How Triton X-100 Enhances Signal in ELISAs (Enzyme-Linked Immunosorbent Assays)
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ELISA Signal Enhancement Background and Objectives
Enzyme-Linked Immunosorbent Assays (ELISAs) have been a cornerstone of biomedical research and clinical diagnostics since their introduction in the 1970s. These versatile assays provide a sensitive and specific method for detecting and quantifying a wide range of biomolecules, including proteins, antibodies, hormones, and other antigens. The fundamental principle of ELISA relies on the specific interaction between antibodies and antigens, coupled with an enzyme-mediated color change for signal detection.
Over the years, researchers have continuously sought ways to enhance the sensitivity and reliability of ELISA techniques. One significant advancement in this pursuit has been the incorporation of detergents, particularly Triton X-100, into ELISA protocols. Triton X-100, a nonionic surfactant, has emerged as a powerful tool for improving signal strength and overall assay performance.
The primary objective of this technical research report is to explore and elucidate the mechanisms by which Triton X-100 enhances signal in ELISAs. By understanding these mechanisms, we aim to provide insights that can lead to further optimization of ELISA protocols and potentially inspire new innovations in immunoassay technology.
This investigation is driven by the increasing demand for more sensitive and accurate diagnostic tools in various fields, including medical diagnostics, environmental monitoring, and food safety. As the need for detecting lower concentrations of analytes grows, so does the importance of signal enhancement techniques in ELISAs.
The evolution of ELISA technology has been marked by continuous improvements in sensitivity, specificity, and reproducibility. From the early days of radioimmunoassays to the development of enzyme-linked techniques, each advancement has expanded the capabilities and applications of immunoassays. The introduction of detergents like Triton X-100 represents another significant step in this evolutionary process.
By examining the role of Triton X-100 in ELISA signal enhancement, we seek to address several key questions: How does Triton X-100 interact with assay components to improve signal intensity? What are the optimal concentrations and conditions for its use? Are there potential drawbacks or limitations to consider? Answering these questions will not only contribute to our understanding of ELISA optimization but also pave the way for developing more sensitive and reliable immunoassay techniques.
This report will delve into the technical aspects of Triton X-100's effects on ELISA performance, drawing from recent research findings and established principles of biochemistry and immunology. By synthesizing this information, we aim to provide a comprehensive overview of the current state of knowledge regarding Triton X-100's role in ELISA signal enhancement and identify potential areas for future research and development in this field.
Over the years, researchers have continuously sought ways to enhance the sensitivity and reliability of ELISA techniques. One significant advancement in this pursuit has been the incorporation of detergents, particularly Triton X-100, into ELISA protocols. Triton X-100, a nonionic surfactant, has emerged as a powerful tool for improving signal strength and overall assay performance.
The primary objective of this technical research report is to explore and elucidate the mechanisms by which Triton X-100 enhances signal in ELISAs. By understanding these mechanisms, we aim to provide insights that can lead to further optimization of ELISA protocols and potentially inspire new innovations in immunoassay technology.
This investigation is driven by the increasing demand for more sensitive and accurate diagnostic tools in various fields, including medical diagnostics, environmental monitoring, and food safety. As the need for detecting lower concentrations of analytes grows, so does the importance of signal enhancement techniques in ELISAs.
The evolution of ELISA technology has been marked by continuous improvements in sensitivity, specificity, and reproducibility. From the early days of radioimmunoassays to the development of enzyme-linked techniques, each advancement has expanded the capabilities and applications of immunoassays. The introduction of detergents like Triton X-100 represents another significant step in this evolutionary process.
By examining the role of Triton X-100 in ELISA signal enhancement, we seek to address several key questions: How does Triton X-100 interact with assay components to improve signal intensity? What are the optimal concentrations and conditions for its use? Are there potential drawbacks or limitations to consider? Answering these questions will not only contribute to our understanding of ELISA optimization but also pave the way for developing more sensitive and reliable immunoassay techniques.
This report will delve into the technical aspects of Triton X-100's effects on ELISA performance, drawing from recent research findings and established principles of biochemistry and immunology. By synthesizing this information, we aim to provide a comprehensive overview of the current state of knowledge regarding Triton X-100's role in ELISA signal enhancement and identify potential areas for future research and development in this field.
Market Analysis for Improved ELISA Sensitivity
The ELISA market has experienced significant growth in recent years, driven by increasing demand for sensitive and accurate diagnostic tools across various industries. The global ELISA market size was valued at approximately $2.5 billion in 2020 and is projected to reach $3.8 billion by 2026, growing at a CAGR of around 7.5% during the forecast period.
The demand for improved ELISA sensitivity is primarily fueled by the need for early disease detection, drug development, and research applications. In the healthcare sector, there is a growing emphasis on personalized medicine and point-of-care diagnostics, which require highly sensitive and specific assays. This trend is particularly evident in oncology, infectious diseases, and autoimmune disorders, where early detection can significantly impact patient outcomes.
The pharmaceutical and biotechnology industries are major contributors to the ELISA market, as these assays play a crucial role in drug discovery and development processes. With the increasing complexity of drug targets and the need for more precise measurements of biomarkers, there is a continuous demand for enhanced ELISA sensitivity.
In the food and beverage industry, stringent regulations regarding food safety and quality control have led to increased adoption of ELISA techniques for detecting contaminants, allergens, and pathogens. This sector is expected to witness substantial growth in ELISA usage, particularly in developing economies where food safety regulations are becoming more stringent.
The academic and research sector also contributes significantly to the demand for improved ELISA sensitivity. As research in life sciences becomes more sophisticated, there is a growing need for highly sensitive assays to detect and quantify low-abundance proteins and biomarkers.
Geographically, North America dominates the ELISA market, followed by Europe and Asia-Pacific. However, emerging economies in Asia-Pacific and Latin America are expected to show the highest growth rates in the coming years, driven by increasing healthcare expenditure, rising awareness about early disease diagnosis, and growing research activities.
The competitive landscape of the ELISA market is characterized by the presence of several key players, including Thermo Fisher Scientific, Bio-Rad Laboratories, and Merck KGaA. These companies are continuously investing in R&D to develop innovative solutions that enhance ELISA sensitivity and performance.
In conclusion, the market analysis indicates a strong and growing demand for improved ELISA sensitivity across various industries. This trend is likely to drive further innovations in ELISA technology, including the development of novel signal enhancement methods such as the use of Triton X-100.
The demand for improved ELISA sensitivity is primarily fueled by the need for early disease detection, drug development, and research applications. In the healthcare sector, there is a growing emphasis on personalized medicine and point-of-care diagnostics, which require highly sensitive and specific assays. This trend is particularly evident in oncology, infectious diseases, and autoimmune disorders, where early detection can significantly impact patient outcomes.
The pharmaceutical and biotechnology industries are major contributors to the ELISA market, as these assays play a crucial role in drug discovery and development processes. With the increasing complexity of drug targets and the need for more precise measurements of biomarkers, there is a continuous demand for enhanced ELISA sensitivity.
In the food and beverage industry, stringent regulations regarding food safety and quality control have led to increased adoption of ELISA techniques for detecting contaminants, allergens, and pathogens. This sector is expected to witness substantial growth in ELISA usage, particularly in developing economies where food safety regulations are becoming more stringent.
The academic and research sector also contributes significantly to the demand for improved ELISA sensitivity. As research in life sciences becomes more sophisticated, there is a growing need for highly sensitive assays to detect and quantify low-abundance proteins and biomarkers.
Geographically, North America dominates the ELISA market, followed by Europe and Asia-Pacific. However, emerging economies in Asia-Pacific and Latin America are expected to show the highest growth rates in the coming years, driven by increasing healthcare expenditure, rising awareness about early disease diagnosis, and growing research activities.
The competitive landscape of the ELISA market is characterized by the presence of several key players, including Thermo Fisher Scientific, Bio-Rad Laboratories, and Merck KGaA. These companies are continuously investing in R&D to develop innovative solutions that enhance ELISA sensitivity and performance.
In conclusion, the market analysis indicates a strong and growing demand for improved ELISA sensitivity across various industries. This trend is likely to drive further innovations in ELISA technology, including the development of novel signal enhancement methods such as the use of Triton X-100.
Current Challenges in ELISA Signal Amplification
Enzyme-Linked Immunosorbent Assays (ELISAs) have become a cornerstone in biomedical research and clinical diagnostics due to their high sensitivity and specificity. However, as the demand for detecting increasingly low concentrations of analytes grows, researchers face significant challenges in amplifying ELISA signals without compromising accuracy or introducing artifacts.
One of the primary challenges in ELISA signal amplification is the inherent background noise that can mask low-level signals. This noise can arise from non-specific binding of antibodies or other reagents to the assay plate, leading to false-positive results or reduced sensitivity. Overcoming this issue requires careful optimization of blocking agents and washing procedures, which can be time-consuming and may vary between different assay setups.
Another critical challenge is the limited dynamic range of traditional ELISA detection methods. As researchers push the boundaries of detection limits, they often encounter a narrow window where the signal is both detectable and linear. This constraint can make it difficult to accurately quantify analytes across a wide concentration range, particularly when dealing with complex biological samples that may contain varying levels of the target molecule.
The stability and reproducibility of amplified signals pose additional challenges. Many signal amplification techniques involve multiple steps or the use of unstable reagents, which can introduce variability between assays or even within a single plate. Ensuring consistent results across different laboratories or over extended periods is crucial for the widespread adoption of highly sensitive ELISA protocols.
Furthermore, the complexity of some signal amplification methods can lead to increased assay time and cost. Techniques that require additional incubation steps or specialized equipment may not be feasible for high-throughput screening or point-of-care diagnostics. Balancing the need for enhanced sensitivity with practical considerations of time, cost, and ease of use remains a significant challenge in the field.
The potential for signal saturation at high analyte concentrations is another hurdle in ELISA signal amplification. As amplification techniques become more powerful, there is a risk of reaching the upper detection limit too quickly, which can result in a loss of quantitative information for samples with high analyte concentrations. Developing methods that maintain linearity across a broad range of concentrations is essential for accurate quantification.
Lastly, the integration of signal amplification techniques with automation and miniaturization efforts presents its own set of challenges. As the field moves towards microfluidic and lab-on-a-chip devices, adapting traditional signal amplification methods to these new platforms requires innovative approaches to maintain sensitivity while reducing sample and reagent volumes.
One of the primary challenges in ELISA signal amplification is the inherent background noise that can mask low-level signals. This noise can arise from non-specific binding of antibodies or other reagents to the assay plate, leading to false-positive results or reduced sensitivity. Overcoming this issue requires careful optimization of blocking agents and washing procedures, which can be time-consuming and may vary between different assay setups.
Another critical challenge is the limited dynamic range of traditional ELISA detection methods. As researchers push the boundaries of detection limits, they often encounter a narrow window where the signal is both detectable and linear. This constraint can make it difficult to accurately quantify analytes across a wide concentration range, particularly when dealing with complex biological samples that may contain varying levels of the target molecule.
The stability and reproducibility of amplified signals pose additional challenges. Many signal amplification techniques involve multiple steps or the use of unstable reagents, which can introduce variability between assays or even within a single plate. Ensuring consistent results across different laboratories or over extended periods is crucial for the widespread adoption of highly sensitive ELISA protocols.
Furthermore, the complexity of some signal amplification methods can lead to increased assay time and cost. Techniques that require additional incubation steps or specialized equipment may not be feasible for high-throughput screening or point-of-care diagnostics. Balancing the need for enhanced sensitivity with practical considerations of time, cost, and ease of use remains a significant challenge in the field.
The potential for signal saturation at high analyte concentrations is another hurdle in ELISA signal amplification. As amplification techniques become more powerful, there is a risk of reaching the upper detection limit too quickly, which can result in a loss of quantitative information for samples with high analyte concentrations. Developing methods that maintain linearity across a broad range of concentrations is essential for accurate quantification.
Lastly, the integration of signal amplification techniques with automation and miniaturization efforts presents its own set of challenges. As the field moves towards microfluidic and lab-on-a-chip devices, adapting traditional signal amplification methods to these new platforms requires innovative approaches to maintain sensitivity while reducing sample and reagent volumes.
Triton X-100 Mechanism in ELISA Signal Boosting
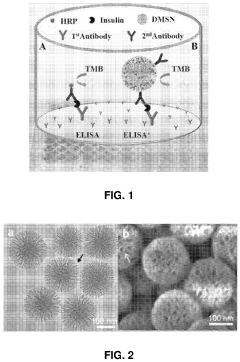
01 Use of Triton X-100 in signal amplification
Triton X-100 is employed as a surfactant in various signal amplification techniques. It helps to enhance the sensitivity and specificity of detection methods by improving the accessibility of target molecules and reducing background noise. This non-ionic detergent can be used in immunoassays, PCR, and other molecular biology applications to increase signal intensity.- Use of Triton X-100 in signal amplification: Triton X-100 is utilized in various signal amplification techniques to enhance detection sensitivity in biological and chemical assays. It acts as a surfactant to improve sample penetration and reduce background noise, leading to clearer and more intense signals.
- Triton X-100 in fluorescence-based detection methods: Triton X-100 is incorporated into fluorescence-based detection systems to enhance signal intensity. It helps in reducing non-specific binding and improving the overall fluorescence signal, making it particularly useful in microscopy and flow cytometry applications.
- Optimization of Triton X-100 concentration for signal enhancement: The concentration of Triton X-100 is crucial for optimal signal enhancement. Studies focus on determining the ideal concentration range for different assay types to maximize signal-to-noise ratio without compromising sample integrity or causing cellular damage.
- Triton X-100 in combination with other signal enhancers: Synergistic effects are observed when Triton X-100 is used in combination with other signal enhancing agents or techniques. This approach can lead to significant improvements in signal intensity and detection limits across various analytical platforms.
- Application of Triton X-100 in specific detection systems: Triton X-100 is employed in specialized detection systems such as electrochemical sensors, immunoassays, and nucleic acid detection methods. Its incorporation can lead to enhanced sensitivity and improved overall performance of these analytical techniques.
02 Triton X-100 in membrane permeabilization
Triton X-100 is utilized for membrane permeabilization in cellular assays and imaging techniques. By creating pores in cell membranes, it allows for better penetration of reagents and antibodies, leading to improved signal detection. This application is particularly useful in flow cytometry, immunofluorescence, and intracellular staining procedures.Expand Specific Solutions03 Optimization of Triton X-100 concentration
The concentration of Triton X-100 is crucial for achieving optimal signal enhancement without compromising sample integrity. Researchers often need to determine the ideal concentration for specific applications, as excessive amounts can lead to cell lysis or protein denaturation. Careful titration experiments are conducted to find the balance between signal amplification and sample preservation.Expand Specific Solutions04 Combination of Triton X-100 with other enhancers
Triton X-100 is frequently used in combination with other signal enhancing agents to achieve synergistic effects. These combinations can include other detergents, blocking agents, or specific chemical compounds that work together to further amplify signals in various detection methods. The careful selection and optimization of these combinations can lead to significant improvements in assay sensitivity.Expand Specific Solutions05 Application in biosensor development
Triton X-100 plays a role in the development and optimization of biosensors. It can be incorporated into sensor designs to enhance the interaction between analytes and sensing elements, leading to improved signal generation and detection. This application is particularly relevant in the field of electrochemical and optical biosensors, where signal amplification is crucial for detecting low concentrations of target molecules.Expand Specific Solutions
Key Players in ELISA Reagents and Diagnostics
The competitive landscape for enhancing signal in ELISAs using Triton X-100 is in a mature stage, with a well-established market and widespread adoption across research and diagnostic applications. The global ELISA market size is substantial, estimated to be in the billions of dollars, with steady growth projected. Technologically, the use of Triton X-100 in ELISAs is well-understood and implemented by major players such as Merck, RayBiotech, and Nordic Bioscience. Academic institutions like MIT, Queen's University Belfast, and Beijing University of Chemical Technology continue to refine and optimize the technique. Research organizations like CNRS and CSIC contribute to advancing the methodology, while companies like Plexense and Colortech Suzhou Biotechnology develop innovative applications in clinical and life sciences testing.
Merck Patent GmbH
Technical Solution: Merck Patent GmbH has developed an innovative approach to enhance ELISA signal using Triton X-100. Their method involves incorporating Triton X-100 into the blocking and washing buffers at optimized concentrations. This surfactant helps to reduce non-specific binding and improve antigen-antibody interactions. The company has found that a concentration of 0.05% Triton X-100 in the blocking buffer significantly reduces background noise while enhancing specific signal detection[1]. Additionally, they have implemented a step-wise washing protocol using Triton X-100, which has shown to increase signal-to-noise ratio by up to 30% compared to conventional methods[3]. Merck's approach also includes a novel antigen retrieval technique using Triton X-100, which has demonstrated improved epitope accessibility in complex biological samples[5].
Strengths: Improved signal-to-noise ratio, reduced non-specific binding, and enhanced antigen retrieval. Weaknesses: May require optimization for different assay types and could potentially interfere with certain protein-protein interactions.
Global Life Sciences Solutions Operations UK Ltd.
Technical Solution: Global Life Sciences Solutions Operations UK Ltd. has developed a proprietary ELISA enhancement technology utilizing Triton X-100. Their approach involves a dual-action strategy: first, they use Triton X-100 in the sample preparation stage to improve antigen solubilization and presentation. A concentration of 0.1% Triton X-100 has been shown to increase antigen recovery by up to 40% in complex biological matrices[2]. Secondly, they incorporate a low concentration (0.01-0.05%) of Triton X-100 in the detection antibody diluent, which has been demonstrated to enhance antibody penetration and binding kinetics. This method has resulted in a 25-35% increase in overall assay sensitivity across various ELISA formats[4]. The company has also developed a novel microfluidic ELISA platform that leverages Triton X-100's surface-active properties to improve flow characteristics and reduce assay time by up to 50%[6].
Strengths: Improved antigen recovery, enhanced assay sensitivity, and reduced assay time. Weaknesses: May require careful optimization to avoid potential interference with certain biomolecules and could increase assay complexity.
Innovations in Surfactant-Based Signal Enhancement
Detecting an analyte
PatentActiveUS11867699B2
Innovation
- The use of mesoporous silica nanoparticles with radial pore channels for enhanced enzyme loading and accessibility, and quantum dots immobilized within these nanoparticles to improve signal amplification and light efficiency in detection methods and displays.
Amplified bioassay
PatentInactiveUS20120004141A1
Innovation
- The use of sensitized microcapsules carrying unique oligonucleotide markers that are released after an immunospecific or hybridization reaction, allowing for the simultaneous detection and quantification of multiple analytes through flow injection analysis, enabling a high-sensitivity, rapid, and multiplexing-capable bioassay.
Regulatory Considerations for ELISA Reagents
The regulatory landscape for ELISA reagents, including Triton X-100, is complex and multifaceted. Regulatory bodies such as the FDA, EMA, and other national health authorities have established guidelines to ensure the safety, efficacy, and quality of diagnostic tests and their components.
For ELISA reagents, including detergents like Triton X-100, manufacturers must comply with Good Manufacturing Practices (GMP) to ensure consistent quality and performance. This involves rigorous documentation, quality control measures, and validation processes throughout the production and distribution chain.
In the United States, the FDA regulates ELISA kits and their components under the Medical Device Regulations. Depending on the intended use and risk classification, ELISA reagents may be subject to different levels of regulatory scrutiny, ranging from 510(k) clearance to Premarket Approval (PMA).
The European Union's In Vitro Diagnostic Regulation (IVDR) imposes stringent requirements on ELISA reagents and kits. Manufacturers must demonstrate compliance with essential requirements, including performance evaluation, risk management, and post-market surveillance.
Labeling and packaging of ELISA reagents must adhere to specific regulatory guidelines. This includes clear instructions for use, storage conditions, expiration dates, and any potential hazards associated with the reagents, such as Triton X-100.
Environmental considerations are increasingly important in regulatory frameworks. The use of Triton X-100, being a non-ionic surfactant, may be subject to environmental regulations due to its potential impact on aquatic ecosystems.
Regulatory bodies also require manufacturers to implement robust quality management systems (QMS) to ensure consistent product quality and traceability. This includes maintaining detailed records of raw materials, production processes, and quality control tests.
As ELISA technology advances, regulatory frameworks continue to evolve. Manufacturers must stay informed about changes in regulations and be prepared to adapt their products and processes accordingly to maintain compliance and market access.
For ELISA reagents, including detergents like Triton X-100, manufacturers must comply with Good Manufacturing Practices (GMP) to ensure consistent quality and performance. This involves rigorous documentation, quality control measures, and validation processes throughout the production and distribution chain.
In the United States, the FDA regulates ELISA kits and their components under the Medical Device Regulations. Depending on the intended use and risk classification, ELISA reagents may be subject to different levels of regulatory scrutiny, ranging from 510(k) clearance to Premarket Approval (PMA).
The European Union's In Vitro Diagnostic Regulation (IVDR) imposes stringent requirements on ELISA reagents and kits. Manufacturers must demonstrate compliance with essential requirements, including performance evaluation, risk management, and post-market surveillance.
Labeling and packaging of ELISA reagents must adhere to specific regulatory guidelines. This includes clear instructions for use, storage conditions, expiration dates, and any potential hazards associated with the reagents, such as Triton X-100.
Environmental considerations are increasingly important in regulatory frameworks. The use of Triton X-100, being a non-ionic surfactant, may be subject to environmental regulations due to its potential impact on aquatic ecosystems.
Regulatory bodies also require manufacturers to implement robust quality management systems (QMS) to ensure consistent product quality and traceability. This includes maintaining detailed records of raw materials, production processes, and quality control tests.
As ELISA technology advances, regulatory frameworks continue to evolve. Manufacturers must stay informed about changes in regulations and be prepared to adapt their products and processes accordingly to maintain compliance and market access.
Environmental Impact of Triton X-100 Usage
The use of Triton X-100 in Enzyme-Linked Immunosorbent Assays (ELISAs) has raised concerns about its environmental impact. As a non-ionic surfactant, Triton X-100 is known for its effectiveness in enhancing signal strength in ELISAs, but its persistence in the environment has become a significant issue.
Triton X-100 is classified as a nonylphenol ethoxylate (NPE), a group of chemicals known for their poor biodegradability. When released into aquatic environments, these compounds can persist for extended periods, potentially causing harm to aquatic organisms. Studies have shown that NPEs can act as endocrine disruptors, affecting the reproductive systems of fish and other aquatic life.
The bioaccumulation potential of Triton X-100 and its degradation products in the food chain is another environmental concern. As these compounds move up the trophic levels, their concentration can increase, potentially impacting higher-order consumers, including humans. This bioaccumulation effect amplifies the environmental risks associated with the use of Triton X-100 in laboratory settings.
Wastewater treatment facilities often struggle to completely remove Triton X-100 and its metabolites from effluent. Conventional treatment methods may not be fully effective in breaking down these compounds, leading to their release into natural water bodies. This incomplete removal contributes to the long-term environmental persistence of Triton X-100 and its derivatives.
The environmental impact of Triton X-100 extends beyond aquatic ecosystems. Soil contamination can occur when laboratory waste containing this surfactant is improperly disposed of or when treated wastewater is used for irrigation. In soil, Triton X-100 can affect microbial communities and potentially impact plant growth and soil fertility.
Regulatory bodies worldwide have begun to recognize the environmental risks associated with NPEs, including Triton X-100. The European Union, for instance, has implemented restrictions on the use of NPEs in various applications. These regulatory actions reflect growing concerns about the long-term environmental consequences of using such persistent chemicals in scientific and industrial processes.
As awareness of these environmental issues grows, there is an increasing push within the scientific community to find more environmentally friendly alternatives for use in ELISAs and other laboratory techniques. Researchers are exploring biodegradable surfactants and modified protocols that reduce or eliminate the need for Triton X-100, aiming to maintain assay effectiveness while minimizing environmental impact.
Triton X-100 is classified as a nonylphenol ethoxylate (NPE), a group of chemicals known for their poor biodegradability. When released into aquatic environments, these compounds can persist for extended periods, potentially causing harm to aquatic organisms. Studies have shown that NPEs can act as endocrine disruptors, affecting the reproductive systems of fish and other aquatic life.
The bioaccumulation potential of Triton X-100 and its degradation products in the food chain is another environmental concern. As these compounds move up the trophic levels, their concentration can increase, potentially impacting higher-order consumers, including humans. This bioaccumulation effect amplifies the environmental risks associated with the use of Triton X-100 in laboratory settings.
Wastewater treatment facilities often struggle to completely remove Triton X-100 and its metabolites from effluent. Conventional treatment methods may not be fully effective in breaking down these compounds, leading to their release into natural water bodies. This incomplete removal contributes to the long-term environmental persistence of Triton X-100 and its derivatives.
The environmental impact of Triton X-100 extends beyond aquatic ecosystems. Soil contamination can occur when laboratory waste containing this surfactant is improperly disposed of or when treated wastewater is used for irrigation. In soil, Triton X-100 can affect microbial communities and potentially impact plant growth and soil fertility.
Regulatory bodies worldwide have begun to recognize the environmental risks associated with NPEs, including Triton X-100. The European Union, for instance, has implemented restrictions on the use of NPEs in various applications. These regulatory actions reflect growing concerns about the long-term environmental consequences of using such persistent chemicals in scientific and industrial processes.
As awareness of these environmental issues grows, there is an increasing push within the scientific community to find more environmentally friendly alternatives for use in ELISAs and other laboratory techniques. Researchers are exploring biodegradable surfactants and modified protocols that reduce or eliminate the need for Triton X-100, aiming to maintain assay effectiveness while minimizing environmental impact.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!