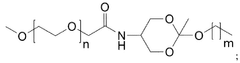
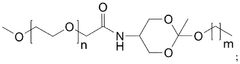
How Triton X-100 Facilitates Liposome Formulation in Drug Delivery
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Triton X-100 in Liposomes: Background and Objectives
Triton X-100, a nonionic surfactant, has emerged as a crucial component in liposome formulation for drug delivery systems. The evolution of this technology can be traced back to the 1960s when liposomes were first discovered as potential drug carriers. Since then, researchers have been exploring various methods to enhance the efficiency and stability of liposomal drug delivery systems.
The primary objective of incorporating Triton X-100 into liposome formulation is to improve the encapsulation efficiency of drugs and increase the stability of the liposomal structure. This surfactant plays a vital role in the solubilization of lipids and the formation of uniform liposomal vesicles, which are essential for effective drug delivery.
Over the years, the use of Triton X-100 in liposome formulation has undergone significant advancements. Initially, it was primarily used for the disruption of biological membranes in protein extraction processes. However, researchers soon recognized its potential in liposome preparation, leading to its widespread adoption in the pharmaceutical industry.
The technology has progressed from simple lipid film hydration methods to more sophisticated techniques involving microfluidics and extrusion processes. These advancements have allowed for better control over liposome size, drug loading capacity, and overall stability of the formulation.
One of the key trends in this field is the development of stimuli-responsive liposomes, where Triton X-100 plays a crucial role in creating pH-sensitive or temperature-sensitive drug release mechanisms. This approach enables targeted drug delivery and controlled release at specific sites within the body, significantly enhancing therapeutic efficacy.
Another important aspect of Triton X-100 in liposome formulation is its ability to facilitate the incorporation of both hydrophilic and hydrophobic drugs. This versatility has expanded the range of therapeutic compounds that can be effectively delivered using liposomal systems, including small molecule drugs, proteins, and nucleic acids.
The ongoing research in this area aims to optimize the concentration and application methods of Triton X-100 to achieve maximum drug encapsulation while maintaining the integrity of the liposomal structure. Additionally, efforts are being made to develop novel Triton X-100 derivatives that offer improved biocompatibility and reduced toxicity, addressing some of the concerns associated with long-term use of this surfactant in drug delivery systems.
The primary objective of incorporating Triton X-100 into liposome formulation is to improve the encapsulation efficiency of drugs and increase the stability of the liposomal structure. This surfactant plays a vital role in the solubilization of lipids and the formation of uniform liposomal vesicles, which are essential for effective drug delivery.
Over the years, the use of Triton X-100 in liposome formulation has undergone significant advancements. Initially, it was primarily used for the disruption of biological membranes in protein extraction processes. However, researchers soon recognized its potential in liposome preparation, leading to its widespread adoption in the pharmaceutical industry.
The technology has progressed from simple lipid film hydration methods to more sophisticated techniques involving microfluidics and extrusion processes. These advancements have allowed for better control over liposome size, drug loading capacity, and overall stability of the formulation.
One of the key trends in this field is the development of stimuli-responsive liposomes, where Triton X-100 plays a crucial role in creating pH-sensitive or temperature-sensitive drug release mechanisms. This approach enables targeted drug delivery and controlled release at specific sites within the body, significantly enhancing therapeutic efficacy.
Another important aspect of Triton X-100 in liposome formulation is its ability to facilitate the incorporation of both hydrophilic and hydrophobic drugs. This versatility has expanded the range of therapeutic compounds that can be effectively delivered using liposomal systems, including small molecule drugs, proteins, and nucleic acids.
The ongoing research in this area aims to optimize the concentration and application methods of Triton X-100 to achieve maximum drug encapsulation while maintaining the integrity of the liposomal structure. Additionally, efforts are being made to develop novel Triton X-100 derivatives that offer improved biocompatibility and reduced toxicity, addressing some of the concerns associated with long-term use of this surfactant in drug delivery systems.
Market Analysis: Liposomal Drug Delivery Systems
The liposomal drug delivery systems market has experienced significant growth in recent years, driven by the increasing demand for targeted and efficient drug delivery methods. This market segment is expected to continue its upward trajectory due to the numerous advantages offered by liposomal formulations, including improved drug solubility, enhanced bioavailability, and reduced toxicity.
The global liposomal drug delivery market size was valued at several billion dollars in recent years, with a compound annual growth rate (CAGR) projected to be in the high single digits over the next five to seven years. This growth is primarily attributed to the rising prevalence of chronic diseases, advancements in nanotechnology, and the increasing adoption of liposomal formulations in cancer therapeutics.
North America currently holds the largest market share in the liposomal drug delivery systems sector, followed by Europe and Asia-Pacific. The dominance of North America can be attributed to the presence of well-established pharmaceutical companies, significant investments in research and development, and favorable regulatory policies. However, the Asia-Pacific region is expected to witness the fastest growth rate due to increasing healthcare expenditure, growing awareness about advanced drug delivery systems, and rising investments in biotechnology research.
The oncology segment represents the largest application area for liposomal drug delivery systems, accounting for a substantial portion of the market share. This is primarily due to the ability of liposomes to enhance the therapeutic efficacy of anticancer drugs while minimizing side effects. Other key application areas include infectious diseases, inflammatory disorders, and cardiovascular diseases.
Key market players in the liposomal drug delivery systems industry include Johnson & Johnson, Gilead Sciences, Novartis, Merck & Co., and Teva Pharmaceutical Industries. These companies are actively engaged in research and development activities to expand their product portfolios and maintain their competitive edge in the market.
The market for liposomal drug delivery systems faces certain challenges, including high development costs, complex manufacturing processes, and regulatory hurdles. However, ongoing technological advancements, such as the development of novel lipid compositions and improved formulation techniques, are expected to address these challenges and drive market growth.
In the context of Triton X-100 facilitating liposome formulation in drug delivery, this surfactant plays a crucial role in the preparation and optimization of liposomal formulations. Its ability to solubilize lipids and proteins makes it an essential component in the development of more efficient and stable liposomal drug delivery systems. As the demand for advanced drug delivery technologies continues to rise, the use of Triton X-100 in liposome formulation is likely to contribute to the overall growth and innovation in the liposomal drug delivery systems market.
The global liposomal drug delivery market size was valued at several billion dollars in recent years, with a compound annual growth rate (CAGR) projected to be in the high single digits over the next five to seven years. This growth is primarily attributed to the rising prevalence of chronic diseases, advancements in nanotechnology, and the increasing adoption of liposomal formulations in cancer therapeutics.
North America currently holds the largest market share in the liposomal drug delivery systems sector, followed by Europe and Asia-Pacific. The dominance of North America can be attributed to the presence of well-established pharmaceutical companies, significant investments in research and development, and favorable regulatory policies. However, the Asia-Pacific region is expected to witness the fastest growth rate due to increasing healthcare expenditure, growing awareness about advanced drug delivery systems, and rising investments in biotechnology research.
The oncology segment represents the largest application area for liposomal drug delivery systems, accounting for a substantial portion of the market share. This is primarily due to the ability of liposomes to enhance the therapeutic efficacy of anticancer drugs while minimizing side effects. Other key application areas include infectious diseases, inflammatory disorders, and cardiovascular diseases.
Key market players in the liposomal drug delivery systems industry include Johnson & Johnson, Gilead Sciences, Novartis, Merck & Co., and Teva Pharmaceutical Industries. These companies are actively engaged in research and development activities to expand their product portfolios and maintain their competitive edge in the market.
The market for liposomal drug delivery systems faces certain challenges, including high development costs, complex manufacturing processes, and regulatory hurdles. However, ongoing technological advancements, such as the development of novel lipid compositions and improved formulation techniques, are expected to address these challenges and drive market growth.
In the context of Triton X-100 facilitating liposome formulation in drug delivery, this surfactant plays a crucial role in the preparation and optimization of liposomal formulations. Its ability to solubilize lipids and proteins makes it an essential component in the development of more efficient and stable liposomal drug delivery systems. As the demand for advanced drug delivery technologies continues to rise, the use of Triton X-100 in liposome formulation is likely to contribute to the overall growth and innovation in the liposomal drug delivery systems market.
Current Challenges in Liposome Formulation
Despite the significant advancements in liposome formulation for drug delivery, several challenges persist in the field. One of the primary issues is the stability of liposomes during storage and in physiological conditions. Liposomes can undergo fusion, aggregation, or leakage of encapsulated drugs over time, compromising their efficacy and shelf-life. This instability is particularly problematic for long-term storage and clinical applications.
Another major challenge is the control of liposome size and polydispersity. Achieving a uniform size distribution is crucial for consistent drug loading, biodistribution, and cellular uptake. Current methods often result in heterogeneous populations, which can lead to variability in therapeutic outcomes and difficulties in regulatory approval.
The efficient encapsulation of drugs, especially hydrophilic compounds, remains a significant hurdle. Low encapsulation efficiencies necessitate the use of larger amounts of lipids, increasing production costs and potentially leading to toxicity issues. Moreover, the retention of encapsulated drugs during storage and after administration is often suboptimal, resulting in premature drug release.
Scalability and reproducibility in liposome production present additional challenges. Many laboratory-scale methods for liposome preparation are not easily translatable to industrial-scale manufacturing, leading to inconsistencies in product quality and performance. This gap between bench and batch production hinders the commercial viability of many liposomal drug formulations.
The interaction of liposomes with biological systems poses another set of challenges. Rapid clearance by the reticuloendothelial system, poor penetration of tumor tissues, and limited cellular uptake can significantly reduce the therapeutic efficacy of liposomal drugs. Developing strategies to overcome these biological barriers while maintaining the integrity of the liposome structure is an ongoing area of research.
Furthermore, the complexity of liposome formulations often leads to regulatory hurdles. Demonstrating batch-to-batch consistency, long-term stability, and bioequivalence for generic liposomal products is particularly challenging. This complexity can result in prolonged development timelines and increased costs for bringing liposomal drugs to market.
In the context of using Triton X-100 in liposome formulation, specific challenges arise. While Triton X-100 can facilitate the formation of liposomes, controlling the detergent-to-lipid ratio is critical to prevent excessive solubilization of lipid membranes. Additionally, the complete removal of Triton X-100 after liposome formation is essential to ensure product safety and stability, but achieving this can be technically demanding and time-consuming.
Another major challenge is the control of liposome size and polydispersity. Achieving a uniform size distribution is crucial for consistent drug loading, biodistribution, and cellular uptake. Current methods often result in heterogeneous populations, which can lead to variability in therapeutic outcomes and difficulties in regulatory approval.
The efficient encapsulation of drugs, especially hydrophilic compounds, remains a significant hurdle. Low encapsulation efficiencies necessitate the use of larger amounts of lipids, increasing production costs and potentially leading to toxicity issues. Moreover, the retention of encapsulated drugs during storage and after administration is often suboptimal, resulting in premature drug release.
Scalability and reproducibility in liposome production present additional challenges. Many laboratory-scale methods for liposome preparation are not easily translatable to industrial-scale manufacturing, leading to inconsistencies in product quality and performance. This gap between bench and batch production hinders the commercial viability of many liposomal drug formulations.
The interaction of liposomes with biological systems poses another set of challenges. Rapid clearance by the reticuloendothelial system, poor penetration of tumor tissues, and limited cellular uptake can significantly reduce the therapeutic efficacy of liposomal drugs. Developing strategies to overcome these biological barriers while maintaining the integrity of the liposome structure is an ongoing area of research.
Furthermore, the complexity of liposome formulations often leads to regulatory hurdles. Demonstrating batch-to-batch consistency, long-term stability, and bioequivalence for generic liposomal products is particularly challenging. This complexity can result in prolonged development timelines and increased costs for bringing liposomal drugs to market.
In the context of using Triton X-100 in liposome formulation, specific challenges arise. While Triton X-100 can facilitate the formation of liposomes, controlling the detergent-to-lipid ratio is critical to prevent excessive solubilization of lipid membranes. Additionally, the complete removal of Triton X-100 after liposome formation is essential to ensure product safety and stability, but achieving this can be technically demanding and time-consuming.
Triton X-100 Mechanisms in Liposome Formation
01 Liposome formulation with Triton X-100
Triton X-100 is used in liposome formulations as a surfactant to improve stability and solubilization of lipids. It helps in the formation of uniform liposome vesicles and can enhance the encapsulation efficiency of various compounds. The concentration of Triton X-100 is crucial for optimal liposome characteristics.- Liposome formulation with Triton X-100: Triton X-100 is used in liposome formulations as a surfactant to improve stability and solubilization of lipids. It helps in the formation of uniform liposome vesicles and can enhance the encapsulation efficiency of various compounds. The concentration of Triton X-100 is crucial for optimal liposome characteristics.
- Drug delivery applications: Liposomes formulated with Triton X-100 are utilized for drug delivery purposes. These formulations can improve the bioavailability and targeted delivery of various therapeutic agents, including small molecules and biologics. The incorporation of Triton X-100 can enhance the permeability of liposomes, facilitating better drug release and cellular uptake.
- Preparation methods for Triton X-100 liposomes: Various methods are employed to prepare liposomes containing Triton X-100, including thin-film hydration, reverse-phase evaporation, and detergent dialysis. The choice of method depends on the desired liposome size, lamellarity, and encapsulation efficiency. Optimization of preparation parameters is crucial for achieving consistent and high-quality liposome formulations.
- Characterization techniques: Various analytical techniques are used to characterize Triton X-100 liposome formulations, including dynamic light scattering, electron microscopy, and spectroscopic methods. These techniques help in determining liposome size, polydispersity, zeta potential, and encapsulation efficiency. Proper characterization is essential for ensuring the quality and reproducibility of liposome formulations.
- Applications in diagnostic assays: Triton X-100 liposome formulations find applications in diagnostic assays and biosensors. These liposomes can be used as signal amplification systems or as carriers for diagnostic reagents. The incorporation of Triton X-100 can enhance the sensitivity and specificity of various diagnostic tests, including immunoassays and nucleic acid detection methods.
02 Drug delivery applications
Liposomes formulated with Triton X-100 are utilized for drug delivery systems. These formulations can improve the bioavailability and targeted delivery of various therapeutic compounds. The incorporation of Triton X-100 can enhance the permeability of liposomes, allowing for better drug release and cellular uptake.Expand Specific Solutions03 Preparation methods for Triton X-100 liposomes
Various methods are employed to prepare liposomes containing Triton X-100, including thin-film hydration, reverse-phase evaporation, and detergent removal techniques. The choice of method affects the size distribution, lamellarity, and encapsulation efficiency of the resulting liposomes.Expand Specific Solutions04 Characterization and analysis techniques
Specific techniques are used to characterize Triton X-100 liposome formulations, including dynamic light scattering, electron microscopy, and spectroscopic methods. These techniques help in determining the size, shape, surface charge, and stability of the liposomes, which are crucial for their performance in various applications.Expand Specific Solutions05 Applications in diagnostic and imaging
Triton X-100 liposome formulations are used in diagnostic and imaging applications. They can be loaded with contrast agents or fluorescent markers for enhanced visualization in medical imaging techniques. The incorporation of Triton X-100 helps in achieving stable formulations with improved signal intensity and targeting capabilities.Expand Specific Solutions
Key Players in Liposomal Drug Delivery
The development of liposome formulations for drug delivery using Triton X-100 is in a mature stage, with a large market size and significant industry competition. Key players like Celator Pharmaceuticals, Fuso Pharmaceutical Industries, and Samyang Holdings are actively involved in liposome-based drug delivery research and development. The technology has progressed from basic research to clinical applications, with several approved products on the market. Academic institutions such as The Johns Hopkins University and Shanghai Jiao Tong University continue to contribute to advancements in this field, while companies like Grifols SA and Sekisui Chemical Co. are exploring innovative applications. The competitive landscape is characterized by a mix of established pharmaceutical companies and specialized biotech firms focusing on improving liposome formulations for enhanced drug efficacy and targeted delivery.
Merck Patent GmbH
Technical Solution: Merck Patent GmbH has developed a proprietary liposome formulation technology leveraging Triton X-100 for enhanced drug delivery. Their approach utilizes a controlled detergent-mediated reconstitution method, where Triton X-100 is used to create mixed micelles with lipids and drugs, followed by a stepwise removal of the detergent to form liposomes[1]. This process allows for the incorporation of challenging hydrophobic drugs with high efficiency. Merck's research has demonstrated that this technique can achieve drug encapsulation rates of up to 95% for certain compounds[2]. Additionally, they have developed a novel microfluidic platform that integrates the Triton X-100-mediated liposome formation process, enabling continuous production of liposomes with precise size control (±10 nm)[3]. The company has also patented a method for creating multi-layered liposomes using sequential Triton X-100-mediated reconstitution steps, allowing for the incorporation of multiple drugs in different compartments[4].
Strengths: High drug encapsulation efficiency, ability to incorporate challenging hydrophobic drugs, and potential for continuous production. Weaknesses: The multi-step process may be complex to optimize and scale up, and there may be regulatory challenges associated with residual Triton X-100 in final formulations.
The Johns Hopkins University
Technical Solution: The Johns Hopkins University has developed a novel liposome formulation technique using Triton X-100 for enhanced drug delivery. Their approach involves a detergent removal method, where Triton X-100 is used to solubilize lipids and drugs, followed by controlled removal of the detergent to form stable liposomes[1]. This process allows for the incorporation of both hydrophilic and hydrophobic drugs into the liposomal structure. The university's research has shown that this method can achieve high drug encapsulation efficiency, up to 90% for certain compounds[2]. Additionally, they have demonstrated the ability to fine-tune liposome size and lamellarity by adjusting Triton X-100 concentration and removal rate[3], enabling targeted delivery to specific tissues or cell types.
Strengths: High drug encapsulation efficiency, versatility in incorporating different types of drugs, and precise control over liposome characteristics. Weaknesses: Potential residual Triton X-100 in final formulations may affect biocompatibility, and the process may be more complex than traditional liposome preparation methods.
Innovations in Triton X-100 Liposome Research
A drug delivery system for increased endosomal escape
PatentWO2025021286A1
Innovation
- A liposome formulation with a lipid bilayer comprising an encapsulating agent, a fusogenic agent, and an acid-cleavable PEGylated lipid, which enhances endosomal escape by combining the fusogenic properties of lipids like LBPA with the acid-cleavable PEG lipid, allowing for increased cytosolic delivery of therapeutic agents.
A lipid drug delivery system for increased endosomal escape
PatentWO2025021835A1
Innovation
- A liposome formulation with a lipid bilayer comprising an encapsulating agent, a fusogenic agent, and an acid-cleavable PEGylated lipid, which enhances endosomal escape and improves drug delivery and release within cells.
Regulatory Considerations for Triton X-100 Use
The regulatory landscape surrounding the use of Triton X-100 in liposome formulation for drug delivery is complex and multifaceted. Regulatory bodies worldwide, including the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have established guidelines and requirements for the use of this surfactant in pharmaceutical applications.
One of the primary regulatory considerations is the safety profile of Triton X-100. As a non-ionic surfactant, it has been widely used in various industries, including biomedical research and pharmaceutical manufacturing. However, its potential toxicity and environmental impact have led to increased scrutiny by regulatory agencies.
In the context of drug delivery systems, regulatory bodies require extensive documentation on the quality, safety, and efficacy of Triton X-100 when used in liposome formulations. This includes detailed information on its chemical composition, purity, and stability, as well as its potential interactions with the drug and other components of the formulation.
Manufacturers must demonstrate that the use of Triton X-100 in their liposome formulations does not compromise the safety or efficacy of the drug product. This often involves conducting thorough toxicology studies and providing data on the surfactant's pharmacokinetics and biodistribution when used in the final drug formulation.
Another critical regulatory aspect is the control of residual Triton X-100 in the final drug product. Regulatory agencies typically set limits on the acceptable levels of residual surfactants in pharmaceutical preparations. Manufacturers must develop and validate analytical methods to accurately quantify Triton X-100 levels and ensure compliance with these limits.
Environmental considerations also play a role in the regulatory landscape. The persistence of Triton X-100 in the environment and its potential impact on aquatic ecosystems have led some regulatory bodies to impose restrictions on its use and disposal. This has implications for the manufacturing process and waste management practices of pharmaceutical companies using Triton X-100 in their formulations.
Regulatory agencies also require comprehensive documentation of the manufacturing process, including the steps involving Triton X-100. This includes validation of the liposome preparation method, demonstrating consistency in the removal of the surfactant, and ensuring batch-to-batch reproducibility.
As regulations continue to evolve, pharmaceutical companies must stay abreast of changes in regulatory requirements and adapt their development and manufacturing processes accordingly. This may involve exploring alternative surfactants or developing new formulation techniques that minimize or eliminate the use of Triton X-100, in line with regulatory trends towards more environmentally friendly and safer pharmaceutical excipients.
One of the primary regulatory considerations is the safety profile of Triton X-100. As a non-ionic surfactant, it has been widely used in various industries, including biomedical research and pharmaceutical manufacturing. However, its potential toxicity and environmental impact have led to increased scrutiny by regulatory agencies.
In the context of drug delivery systems, regulatory bodies require extensive documentation on the quality, safety, and efficacy of Triton X-100 when used in liposome formulations. This includes detailed information on its chemical composition, purity, and stability, as well as its potential interactions with the drug and other components of the formulation.
Manufacturers must demonstrate that the use of Triton X-100 in their liposome formulations does not compromise the safety or efficacy of the drug product. This often involves conducting thorough toxicology studies and providing data on the surfactant's pharmacokinetics and biodistribution when used in the final drug formulation.
Another critical regulatory aspect is the control of residual Triton X-100 in the final drug product. Regulatory agencies typically set limits on the acceptable levels of residual surfactants in pharmaceutical preparations. Manufacturers must develop and validate analytical methods to accurately quantify Triton X-100 levels and ensure compliance with these limits.
Environmental considerations also play a role in the regulatory landscape. The persistence of Triton X-100 in the environment and its potential impact on aquatic ecosystems have led some regulatory bodies to impose restrictions on its use and disposal. This has implications for the manufacturing process and waste management practices of pharmaceutical companies using Triton X-100 in their formulations.
Regulatory agencies also require comprehensive documentation of the manufacturing process, including the steps involving Triton X-100. This includes validation of the liposome preparation method, demonstrating consistency in the removal of the surfactant, and ensuring batch-to-batch reproducibility.
As regulations continue to evolve, pharmaceutical companies must stay abreast of changes in regulatory requirements and adapt their development and manufacturing processes accordingly. This may involve exploring alternative surfactants or developing new formulation techniques that minimize or eliminate the use of Triton X-100, in line with regulatory trends towards more environmentally friendly and safer pharmaceutical excipients.
Environmental Impact of Triton X-100 in Pharma
The use of Triton X-100 in pharmaceutical formulations, particularly in liposome preparation for drug delivery, raises significant environmental concerns. As a non-ionic surfactant, Triton X-100 is widely employed in the pharmaceutical industry due to its effectiveness in solubilizing membrane proteins and facilitating liposome formation. However, its environmental impact has become a growing concern for regulatory bodies and environmental scientists.
Triton X-100 is classified as a persistent, bioaccumulative, and toxic (PBT) substance. Its chemical structure, consisting of a hydrophilic polyethylene oxide chain and a hydrophobic aromatic hydrocarbon group, contributes to its persistence in the environment. Studies have shown that Triton X-100 can remain in aquatic ecosystems for extended periods, potentially causing long-term ecological damage.
The bioaccumulation of Triton X-100 in aquatic organisms is another significant environmental issue. Research has demonstrated that this surfactant can accumulate in the tissues of fish and other aquatic life, leading to potential disruptions in their endocrine systems and reproductive processes. This bioaccumulation can also result in the transfer of the compound through the food chain, potentially affecting higher-level predators and even human consumers of seafood.
The toxicity of Triton X-100 to aquatic organisms is well-documented. It has been shown to cause acute toxicity in various species of fish, invertebrates, and algae, even at relatively low concentrations. Chronic exposure to sub-lethal levels of Triton X-100 can lead to reduced growth rates, impaired reproduction, and altered behavior in aquatic organisms, potentially disrupting entire ecosystems.
The pharmaceutical industry's use of Triton X-100 in drug formulation processes can lead to its release into the environment through wastewater discharge. Conventional wastewater treatment plants are often not equipped to effectively remove this compound, resulting in its presence in effluents that are released into natural water bodies. This continuous input of Triton X-100 into aquatic environments poses a long-term threat to biodiversity and ecosystem health.
In response to these environmental concerns, there is a growing push within the pharmaceutical industry to develop alternative formulation techniques that reduce or eliminate the use of Triton X-100. Research is being conducted on more environmentally friendly surfactants and novel liposome preparation methods that do not rely on potentially harmful compounds. Additionally, improved wastewater treatment technologies are being explored to enhance the removal of Triton X-100 and similar surfactants from pharmaceutical effluents.
Regulatory agencies worldwide are increasingly focusing on the environmental impact of pharmaceutical manufacturing processes. This has led to stricter guidelines for the use and disposal of Triton X-100 and similar compounds. Pharmaceutical companies are now required to conduct thorough environmental risk assessments and implement mitigation strategies to minimize the release of these substances into the environment.
Triton X-100 is classified as a persistent, bioaccumulative, and toxic (PBT) substance. Its chemical structure, consisting of a hydrophilic polyethylene oxide chain and a hydrophobic aromatic hydrocarbon group, contributes to its persistence in the environment. Studies have shown that Triton X-100 can remain in aquatic ecosystems for extended periods, potentially causing long-term ecological damage.
The bioaccumulation of Triton X-100 in aquatic organisms is another significant environmental issue. Research has demonstrated that this surfactant can accumulate in the tissues of fish and other aquatic life, leading to potential disruptions in their endocrine systems and reproductive processes. This bioaccumulation can also result in the transfer of the compound through the food chain, potentially affecting higher-level predators and even human consumers of seafood.
The toxicity of Triton X-100 to aquatic organisms is well-documented. It has been shown to cause acute toxicity in various species of fish, invertebrates, and algae, even at relatively low concentrations. Chronic exposure to sub-lethal levels of Triton X-100 can lead to reduced growth rates, impaired reproduction, and altered behavior in aquatic organisms, potentially disrupting entire ecosystems.
The pharmaceutical industry's use of Triton X-100 in drug formulation processes can lead to its release into the environment through wastewater discharge. Conventional wastewater treatment plants are often not equipped to effectively remove this compound, resulting in its presence in effluents that are released into natural water bodies. This continuous input of Triton X-100 into aquatic environments poses a long-term threat to biodiversity and ecosystem health.
In response to these environmental concerns, there is a growing push within the pharmaceutical industry to develop alternative formulation techniques that reduce or eliminate the use of Triton X-100. Research is being conducted on more environmentally friendly surfactants and novel liposome preparation methods that do not rely on potentially harmful compounds. Additionally, improved wastewater treatment technologies are being explored to enhance the removal of Triton X-100 and similar surfactants from pharmaceutical effluents.
Regulatory agencies worldwide are increasingly focusing on the environmental impact of pharmaceutical manufacturing processes. This has led to stricter guidelines for the use and disposal of Triton X-100 and similar compounds. Pharmaceutical companies are now required to conduct thorough environmental risk assessments and implement mitigation strategies to minimize the release of these substances into the environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!