Triton X-100-Assisted Proteomic Analysis of Monoclonal Antibodies
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Triton X-100 in mAb Analysis: Background and Objectives
Triton X-100, a nonionic surfactant, has emerged as a crucial tool in the proteomic analysis of monoclonal antibodies (mAbs). The use of this detergent in mAb analysis represents a significant advancement in the field of biopharmaceuticals, particularly in the development and quality control of therapeutic antibodies.
The background of Triton X-100 in mAb analysis dates back to the early 2000s when researchers began exploring more efficient methods for characterizing complex protein structures. Monoclonal antibodies, with their intricate molecular composition, posed significant challenges in terms of structural analysis and quality assessment. Traditional analytical techniques often fell short in providing comprehensive insights into mAb properties, leading to a pressing need for innovative approaches.
Triton X-100's unique properties, including its ability to solubilize membrane proteins without denaturing them, made it an attractive candidate for enhancing mAb analysis. Its introduction into proteomic workflows marked a turning point in the field, enabling more detailed and accurate characterization of antibody structures and functions.
The primary objective of incorporating Triton X-100 into mAb analysis is to improve the depth and accuracy of proteomic studies. This includes enhancing the detection and quantification of post-translational modifications, which are critical for the efficacy and safety of therapeutic antibodies. Additionally, Triton X-100 aids in revealing hidden epitopes and structural features that may be obscured in conventional analytical methods.
Another key goal is to streamline the analytical process, reducing the time and resources required for comprehensive mAb characterization. By facilitating more efficient protein extraction and sample preparation, Triton X-100 contributes to faster turnaround times in both research and quality control settings.
The integration of Triton X-100 into mAb analysis also aims to improve the reproducibility and reliability of proteomic data. This is particularly crucial in the biopharmaceutical industry, where consistent product quality is paramount. By providing a more robust analytical platform, Triton X-100 helps ensure that mAb-based therapeutics meet stringent regulatory standards.
Furthermore, the use of Triton X-100 in mAb analysis aligns with the broader trend towards more sophisticated and comprehensive characterization techniques in biopharmaceutical development. As the complexity of mAb-based therapies continues to increase, the need for advanced analytical tools becomes more pronounced. Triton X-100 represents a step towards meeting these evolving demands, potentially paving the way for the development of even more targeted and effective antibody therapies.
In conclusion, the background and objectives of Triton X-100 in mAb analysis reflect a significant evolution in proteomic techniques. By addressing key challenges in antibody characterization, this approach promises to enhance our understanding of mAb structures and functions, ultimately contributing to the advancement of biopharmaceutical research and development.
The background of Triton X-100 in mAb analysis dates back to the early 2000s when researchers began exploring more efficient methods for characterizing complex protein structures. Monoclonal antibodies, with their intricate molecular composition, posed significant challenges in terms of structural analysis and quality assessment. Traditional analytical techniques often fell short in providing comprehensive insights into mAb properties, leading to a pressing need for innovative approaches.
Triton X-100's unique properties, including its ability to solubilize membrane proteins without denaturing them, made it an attractive candidate for enhancing mAb analysis. Its introduction into proteomic workflows marked a turning point in the field, enabling more detailed and accurate characterization of antibody structures and functions.
The primary objective of incorporating Triton X-100 into mAb analysis is to improve the depth and accuracy of proteomic studies. This includes enhancing the detection and quantification of post-translational modifications, which are critical for the efficacy and safety of therapeutic antibodies. Additionally, Triton X-100 aids in revealing hidden epitopes and structural features that may be obscured in conventional analytical methods.
Another key goal is to streamline the analytical process, reducing the time and resources required for comprehensive mAb characterization. By facilitating more efficient protein extraction and sample preparation, Triton X-100 contributes to faster turnaround times in both research and quality control settings.
The integration of Triton X-100 into mAb analysis also aims to improve the reproducibility and reliability of proteomic data. This is particularly crucial in the biopharmaceutical industry, where consistent product quality is paramount. By providing a more robust analytical platform, Triton X-100 helps ensure that mAb-based therapeutics meet stringent regulatory standards.
Furthermore, the use of Triton X-100 in mAb analysis aligns with the broader trend towards more sophisticated and comprehensive characterization techniques in biopharmaceutical development. As the complexity of mAb-based therapies continues to increase, the need for advanced analytical tools becomes more pronounced. Triton X-100 represents a step towards meeting these evolving demands, potentially paving the way for the development of even more targeted and effective antibody therapies.
In conclusion, the background and objectives of Triton X-100 in mAb analysis reflect a significant evolution in proteomic techniques. By addressing key challenges in antibody characterization, this approach promises to enhance our understanding of mAb structures and functions, ultimately contributing to the advancement of biopharmaceutical research and development.
Market Demand for Advanced mAb Characterization
The market demand for advanced monoclonal antibody (mAb) characterization has been steadily increasing due to the growing importance of mAbs in therapeutic applications. As the biopharmaceutical industry continues to expand, there is a pressing need for more sophisticated and efficient analytical techniques to ensure the quality, safety, and efficacy of mAb-based drugs.
One of the key drivers of this demand is the complexity of mAb structures and their potential for post-translational modifications. These modifications can significantly impact the drug's efficacy and safety profile, making thorough characterization essential. Traditional analytical methods often fall short in providing comprehensive insights into these complex molecules, creating a gap in the market for more advanced techniques.
The rise of biosimilars and biobetters has further fueled the need for advanced mAb characterization. As more companies enter this space, there is an increased focus on demonstrating comparability and superiority to existing products. This requires highly sensitive and specific analytical methods that can detect even subtle differences between mAb products.
Regulatory agencies worldwide have also raised their standards for mAb characterization, driving pharmaceutical companies to invest in more advanced analytical technologies. The FDA and EMA, for instance, now require more detailed structural and functional analyses of mAbs throughout their development and manufacturing processes.
The demand for high-throughput screening methods in early-stage drug discovery has created another market opportunity for advanced mAb characterization techniques. Pharmaceutical companies are seeking ways to rapidly assess large numbers of mAb candidates, necessitating faster and more efficient analytical tools.
Personalized medicine is another trend driving the demand for advanced mAb characterization. As treatments become more tailored to individual patients, there is a growing need for techniques that can provide detailed molecular-level information about mAbs and their interactions with specific patient populations.
The emergence of novel mAb formats, such as bispecific antibodies and antibody-drug conjugates, has further complicated the characterization landscape. These complex molecules require even more sophisticated analytical approaches, creating a niche market for advanced characterization technologies.
In response to these market demands, there has been significant investment in developing new analytical techniques and improving existing ones. Mass spectrometry-based methods, in particular, have gained traction due to their ability to provide detailed structural information. The integration of artificial intelligence and machine learning into data analysis has also become a focus area, promising to enhance the speed and accuracy of mAb characterization.
One of the key drivers of this demand is the complexity of mAb structures and their potential for post-translational modifications. These modifications can significantly impact the drug's efficacy and safety profile, making thorough characterization essential. Traditional analytical methods often fall short in providing comprehensive insights into these complex molecules, creating a gap in the market for more advanced techniques.
The rise of biosimilars and biobetters has further fueled the need for advanced mAb characterization. As more companies enter this space, there is an increased focus on demonstrating comparability and superiority to existing products. This requires highly sensitive and specific analytical methods that can detect even subtle differences between mAb products.
Regulatory agencies worldwide have also raised their standards for mAb characterization, driving pharmaceutical companies to invest in more advanced analytical technologies. The FDA and EMA, for instance, now require more detailed structural and functional analyses of mAbs throughout their development and manufacturing processes.
The demand for high-throughput screening methods in early-stage drug discovery has created another market opportunity for advanced mAb characterization techniques. Pharmaceutical companies are seeking ways to rapidly assess large numbers of mAb candidates, necessitating faster and more efficient analytical tools.
Personalized medicine is another trend driving the demand for advanced mAb characterization. As treatments become more tailored to individual patients, there is a growing need for techniques that can provide detailed molecular-level information about mAbs and their interactions with specific patient populations.
The emergence of novel mAb formats, such as bispecific antibodies and antibody-drug conjugates, has further complicated the characterization landscape. These complex molecules require even more sophisticated analytical approaches, creating a niche market for advanced characterization technologies.
In response to these market demands, there has been significant investment in developing new analytical techniques and improving existing ones. Mass spectrometry-based methods, in particular, have gained traction due to their ability to provide detailed structural information. The integration of artificial intelligence and machine learning into data analysis has also become a focus area, promising to enhance the speed and accuracy of mAb characterization.
Current Challenges in Proteomic Analysis of mAbs
Proteomic analysis of monoclonal antibodies (mAbs) faces several significant challenges that hinder comprehensive characterization and quality control in biopharmaceutical development. One of the primary obstacles is the complexity of mAb structures, which consist of multiple domains and post-translational modifications. This intricate architecture makes it difficult to achieve complete protein coverage and accurate identification of all structural elements.
Sample preparation remains a critical bottleneck in mAb proteomics. Traditional methods often struggle to efficiently solubilize and digest these large, stable proteins without introducing artifacts or losing important structural information. The presence of disulfide bonds and glycosylation further complicates the digestion process, leading to incomplete peptide recovery and biased results.
Mass spectrometry (MS) analysis of mAbs presents its own set of challenges. The dynamic range of peptide abundances can span several orders of magnitude, making it challenging to detect low-abundance species or modifications. Additionally, the similarity between different mAb sequences, especially in the constant regions, can lead to ambiguities in peptide assignment and protein identification.
Quantification of mAbs and their variants is another area of concern. Label-free quantification methods may suffer from inconsistencies due to variations in ionization efficiency and matrix effects. Labeled approaches, while more accurate, can be costly and time-consuming, limiting their applicability in high-throughput settings.
Data analysis and interpretation pose significant challenges due to the vast amount of information generated in proteomic experiments. Distinguishing between true biological variants and artifacts introduced during sample preparation or analysis requires sophisticated bioinformatics tools and expert knowledge. Moreover, the lack of standardized data analysis pipelines hampers reproducibility and comparability between different laboratories and studies.
The characterization of post-translational modifications (PTMs) on mAbs, such as glycosylation, deamidation, and oxidation, remains particularly challenging. These modifications can significantly impact mAb efficacy and safety, yet their comprehensive analysis requires specialized techniques and often multiple complementary approaches.
Emerging challenges include the analysis of novel mAb formats, such as bispecific antibodies and antibody-drug conjugates, which add layers of complexity to proteomic workflows. Additionally, the increasing demand for higher sensitivity and faster turnaround times in quality control processes puts pressure on analytical methods to evolve rapidly.
Addressing these challenges requires innovative approaches that combine advances in sample preparation, MS instrumentation, and data analysis. The integration of Triton X-100 in proteomic workflows shows promise in improving mAb solubilization and digestion efficiency, potentially overcoming some of the current limitations in sample preparation and protein coverage.
Sample preparation remains a critical bottleneck in mAb proteomics. Traditional methods often struggle to efficiently solubilize and digest these large, stable proteins without introducing artifacts or losing important structural information. The presence of disulfide bonds and glycosylation further complicates the digestion process, leading to incomplete peptide recovery and biased results.
Mass spectrometry (MS) analysis of mAbs presents its own set of challenges. The dynamic range of peptide abundances can span several orders of magnitude, making it challenging to detect low-abundance species or modifications. Additionally, the similarity between different mAb sequences, especially in the constant regions, can lead to ambiguities in peptide assignment and protein identification.
Quantification of mAbs and their variants is another area of concern. Label-free quantification methods may suffer from inconsistencies due to variations in ionization efficiency and matrix effects. Labeled approaches, while more accurate, can be costly and time-consuming, limiting their applicability in high-throughput settings.
Data analysis and interpretation pose significant challenges due to the vast amount of information generated in proteomic experiments. Distinguishing between true biological variants and artifacts introduced during sample preparation or analysis requires sophisticated bioinformatics tools and expert knowledge. Moreover, the lack of standardized data analysis pipelines hampers reproducibility and comparability between different laboratories and studies.
The characterization of post-translational modifications (PTMs) on mAbs, such as glycosylation, deamidation, and oxidation, remains particularly challenging. These modifications can significantly impact mAb efficacy and safety, yet their comprehensive analysis requires specialized techniques and often multiple complementary approaches.
Emerging challenges include the analysis of novel mAb formats, such as bispecific antibodies and antibody-drug conjugates, which add layers of complexity to proteomic workflows. Additionally, the increasing demand for higher sensitivity and faster turnaround times in quality control processes puts pressure on analytical methods to evolve rapidly.
Addressing these challenges requires innovative approaches that combine advances in sample preparation, MS instrumentation, and data analysis. The integration of Triton X-100 in proteomic workflows shows promise in improving mAb solubilization and digestion efficiency, potentially overcoming some of the current limitations in sample preparation and protein coverage.
Triton X-100 Protocols for mAb Proteomic Analysis
01 Proteomic analysis techniques for monoclonal antibodies
Various proteomic analysis techniques are employed to study monoclonal antibodies, including mass spectrometry, chromatography, and electrophoresis. These methods allow for the identification, characterization, and quantification of antibody proteins, their modifications, and interactions with other molecules.- Proteomic analysis techniques for monoclonal antibodies: Various proteomic analysis techniques are employed to study monoclonal antibodies, including mass spectrometry, chromatography, and electrophoresis. These methods allow for the identification, characterization, and quantification of antibody proteins and their modifications, providing valuable insights into their structure and function.
- Production and purification of monoclonal antibodies for analysis: Methods for producing and purifying monoclonal antibodies are crucial for subsequent proteomic analysis. This includes cell culture techniques, hybridoma technology, and various purification steps such as affinity chromatography and filtration to obtain high-quality antibody samples suitable for detailed proteomic studies.
- Structural characterization of monoclonal antibodies: Proteomic analysis is used to determine the structural characteristics of monoclonal antibodies, including amino acid sequences, post-translational modifications, and higher-order structures. This information is crucial for understanding antibody function and optimizing their therapeutic potential.
- Functional analysis of monoclonal antibodies using proteomics: Proteomic approaches are applied to study the functional aspects of monoclonal antibodies, such as antigen binding, effector functions, and interactions with other proteins. This includes techniques like protein-protein interaction studies and epitope mapping to elucidate the mechanisms of antibody action.
- Application of proteomic analysis in monoclonal antibody development: Proteomic analysis plays a crucial role in the development and optimization of monoclonal antibodies for therapeutic and diagnostic applications. It is used for quality control, stability studies, and comparability assessments of different antibody batches or biosimilars, ensuring consistency and efficacy of antibody-based products.
02 Production and purification of monoclonal antibodies for analysis
Methods for producing and purifying monoclonal antibodies suitable for proteomic analysis are described. These include cell culture techniques, hybridoma technology, and various purification steps to obtain high-quality antibody samples for subsequent analysis.Expand Specific Solutions03 Structural characterization of monoclonal antibodies
Techniques for determining the structural characteristics of monoclonal antibodies, including primary sequence analysis, post-translational modifications, and higher-order structure determination. These methods provide insights into antibody function and stability.Expand Specific Solutions04 Functional analysis of monoclonal antibodies using proteomics
Proteomic approaches for studying the functional properties of monoclonal antibodies, including binding affinity, specificity, and effector functions. These methods help in understanding the mechanisms of action and optimizing antibody therapeutics.Expand Specific Solutions05 Application of proteomic analysis in monoclonal antibody development
The use of proteomic analysis techniques in various stages of monoclonal antibody development, including discovery, optimization, and quality control. These methods contribute to the design and production of more effective and safer antibody-based therapeutics.Expand Specific Solutions
Key Players in Antibody Analytics and Proteomics
The competitive landscape for "Triton X-100-Assisted Proteomic Analysis of Monoclonal Antibodies" is in a growth phase, with increasing market size and technological advancements. This field intersects pharmaceutical research, proteomics, and antibody development, attracting both established pharmaceutical companies and specialized biotech firms. Key players like F. Hoffmann-La Roche, AstraZeneca, and Bristol Myers Squibb are investing heavily in this area, leveraging their extensive R&D capabilities. Emerging companies such as LC-Bio Technologies and Novacellum are also making significant contributions, focusing on innovative approaches to proteomic analysis. The technology's maturity is advancing rapidly, with companies like Bio-Rad Laboratories providing essential tools and services, indicating a shift towards more sophisticated and efficient analytical methods in antibody research and development.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed an advanced Triton X-100-assisted proteomic analysis method for monoclonal antibodies. Their approach utilizes a combination of high-resolution mass spectrometry and Triton X-100 detergent-based sample preparation to enhance protein solubilization and digestion efficiency. This method allows for comprehensive characterization of monoclonal antibodies, including post-translational modifications and sequence variants. Roche's technique incorporates a multi-dimensional liquid chromatography separation coupled with tandem mass spectrometry (LC-MS/MS) for improved peptide identification and quantification[1][3]. The company has also implemented advanced data analysis algorithms to interpret the complex proteomic data, enabling rapid and accurate antibody profiling.
Strengths: High sensitivity and specificity in antibody characterization, comprehensive analysis of post-translational modifications, and improved throughput. Weaknesses: Potential interference from Triton X-100 in mass spectrometry, requiring careful optimization of detergent removal steps.
AstraZeneca AB
Technical Solution: AstraZeneca has developed a comprehensive Triton X-100-assisted proteomic analysis platform for monoclonal antibodies, focusing on high-throughput characterization for biotherapeutic development. Their method employs a optimized Triton X-100-based extraction protocol combined with automated sample preparation systems. The platform integrates nano-flow liquid chromatography with high-resolution Orbitrap mass spectrometry for deep proteomic analysis. AstraZeneca's approach includes innovative peptide mapping techniques that allow for detailed characterization of post-translational modifications and sequence variants[7]. The company has also implemented machine learning algorithms for data analysis, enabling rapid identification of critical quality attributes in antibody candidates[8]. This platform has been successfully applied in AstraZeneca's antibody discovery and development pipeline, significantly reducing the time required for antibody characterization.
Strengths: High-throughput capability, advanced data analysis using machine learning, comprehensive characterization of post-translational modifications. Weaknesses: Potential high cost of implementation, may require specialized expertise for data interpretation.
Innovations in Detergent-Based mAb Characterization
Method for evaluating fibrinolysis resistance activity in blood plasma
PatentWO2023074864A1
Innovation
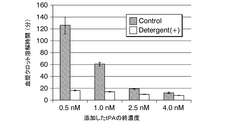
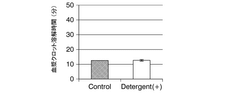
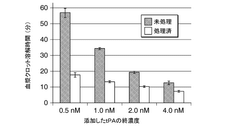
- A method involving the addition of tPA to SDS-treated plasma in the presence of Triton X-100, measuring PA-dependent plasma clot lysis time and plasmin production rate using fluorescent substrates to evaluate fibrinolytic resistance activity, including pretreatment with anionic surfactants and subsequent measurements to assess index values.
Method for measuring protein kinase activity and kit for same
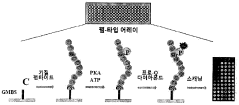
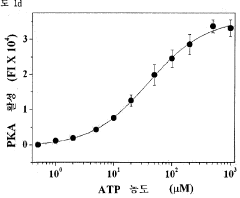
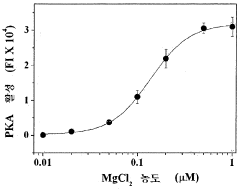
PatentWO2015133680A1
Innovation
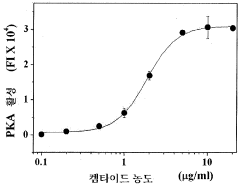
- A method involving the attachment of GMBS to a substrate, followed by reaction with protein kinase, and the use of a buffer containing Triton X-100 to measure kinase activity using a phosphorylation probe, which enhances sensitivity and ease of operation.
Regulatory Considerations for mAb Analytical Methods
Regulatory considerations play a crucial role in the development and implementation of analytical methods for monoclonal antibodies (mAbs), including those involving Triton X-100-assisted proteomic analysis. The regulatory landscape for mAb analytical methods is complex and evolving, with various agencies and guidelines influencing the process.
The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are the primary regulatory bodies overseeing mAb analytical methods. These agencies have established guidelines and requirements for method validation, data integrity, and quality control. The International Conference on Harmonisation (ICH) also provides harmonized guidelines that are widely adopted across regions.
For Triton X-100-assisted proteomic analysis of mAbs, regulatory considerations focus on several key aspects. Method validation is a critical component, requiring demonstration of specificity, accuracy, precision, linearity, and robustness. Regulatory agencies expect comprehensive validation data to support the use of novel analytical techniques in mAb characterization.
Data integrity is another crucial regulatory consideration. Agencies require that all data generated during method development, validation, and routine use be traceable, complete, and secure. This includes implementing appropriate electronic data management systems and maintaining detailed records of all experimental procedures and results.
Regulatory bodies also emphasize the importance of quality control measures in mAb analytical methods. This includes the use of appropriate reference standards, system suitability tests, and ongoing method performance monitoring. For Triton X-100-assisted proteomic analysis, specific considerations may include the purity and consistency of the Triton X-100 reagent used and its potential impact on mAb structure and function.
The use of Triton X-100 in mAb analysis may require additional regulatory scrutiny due to its potential effects on protein structure and the possibility of introducing artifacts. Regulatory agencies may request extensive data demonstrating that the use of Triton X-100 does not compromise the integrity of the mAb or interfere with the accuracy of the analytical results.
As analytical technologies continue to advance, regulatory agencies are adapting their guidelines to accommodate new methodologies. This includes the increasing use of high-resolution mass spectrometry and other advanced proteomic techniques in mAb characterization. Regulatory bodies are encouraging the adoption of these technologies while ensuring that appropriate validation and quality control measures are in place.
The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are the primary regulatory bodies overseeing mAb analytical methods. These agencies have established guidelines and requirements for method validation, data integrity, and quality control. The International Conference on Harmonisation (ICH) also provides harmonized guidelines that are widely adopted across regions.
For Triton X-100-assisted proteomic analysis of mAbs, regulatory considerations focus on several key aspects. Method validation is a critical component, requiring demonstration of specificity, accuracy, precision, linearity, and robustness. Regulatory agencies expect comprehensive validation data to support the use of novel analytical techniques in mAb characterization.
Data integrity is another crucial regulatory consideration. Agencies require that all data generated during method development, validation, and routine use be traceable, complete, and secure. This includes implementing appropriate electronic data management systems and maintaining detailed records of all experimental procedures and results.
Regulatory bodies also emphasize the importance of quality control measures in mAb analytical methods. This includes the use of appropriate reference standards, system suitability tests, and ongoing method performance monitoring. For Triton X-100-assisted proteomic analysis, specific considerations may include the purity and consistency of the Triton X-100 reagent used and its potential impact on mAb structure and function.
The use of Triton X-100 in mAb analysis may require additional regulatory scrutiny due to its potential effects on protein structure and the possibility of introducing artifacts. Regulatory agencies may request extensive data demonstrating that the use of Triton X-100 does not compromise the integrity of the mAb or interfere with the accuracy of the analytical results.
As analytical technologies continue to advance, regulatory agencies are adapting their guidelines to accommodate new methodologies. This includes the increasing use of high-resolution mass spectrometry and other advanced proteomic techniques in mAb characterization. Regulatory bodies are encouraging the adoption of these technologies while ensuring that appropriate validation and quality control measures are in place.
Environmental Impact of Triton X-100 Usage
The use of Triton X-100 in proteomic analysis of monoclonal antibodies raises significant environmental concerns due to its potential impact on aquatic ecosystems. Triton X-100, a nonionic surfactant, is known for its persistence in the environment and its ability to bioaccumulate in organisms. When released into water systems, it can disrupt the natural balance of aquatic life, particularly affecting the membranes of fish and other aquatic organisms.
The biodegradation of Triton X-100 in the environment is relatively slow, with studies showing that it can persist for extended periods in water and sediments. This prolonged presence increases the likelihood of exposure to various aquatic species, potentially leading to long-term ecological effects. The compound's surfactant properties can also alter the surface tension of water, affecting the behavior and survival of water-dwelling insects and microorganisms.
Furthermore, the breakdown products of Triton X-100, particularly nonylphenol, have been identified as endocrine disruptors. These substances can interfere with the hormonal systems of wildlife, potentially affecting reproduction and development in exposed populations. This is particularly concerning in aquatic environments where the concentration of these compounds can accumulate over time.
The use of Triton X-100 in laboratory settings also raises concerns about its disposal. Improper handling and disposal of laboratory waste containing this surfactant can lead to its introduction into wastewater systems. Conventional wastewater treatment processes may not completely remove Triton X-100, resulting in its release into natural water bodies.
To mitigate these environmental risks, researchers and laboratories are exploring alternative surfactants with lower environmental impacts. Additionally, improved waste management protocols and more efficient wastewater treatment technologies are being developed to reduce the release of Triton X-100 and similar compounds into the environment.
Regulatory bodies in various countries have begun to recognize the potential environmental hazards of Triton X-100 and related substances. Some regions have implemented restrictions on its use and disposal, encouraging the development of more environmentally friendly alternatives for scientific and industrial applications.
The biodegradation of Triton X-100 in the environment is relatively slow, with studies showing that it can persist for extended periods in water and sediments. This prolonged presence increases the likelihood of exposure to various aquatic species, potentially leading to long-term ecological effects. The compound's surfactant properties can also alter the surface tension of water, affecting the behavior and survival of water-dwelling insects and microorganisms.
Furthermore, the breakdown products of Triton X-100, particularly nonylphenol, have been identified as endocrine disruptors. These substances can interfere with the hormonal systems of wildlife, potentially affecting reproduction and development in exposed populations. This is particularly concerning in aquatic environments where the concentration of these compounds can accumulate over time.
The use of Triton X-100 in laboratory settings also raises concerns about its disposal. Improper handling and disposal of laboratory waste containing this surfactant can lead to its introduction into wastewater systems. Conventional wastewater treatment processes may not completely remove Triton X-100, resulting in its release into natural water bodies.
To mitigate these environmental risks, researchers and laboratories are exploring alternative surfactants with lower environmental impacts. Additionally, improved waste management protocols and more efficient wastewater treatment technologies are being developed to reduce the release of Triton X-100 and similar compounds into the environment.
Regulatory bodies in various countries have begun to recognize the potential environmental hazards of Triton X-100 and related substances. Some regions have implemented restrictions on its use and disposal, encouraging the development of more environmentally friendly alternatives for scientific and industrial applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!