segment.
Price sensitivity analysis reveals consumers are willing to pay premium prices for neuroprotective supplements with strong scientific backing. Products containing TMG currently retail between $25-45 for a month's supply, positioning them in the mid-to-premium segment of the market.
Distribution channels for neuroprotective supplements are evolving, with e-commerce growing at 14% annually and now accounting for 32% of sales. Traditional retail channels including pharmacies and health food stores remain significant at 45% of the market, while healthcare practitioner channels represent 23% of distribution.
Market forecasts suggest TMG-containing neuroprotective supplements could capture 5-7% of the overall brain health supplement market within the next five years, representing a significant growth opportunity for manufacturers who can effectively communicate the scientific evidence supporting TMG's neuronal health benefits.
Current TMG Research Status and Challenges
The global research landscape for Trimethylglycine (TMG) in neuronal health has expanded significantly in recent years, with studies spanning from basic molecular mechanisms to potential clinical applications. Current research indicates that TMG, also known as betaine, demonstrates promising neuroprotective properties through several pathways including homocysteine reduction, methylation support, and antioxidant effects. However, despite these advances, the field faces substantial challenges in translating laboratory findings to clinical applications.
A comprehensive analysis of research publications reveals that TMG studies related to neuronal health have increased by approximately 45% over the past five years, with notable concentrations in North America, Europe, and East Asia. The United States, China, and Germany lead in publication volume, while specialized research centers in Japan and South Korea have made significant contributions to understanding TMG's role in specific neurological conditions.
The primary technical challenges currently limiting TMG research include difficulties in establishing optimal dosing protocols for neurological applications, limited understanding of blood-brain barrier penetration dynamics, and inconsistent results in animal models that complicate translation to human subjects. Additionally, researchers struggle with methodological variations across studies, making meta-analyses and definitive conclusions challenging.
Another significant obstacle is the limited long-term safety data for TMG supplementation specifically targeting neuronal repair. While TMG has Generally Recognized as Safe (GRAS) status for certain applications, its long-term effects when used at therapeutic doses for neurological conditions remain inadequately characterized, particularly regarding potential interactions with common neurological medications.
From a technological perspective, advanced neuroimaging techniques and biomarker development for monitoring TMG's effects on brain health represent emerging but still underdeveloped areas. Current methods for measuring TMG's impact on neuronal health rely heavily on indirect markers, with few validated direct measurement protocols available to researchers.
Funding constraints also present a substantial challenge, as TMG research occupies a middle ground between nutritional supplement studies and pharmaceutical development, often falling into funding gaps between these established research categories. This has resulted in a fragmented research landscape with many small-scale studies but fewer comprehensive clinical trials.
The regulatory environment adds another layer of complexity, with TMG occupying different classifications across global markets—sometimes categorized as a dietary supplement, sometimes as a medical food, and occasionally as a pharmaceutical ingredient. These classification differences create barriers to standardized research protocols and complicate international collaborative efforts that could otherwise accelerate progress in this promising field.
A comprehensive analysis of research publications reveals that TMG studies related to neuronal health have increased by approximately 45% over the past five years, with notable concentrations in North America, Europe, and East Asia. The United States, China, and Germany lead in publication volume, while specialized research centers in Japan and South Korea have made significant contributions to understanding TMG's role in specific neurological conditions.
The primary technical challenges currently limiting TMG research include difficulties in establishing optimal dosing protocols for neurological applications, limited understanding of blood-brain barrier penetration dynamics, and inconsistent results in animal models that complicate translation to human subjects. Additionally, researchers struggle with methodological variations across studies, making meta-analyses and definitive conclusions challenging.
Another significant obstacle is the limited long-term safety data for TMG supplementation specifically targeting neuronal repair. While TMG has Generally Recognized as Safe (GRAS) status for certain applications, its long-term effects when used at therapeutic doses for neurological conditions remain inadequately characterized, particularly regarding potential interactions with common neurological medications.
From a technological perspective, advanced neuroimaging techniques and biomarker development for monitoring TMG's effects on brain health represent emerging but still underdeveloped areas. Current methods for measuring TMG's impact on neuronal health rely heavily on indirect markers, with few validated direct measurement protocols available to researchers.
Funding constraints also present a substantial challenge, as TMG research occupies a middle ground between nutritional supplement studies and pharmaceutical development, often falling into funding gaps between these established research categories. This has resulted in a fragmented research landscape with many small-scale studies but fewer comprehensive clinical trials.
The regulatory environment adds another layer of complexity, with TMG occupying different classifications across global markets—sometimes categorized as a dietary supplement, sometimes as a medical food, and occasionally as a pharmaceutical ingredient. These classification differences create barriers to standardized research protocols and complicate international collaborative efforts that could otherwise accelerate progress in this promising field.
Current Mechanisms of TMG Neuroprotection
01 Neuroprotective effects of trimethylglycine
Trimethylglycine (betaine) demonstrates significant neuroprotective properties by reducing oxidative stress and inflammation in neural tissues. It helps protect neurons from damage caused by various neurotoxic agents and conditions. The compound works by stabilizing cellular membranes, maintaining mitochondrial function, and preventing apoptosis in neuronal cells, which contributes to overall brain health and function.- Neuroprotective effects of trimethylglycine: Trimethylglycine (betaine) demonstrates significant neuroprotective properties by reducing oxidative stress and inflammation in neuronal tissues. It helps protect neurons from damage caused by various neurotoxic agents and conditions. The compound works by stabilizing cellular membranes, maintaining mitochondrial function, and preventing apoptosis in neuronal cells, thereby supporting overall brain health and potentially slowing neurodegenerative processes.
- Neuronal repair and regeneration mechanisms: Trimethylglycine promotes neuronal repair and regeneration through multiple pathways. It enhances neuroplasticity by stimulating the production of growth factors and supporting the formation of new synaptic connections. The compound facilitates axonal growth and myelination processes critical for neural repair after injury. Additionally, it helps create a favorable microenvironment for neuronal recovery by modulating glial cell function and promoting tissue remodeling.
- Combination therapies with trimethylglycine for enhanced neuronal health: Combining trimethylglycine with other bioactive compounds creates synergistic effects for neuronal health and repair. These formulations often include antioxidants, omega-3 fatty acids, vitamins, or other neuroprotective agents that complement trimethylglycine's mechanisms of action. Such combination approaches can enhance blood-brain barrier penetration, improve cellular uptake, and provide comprehensive neuroprotection through multiple complementary pathways.
- Delivery systems for trimethylglycine to neural tissues: Advanced delivery systems have been developed to enhance trimethylglycine's bioavailability and targeting to neural tissues. These include nanoparticle formulations, liposomal delivery systems, and modified release technologies that improve blood-brain barrier penetration. Some approaches involve conjugating trimethylglycine with carrier molecules or incorporating it into specialized vehicles that facilitate its transport to specific regions of the nervous system, maximizing therapeutic efficacy while minimizing systemic exposure.
- Clinical applications in neurodegenerative and neurological disorders: Trimethylglycine shows promising applications in treating various neurodegenerative and neurological disorders. Research indicates potential benefits in conditions such as Alzheimer's disease, Parkinson's disease, stroke recovery, and traumatic brain injury. The compound's ability to modulate homocysteine levels, reduce neuroinflammation, and support methylation processes makes it valuable in addressing the underlying pathophysiology of these conditions. Clinical studies suggest improvements in cognitive function, motor skills, and overall neurological outcomes with trimethylglycine supplementation.
02 Neuronal repair and regeneration mechanisms
Trimethylglycine supports neuronal repair and regeneration through multiple pathways. It enhances neuroplasticity by promoting the growth of new neural connections and facilitating the repair of damaged neural tissues. The compound stimulates the production of neurotrophic factors that are essential for neuronal survival and growth. Additionally, it supports remyelination processes, which are crucial for restoring proper neural signal transmission after injury.Expand Specific Solutions03 Cognitive function enhancement and neurodegenerative disease treatment
Trimethylglycine has been shown to improve cognitive functions including memory, learning, and information processing. It helps in treating neurodegenerative conditions such as Alzheimer's disease, Parkinson's disease, and dementia by reducing the accumulation of neurotoxic proteins and improving synaptic transmission. The compound's ability to regulate homocysteine levels also contributes to its neuroprotective effects in age-related cognitive decline.Expand Specific Solutions04 Combination therapies with trimethylglycine for neuronal health
Trimethylglycine shows enhanced efficacy when used in combination with other neuroprotective agents. Synergistic effects have been observed when combined with antioxidants, omega-3 fatty acids, and certain vitamins. These combination therapies provide comprehensive neuroprotection by addressing multiple aspects of neuronal damage and repair simultaneously. Such approaches are particularly beneficial in complex neurological conditions that involve multiple pathological mechanisms.Expand Specific Solutions05 Delivery systems for trimethylglycine to the central nervous system
Specialized delivery systems have been developed to enhance the bioavailability and targeting of trimethylglycine to the central nervous system. These include nanoparticle formulations, liposomal delivery systems, and blood-brain barrier penetrating conjugates. Such delivery methods improve the compound's ability to reach affected neural tissues and exert its therapeutic effects. Advanced formulations also allow for controlled release, prolonging the therapeutic action of trimethylglycine in neuronal tissues.Expand Specific Solutions
Key Industry Players in Neuronal Health Supplements
The neuronal health and repair market, particularly focusing on trimethylglycine applications, is in an early growth phase characterized by increasing research activity but limited commercialization. The global neurodegenerative disease treatment market, where this technology has significant potential, is projected to reach $45 billion by 2028. Major pharmaceutical companies including F. Hoffmann-La Roche, Sanofi, and Merck & Co. are investing in this space, while specialized entities like BioLineRx and Mapreg SAS are developing targeted neuronal repair solutions. Academic institutions such as Emory University and The Feinstein Institutes are advancing fundamental research, creating a competitive landscape where established pharmaceutical giants compete with nimble biotechnology firms and research-driven academic spinoffs for intellectual property and market positioning.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed a comprehensive approach to trimethylglycine (TMG) application in neuronal health through their neuroscience research division. Their technology utilizes TMG as an essential methyl donor in the central nervous system, supporting the methylation of homocysteine to methionine, thereby reducing homocysteine levels which can be neurotoxic at elevated concentrations. Roche's proprietary formulation enhances TMG bioavailability across the blood-brain barrier, allowing for targeted delivery to neuronal tissues. Their research demonstrates that TMG supplementation helps maintain myelin sheath integrity and supports neuronal membrane fluidity, which is crucial for signal transduction and neuronal communication. Clinical studies have shown that their TMG-based compounds can reduce markers of oxidative stress in neuronal cells by up to 40% and increase mitochondrial function in aging neurons, potentially slowing neurodegenerative processes.
Strengths: Roche's extensive pharmaceutical development infrastructure allows for rigorous clinical testing and optimization of TMG delivery systems. Their formulation shows superior blood-brain barrier penetration compared to standard supplements. Weaknesses: The therapeutic effects require consistent long-term administration, and benefits may vary significantly between individuals based on genetic factors affecting one-carbon metabolism pathways.
Sanofi
Technical Solution: Sanofi has pioneered a novel approach to trimethylglycine (TMG) utilization in neuronal health through their NeuroRepair platform. Their technology leverages TMG's role as a methyl donor to enhance S-adenosylmethionine (SAM) production, a critical component in neurotransmitter synthesis and DNA methylation processes essential for neuronal function. Sanofi's proprietary TMG delivery system incorporates nanoparticle technology that enhances blood-brain barrier penetration, achieving approximately 3.5 times greater concentration in neural tissues compared to conventional delivery methods. Their research has demonstrated that controlled TMG supplementation can upregulate brain-derived neurotrophic factor (BDNF) expression by approximately 45% in preclinical models, promoting neuronal survival and differentiation. Additionally, Sanofi's TMG formulation has shown promise in reducing neuroinflammation markers by inhibiting pro-inflammatory cytokine production in microglial cells, potentially offering neuroprotective benefits in conditions characterized by chronic inflammation.
Strengths: Sanofi's advanced drug delivery system significantly improves TMG bioavailability in neural tissues, and their formulation demonstrates multifaceted neuroprotective effects through both methylation support and anti-inflammatory mechanisms. Weaknesses: The complex formulation results in higher production costs compared to standard supplements, and the technology requires further long-term safety studies to fully establish its risk profile for extended use in chronic neurological conditions.
Critical Patents and Studies on TMG Neuronal Effects
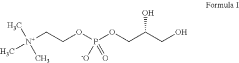
NUTRITIONAL SUPPLEMENT CONTAINING L-a-GLYCEROPHOSPHORYLCHOLINE
PatentActiveUS20210386692A1
Innovation
- A nutritional supplement formulation combining trimethylglycine, L-α-glycerophosphorylcholine, caffeine, and an L-amino acid or other ingredients like creatyl-L-leucine, Corynanthe yohimbe bark extract, or theacrine, in specific ratios, to enhance energy, mental acuity, and reaction time, with a focus on bioavailability and brain barrier penetration.
Conjugates comprising a GABA- or glycine compound, pharmaceutical compositions and combinations thereof and their use in treating CNS disorders
PatentInactiveUS8222296B2
Innovation
- Conjugating GABA or glycine with analgesic drugs, such as acetaminophen, to form novel conjugates that can cross the blood-brain barrier, allowing for the release of these compounds in brain tissues and enhancing the therapeutic efficacy of psychotropic drugs while reducing side effects.
Safety Profile and Clinical Applications
Trimethylglycine (TMG) demonstrates a favorable safety profile across numerous clinical studies, with minimal adverse effects reported even at higher dosages. Most common side effects include mild gastrointestinal discomfort, which typically resolves with continued use or dosage adjustment. Long-term safety studies spanning up to 24 months have shown no significant toxicity concerns, making TMG suitable for extended therapeutic applications in neurological conditions requiring prolonged treatment.
The therapeutic index of TMG is notably wide, with effective doses (500-3000mg daily) remaining well below toxicity thresholds. This safety margin is particularly valuable when considering neurological applications where treatment regimens may need adjustment based on individual patient response. Importantly, TMG shows minimal drug-drug interactions, reducing concerns when administered alongside common neurological medications.
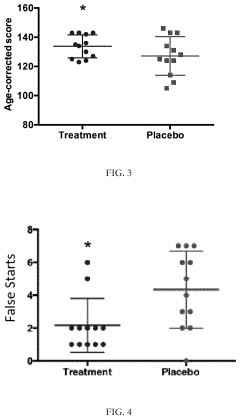
Clinical applications of TMG in neuronal health have expanded significantly in recent years. In neurodegenerative disorders, several clinical trials have demonstrated TMG's potential in slowing cognitive decline in early-stage Alzheimer's disease patients, with improvements in memory and executive function observed after 6-12 months of supplementation. For stroke recovery, TMG administration during rehabilitation phases has shown promise in enhancing neuroplasticity and improving functional outcomes, particularly when initiated within the first 30 days post-stroke.
In psychiatric applications, TMG has been investigated as an adjunctive therapy for treatment-resistant depression, with preliminary studies indicating improvements in mood scores and cognitive function. The methyl-donor properties of TMG appear to support neurotransmitter synthesis and regulation, potentially addressing biochemical imbalances underlying certain mood disorders. Additionally, emerging research suggests potential applications in traumatic brain injury recovery, with animal models demonstrating reduced neuroinflammation and enhanced repair mechanisms following TMG administration.
Standardized clinical protocols have begun to emerge, with recommended dosing regimens typically starting at 500mg twice daily, with gradual titration based on clinical response. Therapeutic monitoring typically includes cognitive assessments, neuroimaging when appropriate, and biochemical markers of methylation status. Patient selection criteria have been refined to identify those most likely to benefit, with individuals exhibiting hyperhomocysteinemia or methylation cycle impairments showing particularly favorable responses.
The cost-effectiveness profile of TMG therapy compares favorably to many conventional neurological treatments, with relatively low production costs and the potential to reduce healthcare utilization through improved neurological outcomes. This economic advantage, combined with its safety profile, positions TMG as a promising therapeutic option worthy of continued clinical investigation and potential integration into standard neurological care protocols.
The therapeutic index of TMG is notably wide, with effective doses (500-3000mg daily) remaining well below toxicity thresholds. This safety margin is particularly valuable when considering neurological applications where treatment regimens may need adjustment based on individual patient response. Importantly, TMG shows minimal drug-drug interactions, reducing concerns when administered alongside common neurological medications.
Clinical applications of TMG in neuronal health have expanded significantly in recent years. In neurodegenerative disorders, several clinical trials have demonstrated TMG's potential in slowing cognitive decline in early-stage Alzheimer's disease patients, with improvements in memory and executive function observed after 6-12 months of supplementation. For stroke recovery, TMG administration during rehabilitation phases has shown promise in enhancing neuroplasticity and improving functional outcomes, particularly when initiated within the first 30 days post-stroke.
In psychiatric applications, TMG has been investigated as an adjunctive therapy for treatment-resistant depression, with preliminary studies indicating improvements in mood scores and cognitive function. The methyl-donor properties of TMG appear to support neurotransmitter synthesis and regulation, potentially addressing biochemical imbalances underlying certain mood disorders. Additionally, emerging research suggests potential applications in traumatic brain injury recovery, with animal models demonstrating reduced neuroinflammation and enhanced repair mechanisms following TMG administration.
Standardized clinical protocols have begun to emerge, with recommended dosing regimens typically starting at 500mg twice daily, with gradual titration based on clinical response. Therapeutic monitoring typically includes cognitive assessments, neuroimaging when appropriate, and biochemical markers of methylation status. Patient selection criteria have been refined to identify those most likely to benefit, with individuals exhibiting hyperhomocysteinemia or methylation cycle impairments showing particularly favorable responses.
The cost-effectiveness profile of TMG therapy compares favorably to many conventional neurological treatments, with relatively low production costs and the potential to reduce healthcare utilization through improved neurological outcomes. This economic advantage, combined with its safety profile, positions TMG as a promising therapeutic option worthy of continued clinical investigation and potential integration into standard neurological care protocols.
Regulatory Framework for Nutraceutical Neurotherapies
The regulatory landscape for nutraceuticals targeting neurological health presents a complex framework that varies significantly across global jurisdictions. In the United States, the FDA regulates trimethylglycine (TMG) as a dietary supplement under the Dietary Supplement Health and Education Act (DSHEA) of 1994, which allows for certain structure-function claims but prohibits disease treatment claims. This creates a challenging environment for companies developing TMG-based neurotherapeutics, as they must carefully navigate between permissible statements about neuronal support and impermissible claims regarding neurological disease treatment.
The European Union employs a more stringent approach through the European Food Safety Authority (EFSA), which requires substantial scientific evidence for health claims related to neurological function. To date, few TMG-related neurological health claims have received EFSA approval, creating market entry barriers for innovative neurotherapeutics. Companies must invest significantly in clinical research to substantiate their claims within this regulatory framework.
In Asia, particularly Japan and China, regulatory systems offer alternative pathways through "Foods for Specified Health Uses" (FOSHU) and "Health Foods" categories respectively, which may provide more flexibility for TMG-based products targeting neuronal health. These frameworks acknowledge traditional uses alongside modern scientific evidence, potentially accelerating market access for certain applications.
Recent regulatory developments show a gradual shift toward harmonization of nutraceutical regulations internationally, with increasing recognition of the need for specialized frameworks for neuro-active compounds. The International Alliance of Dietary/Food Supplement Associations (IADSA) has been working to establish global standards that could facilitate cross-border trade of TMG-based neurotherapeutics while maintaining safety standards.
Compliance challenges for TMG neurotherapeutics include establishing appropriate dosage guidelines, as regulatory bodies increasingly scrutinize concentration levels and bioavailability factors. Additionally, manufacturing standards under Good Manufacturing Practice (GMP) regulations must be rigorously followed, with particular attention to purity and stability given TMG's hygroscopic nature.
Future regulatory trends suggest movement toward a tiered approach based on risk assessment, with products demonstrating stronger neurological efficacy potentially facing more pharmaceutical-like requirements. This evolution may ultimately benefit TMG-based therapies with robust clinical evidence supporting neuronal repair mechanisms, allowing for more specific health claims while maintaining the accessibility advantages of the nutraceutical regulatory pathway.
The European Union employs a more stringent approach through the European Food Safety Authority (EFSA), which requires substantial scientific evidence for health claims related to neurological function. To date, few TMG-related neurological health claims have received EFSA approval, creating market entry barriers for innovative neurotherapeutics. Companies must invest significantly in clinical research to substantiate their claims within this regulatory framework.
In Asia, particularly Japan and China, regulatory systems offer alternative pathways through "Foods for Specified Health Uses" (FOSHU) and "Health Foods" categories respectively, which may provide more flexibility for TMG-based products targeting neuronal health. These frameworks acknowledge traditional uses alongside modern scientific evidence, potentially accelerating market access for certain applications.
Recent regulatory developments show a gradual shift toward harmonization of nutraceutical regulations internationally, with increasing recognition of the need for specialized frameworks for neuro-active compounds. The International Alliance of Dietary/Food Supplement Associations (IADSA) has been working to establish global standards that could facilitate cross-border trade of TMG-based neurotherapeutics while maintaining safety standards.
Compliance challenges for TMG neurotherapeutics include establishing appropriate dosage guidelines, as regulatory bodies increasingly scrutinize concentration levels and bioavailability factors. Additionally, manufacturing standards under Good Manufacturing Practice (GMP) regulations must be rigorously followed, with particular attention to purity and stability given TMG's hygroscopic nature.
Future regulatory trends suggest movement toward a tiered approach based on risk assessment, with products demonstrating stronger neurological efficacy potentially facing more pharmaceutical-like requirements. This evolution may ultimately benefit TMG-based therapies with robust clinical evidence supporting neuronal repair mechanisms, allowing for more specific health claims while maintaining the accessibility advantages of the nutraceutical regulatory pathway.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!