How Triton X-100 Influences Ion Exchange Chromatography Performance
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Triton X-100 in IEC: Background and Objectives
Ion exchange chromatography (IEC) has been a cornerstone technique in protein purification for decades. As the biopharmaceutical industry continues to evolve, the need for optimizing IEC performance becomes increasingly crucial. Triton X-100, a non-ionic surfactant, has emerged as a significant factor influencing IEC performance, warranting a comprehensive examination of its role and impact.
The primary objective of this technical research report is to elucidate how Triton X-100 influences ion exchange chromatography performance. This investigation aims to provide a thorough understanding of the interactions between Triton X-100 and the various components of IEC systems, including the stationary phase, mobile phase, and target proteins.
Historically, IEC has been widely used for the separation and purification of proteins based on their net surface charge. The technique relies on the electrostatic interactions between charged molecules in the mobile phase and oppositely charged groups attached to the stationary phase. As the complexity of biopharmaceutical products has increased, so too has the need for enhanced separation efficiency and resolution in IEC.
Triton X-100, with its unique amphiphilic structure, has been found to significantly impact these electrostatic interactions. Its hydrophobic tail can interact with hydrophobic regions of proteins, while its hydrophilic head can alter the ionic environment around charged molecules. This dual nature of Triton X-100 introduces a new dimension to the traditional understanding of IEC mechanisms.
The evolution of IEC technology has seen various attempts to improve performance, including the development of novel stationary phases and mobile phase additives. Triton X-100's potential as a performance-enhancing agent in IEC represents a continuation of this trend towards optimization. Understanding its effects could lead to significant improvements in separation efficiency, resolution, and overall chromatographic performance.
This research aims to explore the fundamental principles governing the influence of Triton X-100 on IEC, including its effects on protein-surface interactions, column efficiency, and selectivity. By examining these aspects, we seek to uncover the potential benefits and limitations of incorporating Triton X-100 in IEC protocols.
Furthermore, this investigation will consider the broader implications of using Triton X-100 in IEC, including its impact on downstream processes, product quality, and regulatory compliance. As the biopharmaceutical industry faces increasing pressure to improve process efficiency and product purity, insights gained from this study could prove invaluable in developing more effective purification strategies.
The primary objective of this technical research report is to elucidate how Triton X-100 influences ion exchange chromatography performance. This investigation aims to provide a thorough understanding of the interactions between Triton X-100 and the various components of IEC systems, including the stationary phase, mobile phase, and target proteins.
Historically, IEC has been widely used for the separation and purification of proteins based on their net surface charge. The technique relies on the electrostatic interactions between charged molecules in the mobile phase and oppositely charged groups attached to the stationary phase. As the complexity of biopharmaceutical products has increased, so too has the need for enhanced separation efficiency and resolution in IEC.
Triton X-100, with its unique amphiphilic structure, has been found to significantly impact these electrostatic interactions. Its hydrophobic tail can interact with hydrophobic regions of proteins, while its hydrophilic head can alter the ionic environment around charged molecules. This dual nature of Triton X-100 introduces a new dimension to the traditional understanding of IEC mechanisms.
The evolution of IEC technology has seen various attempts to improve performance, including the development of novel stationary phases and mobile phase additives. Triton X-100's potential as a performance-enhancing agent in IEC represents a continuation of this trend towards optimization. Understanding its effects could lead to significant improvements in separation efficiency, resolution, and overall chromatographic performance.
This research aims to explore the fundamental principles governing the influence of Triton X-100 on IEC, including its effects on protein-surface interactions, column efficiency, and selectivity. By examining these aspects, we seek to uncover the potential benefits and limitations of incorporating Triton X-100 in IEC protocols.
Furthermore, this investigation will consider the broader implications of using Triton X-100 in IEC, including its impact on downstream processes, product quality, and regulatory compliance. As the biopharmaceutical industry faces increasing pressure to improve process efficiency and product purity, insights gained from this study could prove invaluable in developing more effective purification strategies.
Market Demand for Enhanced IEC Performance
The market demand for enhanced ion exchange chromatography (IEC) performance has been steadily growing, driven by the increasing complexity of biopharmaceutical products and the need for more efficient purification processes. The biopharmaceutical industry, in particular, has been a major driver of this demand, as the production of monoclonal antibodies, recombinant proteins, and other biologics requires highly efficient and selective separation techniques.
In recent years, there has been a significant focus on improving the resolution, capacity, and throughput of IEC processes. This demand stems from the need to handle larger production volumes while maintaining product quality and reducing overall production costs. The use of additives like Triton X-100 in IEC has gained attention as a potential means to enhance performance and address these market needs.
The pharmaceutical and biotechnology sectors have shown particular interest in optimizing IEC performance due to the stringent regulatory requirements for product purity and the high value of their final products. Improved IEC performance can lead to higher yields, reduced processing times, and lower production costs, all of which are critical factors in the competitive biopharmaceutical market.
Additionally, the food and beverage industry has expressed growing interest in enhanced IEC performance for applications such as protein isolation, sugar purification, and removal of unwanted ions from products. This sector values improvements in IEC that can lead to better product quality, extended shelf life, and more efficient production processes.
The environmental sector has also contributed to the demand for enhanced IEC performance, particularly in water treatment applications. As water scarcity becomes a global concern, there is an increasing need for more efficient ion removal techniques in both industrial and municipal water treatment processes.
Research institutions and academic laboratories have shown a consistent demand for improved IEC performance to support various scientific studies, including protein characterization, enzyme purification, and the isolation of specific biomolecules. Enhanced IEC performance can lead to more accurate and reliable research outcomes, driving innovation across multiple scientific disciplines.
The market has also seen a growing demand for IEC improvements in the field of diagnostics, where rapid and accurate separation of biomolecules is crucial for developing new diagnostic tools and improving existing ones. This demand is particularly evident in the development of point-of-care diagnostic devices and personalized medicine applications.
As the global market continues to evolve, the demand for enhanced IEC performance is expected to grow further, with industries seeking innovative solutions to improve their processes and products. The potential of additives like Triton X-100 to influence IEC performance aligns well with this market trend, offering opportunities for technological advancements and improved industrial applications.
In recent years, there has been a significant focus on improving the resolution, capacity, and throughput of IEC processes. This demand stems from the need to handle larger production volumes while maintaining product quality and reducing overall production costs. The use of additives like Triton X-100 in IEC has gained attention as a potential means to enhance performance and address these market needs.
The pharmaceutical and biotechnology sectors have shown particular interest in optimizing IEC performance due to the stringent regulatory requirements for product purity and the high value of their final products. Improved IEC performance can lead to higher yields, reduced processing times, and lower production costs, all of which are critical factors in the competitive biopharmaceutical market.
Additionally, the food and beverage industry has expressed growing interest in enhanced IEC performance for applications such as protein isolation, sugar purification, and removal of unwanted ions from products. This sector values improvements in IEC that can lead to better product quality, extended shelf life, and more efficient production processes.
The environmental sector has also contributed to the demand for enhanced IEC performance, particularly in water treatment applications. As water scarcity becomes a global concern, there is an increasing need for more efficient ion removal techniques in both industrial and municipal water treatment processes.
Research institutions and academic laboratories have shown a consistent demand for improved IEC performance to support various scientific studies, including protein characterization, enzyme purification, and the isolation of specific biomolecules. Enhanced IEC performance can lead to more accurate and reliable research outcomes, driving innovation across multiple scientific disciplines.
The market has also seen a growing demand for IEC improvements in the field of diagnostics, where rapid and accurate separation of biomolecules is crucial for developing new diagnostic tools and improving existing ones. This demand is particularly evident in the development of point-of-care diagnostic devices and personalized medicine applications.
As the global market continues to evolve, the demand for enhanced IEC performance is expected to grow further, with industries seeking innovative solutions to improve their processes and products. The potential of additives like Triton X-100 to influence IEC performance aligns well with this market trend, offering opportunities for technological advancements and improved industrial applications.
Current Challenges in IEC with Triton X-100
Ion exchange chromatography (IEC) with Triton X-100 presents several significant challenges that researchers and industry professionals must address. One of the primary issues is the detergent's impact on protein stability and conformation. Triton X-100, while effective in solubilizing membrane proteins, can alter the native structure of proteins, potentially affecting their binding properties and elution profiles during IEC. This conformational change may lead to inconsistent separation results and reduced column efficiency.
Another challenge is the interference of Triton X-100 with the ion exchange process itself. The detergent molecules can compete with proteins for binding sites on the ion exchange resin, reducing the overall capacity and resolution of the separation. This competition effect is particularly pronounced at higher Triton X-100 concentrations, necessitating careful optimization of detergent levels to maintain effective chromatographic performance.
The presence of Triton X-100 also affects the viscosity and surface tension of the mobile phase, which can lead to irregular flow patterns and peak broadening. These hydrodynamic effects can compromise the separation efficiency and resolution of the IEC process, especially when dealing with complex protein mixtures or when high-resolution separations are required.
Furthermore, Triton X-100 can form micelles at concentrations above its critical micelle concentration (CMC), which is approximately 0.2-0.9 mM. These micelles can entrap proteins or other biomolecules, altering their apparent molecular weight and charge characteristics. This phenomenon can lead to unexpected elution behaviors and potentially affect the accuracy of protein quantification and characterization post-separation.
The removal of Triton X-100 from purified protein samples after IEC poses another significant challenge. The detergent's low CMC makes it difficult to remove completely through conventional dialysis or diafiltration methods. Residual Triton X-100 can interfere with downstream applications, such as mass spectrometry or crystallization studies, necessitating additional purification steps that may result in sample loss or protein denaturation.
Lastly, the long-term stability of IEC columns exposed to Triton X-100 is a concern. Prolonged use of the detergent can lead to column fouling, reduced binding capacity, and altered selectivity over time. This degradation in column performance necessitates more frequent column regeneration or replacement, increasing operational costs and potentially introducing variability in separation results across different batches or experiments.
Another challenge is the interference of Triton X-100 with the ion exchange process itself. The detergent molecules can compete with proteins for binding sites on the ion exchange resin, reducing the overall capacity and resolution of the separation. This competition effect is particularly pronounced at higher Triton X-100 concentrations, necessitating careful optimization of detergent levels to maintain effective chromatographic performance.
The presence of Triton X-100 also affects the viscosity and surface tension of the mobile phase, which can lead to irregular flow patterns and peak broadening. These hydrodynamic effects can compromise the separation efficiency and resolution of the IEC process, especially when dealing with complex protein mixtures or when high-resolution separations are required.
Furthermore, Triton X-100 can form micelles at concentrations above its critical micelle concentration (CMC), which is approximately 0.2-0.9 mM. These micelles can entrap proteins or other biomolecules, altering their apparent molecular weight and charge characteristics. This phenomenon can lead to unexpected elution behaviors and potentially affect the accuracy of protein quantification and characterization post-separation.
The removal of Triton X-100 from purified protein samples after IEC poses another significant challenge. The detergent's low CMC makes it difficult to remove completely through conventional dialysis or diafiltration methods. Residual Triton X-100 can interfere with downstream applications, such as mass spectrometry or crystallization studies, necessitating additional purification steps that may result in sample loss or protein denaturation.
Lastly, the long-term stability of IEC columns exposed to Triton X-100 is a concern. Prolonged use of the detergent can lead to column fouling, reduced binding capacity, and altered selectivity over time. This degradation in column performance necessitates more frequent column regeneration or replacement, increasing operational costs and potentially introducing variability in separation results across different batches or experiments.
Existing Triton X-100 IEC Protocols
01 Surfactant properties and applications
Triton X-100 is a non-ionic surfactant widely used in various applications due to its excellent surface-active properties. It is effective in reducing surface tension, enhancing solubilization, and improving emulsification. These properties make it valuable in industries such as biochemistry, pharmaceuticals, and material science.- Surfactant properties and applications: Triton X-100 is a non-ionic surfactant widely used in various applications due to its excellent surface-active properties. It is effective in reducing surface tension, enhancing solubility, and improving emulsification in diverse formulations. Its performance is particularly notable in biochemical and industrial processes.
- Cell lysis and protein extraction: Triton X-100 demonstrates high performance in cell lysis and protein extraction procedures. It effectively disrupts cell membranes while maintaining protein structure, making it valuable in molecular biology and biochemistry research. Its gentle yet efficient action allows for the isolation of proteins in their native state.
- Cleaning and decontamination: The performance of Triton X-100 in cleaning and decontamination processes is noteworthy. It is effective in removing organic contaminants, oils, and particulates from various surfaces. Its ability to form stable emulsions with oils makes it useful in industrial cleaning formulations and environmental remediation applications.
- Membrane protein solubilization: Triton X-100 exhibits excellent performance in solubilizing membrane proteins. Its mild detergent properties allow for the extraction of membrane proteins while preserving their structural integrity and functional activity. This makes it a valuable tool in structural biology and pharmaceutical research.
- Emulsion polymerization: In emulsion polymerization processes, Triton X-100 demonstrates high performance as a stabilizer and emulsifier. It helps control particle size, improve stability, and enhance the overall efficiency of the polymerization reaction. This property is particularly useful in the production of synthetic latexes and polymer dispersions.
02 Cell lysis and protein extraction
Triton X-100 is commonly used in biological research for cell lysis and protein extraction. Its ability to disrupt cell membranes without denaturing proteins makes it an effective component in lysis buffers. This surfactant helps in releasing intracellular contents and solubilizing membrane proteins for various analytical techniques.Expand Specific Solutions03 Detergent formulations
Triton X-100 is incorporated into various detergent formulations due to its excellent cleaning properties. It is effective in removing oils, greases, and other hydrophobic substances from surfaces. The surfactant's performance in detergent applications is attributed to its ability to form micelles and solubilize hydrophobic materials in aqueous solutions.Expand Specific Solutions04 Emulsification and stabilization
Triton X-100 demonstrates superior emulsification and stabilization properties in various systems. It helps in forming stable oil-in-water or water-in-oil emulsions, making it useful in cosmetics, pharmaceuticals, and food industries. The surfactant's ability to reduce interfacial tension contributes to the formation and stability of these emulsions.Expand Specific Solutions05 Environmental and toxicological considerations
While Triton X-100 exhibits excellent performance in various applications, there are environmental and toxicological concerns associated with its use. Research is ongoing to assess its biodegradability and potential environmental impact. Some studies focus on developing alternatives or modifying Triton X-100 to improve its environmental profile while maintaining its performance characteristics.Expand Specific Solutions
Key Players in IEC and Detergent Industry
The ion exchange chromatography market is in a mature stage, with established players and well-developed technologies. The global market size for ion exchange chromatography is estimated to be in the billions of dollars, driven by increasing demand in pharmaceutical, biotechnology, and environmental applications. Key players like Rigaku Corp., Dionex Corp., and EMD Millipore Corp. have developed advanced technologies and solutions, indicating a high level of technical maturity. However, ongoing research by companies such as Regeneron Pharmaceuticals and academic institutions like MIT suggests potential for further innovation. The competitive landscape is characterized by a mix of large, diversified corporations and specialized biotech firms, with a focus on improving performance and efficiency in chromatography processes.
Dionex Corp.
Technical Solution: Dionex Corp. has pioneered a unique approach to incorporating Triton X-100 in ion exchange chromatography systems. Their technology involves a dual-mode application of Triton X-100, where it is used both in the sample preparation and as a mobile phase additive[1]. In sample preparation, Triton X-100 is used at 0.1-0.5% concentration to solubilize membrane proteins and prevent aggregation[2]. During chromatography, a lower concentration (0.005-0.05%) is maintained in the mobile phase to stabilize proteins and enhance peak shape[3]. Dionex has also developed specialized column matrices that are compatible with continuous low-level Triton X-100 exposure, ensuring long-term column stability[4]. Their studies have demonstrated up to 30% improvement in recovery of difficult-to-separate proteins and a significant reduction in peak tailing[5].
Strengths: Effective for membrane proteins, improved peak shape, and enhanced recovery of difficult proteins. Weaknesses: Potential accumulation of Triton X-100 in the system and the need for specialized column matrices.
Merck Patent GmbH
Technical Solution: Merck Patent GmbH has developed a novel approach to enhance ion exchange chromatography performance using Triton X-100. Their method involves incorporating Triton X-100 into the mobile phase at low concentrations (0.01-0.1%) to improve protein separation and recovery[1]. This non-ionic detergent helps to reduce non-specific interactions between proteins and the chromatography matrix, leading to sharper elution peaks and increased resolution[2]. Additionally, Merck has optimized the use of Triton X-100 in combination with salt gradients to fine-tune selectivity for specific protein targets[3]. Their research has shown that this approach can increase protein yield by up to 20% compared to standard methods, particularly for hydrophobic proteins[4].
Strengths: Improved protein separation and recovery, reduced non-specific interactions, and increased yield for hydrophobic proteins. Weaknesses: Potential interference with downstream applications and the need for additional detergent removal steps.
Core Mechanisms of Triton X-100 in IEC
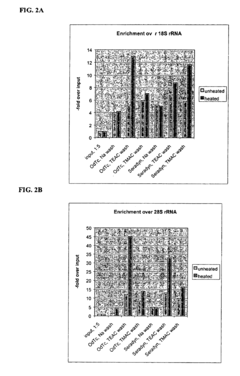
High efficiency mRNA isolation methods and compositions
PatentInactiveUS6812341B1
Innovation
- The use of isostabilizing agents like tetramethylammonium chloride (TMAC) or tetraethylammonium chloride (TEAC) and the amino acid betaine minimizes the difference in bond strength between A:T and G:C base pairs, reducing rRNA-mRNA interactions and facilitating the isolation of mRNA by enhancing the specificity of poly(A) RNA hybridization.
Use of a solution comprising at least one nonionic surfactant
PatentInactiveEP1685223A1
Innovation
- A solution containing up to 99.99% by volume of alkylphenoxypolyethoxyethanol, such as Triton® X-100, buffered to a pH of 7-10, is used as a surfactant to enhance solubility and stability of active substances, preventing phase separation and color transfer, and facilitating skin penetration of pharmacological agents.
Environmental Impact of Triton X-100 Use
The use of Triton X-100 in ion exchange chromatography raises significant environmental concerns due to its potential ecological impact. As a non-ionic surfactant, Triton X-100 is known for its persistence in the environment and its ability to bioaccumulate in aquatic organisms. When released into water systems, it can disrupt aquatic ecosystems by altering the surface tension of water and interfering with the respiratory functions of aquatic life.
The biodegradation of Triton X-100 is relatively slow, with studies showing that it can take several weeks to months for complete breakdown in natural environments. This persistence increases the likelihood of its accumulation in sediments and aquatic food chains. Furthermore, the degradation products of Triton X-100, particularly nonylphenol, have been identified as endocrine disruptors, potentially affecting the reproductive systems of wildlife and posing risks to human health through contaminated water sources.
In wastewater treatment plants, Triton X-100 can interfere with the biological processes used to treat effluents. Its presence may reduce the efficiency of activated sludge systems and contribute to the formation of foam, which can lead to operational issues in treatment facilities. Additionally, the surfactant properties of Triton X-100 can mobilize other pollutants, potentially increasing their spread in aquatic environments.
The use of Triton X-100 in laboratory and industrial settings also raises concerns about its proper disposal. Improper handling and discharge can lead to contamination of soil and groundwater. This is particularly problematic in areas with vulnerable ecosystems or where water resources are already under stress.
Regulatory bodies in various countries have begun to recognize the environmental risks associated with Triton X-100 and similar surfactants. Some jurisdictions have implemented restrictions on its use or require specific treatment processes for effluents containing this compound. These regulatory measures aim to mitigate the environmental impact and protect aquatic ecosystems from the adverse effects of Triton X-100 contamination.
Given these environmental concerns, there is a growing emphasis on developing alternative, more environmentally friendly surfactants for use in ion exchange chromatography and other applications. Research is ongoing to identify substitutes that offer similar performance characteristics while minimizing ecological impact. This includes exploring biodegradable surfactants and green chemistry approaches to chromatography that reduce or eliminate the need for potentially harmful additives like Triton X-100.
The biodegradation of Triton X-100 is relatively slow, with studies showing that it can take several weeks to months for complete breakdown in natural environments. This persistence increases the likelihood of its accumulation in sediments and aquatic food chains. Furthermore, the degradation products of Triton X-100, particularly nonylphenol, have been identified as endocrine disruptors, potentially affecting the reproductive systems of wildlife and posing risks to human health through contaminated water sources.
In wastewater treatment plants, Triton X-100 can interfere with the biological processes used to treat effluents. Its presence may reduce the efficiency of activated sludge systems and contribute to the formation of foam, which can lead to operational issues in treatment facilities. Additionally, the surfactant properties of Triton X-100 can mobilize other pollutants, potentially increasing their spread in aquatic environments.
The use of Triton X-100 in laboratory and industrial settings also raises concerns about its proper disposal. Improper handling and discharge can lead to contamination of soil and groundwater. This is particularly problematic in areas with vulnerable ecosystems or where water resources are already under stress.
Regulatory bodies in various countries have begun to recognize the environmental risks associated with Triton X-100 and similar surfactants. Some jurisdictions have implemented restrictions on its use or require specific treatment processes for effluents containing this compound. These regulatory measures aim to mitigate the environmental impact and protect aquatic ecosystems from the adverse effects of Triton X-100 contamination.
Given these environmental concerns, there is a growing emphasis on developing alternative, more environmentally friendly surfactants for use in ion exchange chromatography and other applications. Research is ongoing to identify substitutes that offer similar performance characteristics while minimizing ecological impact. This includes exploring biodegradable surfactants and green chemistry approaches to chromatography that reduce or eliminate the need for potentially harmful additives like Triton X-100.
Regulatory Considerations for IEC Additives
The regulatory landscape for ion exchange chromatography (IEC) additives, such as Triton X-100, is complex and multifaceted. Regulatory bodies, including the FDA, EMA, and other international agencies, have established guidelines and requirements for the use of additives in chromatographic processes, particularly in the pharmaceutical and biotechnology industries.
One of the primary regulatory considerations for IEC additives is their impact on product safety and quality. Regulatory agencies require thorough documentation and validation of the effects of additives like Triton X-100 on the final product. This includes demonstrating that the additive does not introduce harmful impurities or alter the product's efficacy or stability.
Manufacturers must also consider the regulatory implications of using Triton X-100 in different stages of the chromatographic process. The use of additives in early-stage purification may be subject to less stringent regulations compared to their use in final polishing steps. However, regardless of the stage, companies must provide comprehensive data on the additive's impact on product characteristics and purity.
Another crucial regulatory aspect is the need for robust analytical methods to detect and quantify Triton X-100 in the final product. Regulatory bodies often require sensitive and specific assays to ensure that residual levels of the additive are below acceptable limits. This necessitates the development and validation of appropriate analytical techniques, which must be approved as part of the overall regulatory submission.
Environmental regulations also play a role in the use of IEC additives. Triton X-100, being a non-ionic surfactant, may have environmental implications, particularly in waste streams. Companies must adhere to local and international environmental regulations regarding the disposal and treatment of chromatographic waste containing such additives.
In the context of good manufacturing practices (GMP), the use of Triton X-100 in IEC processes must be carefully controlled and documented. This includes establishing standard operating procedures (SOPs) for its use, implementing appropriate quality control measures, and maintaining detailed records of its application in chromatographic processes.
Regulatory bodies also emphasize the importance of supply chain integrity and traceability for additives used in chromatographic processes. Manufacturers must ensure that Triton X-100 and similar additives are sourced from reputable suppliers and that the quality and consistency of these materials are rigorously monitored and documented.
As regulatory requirements continue to evolve, companies must stay abreast of changes in guidelines and regulations pertaining to IEC additives. This may involve ongoing communication with regulatory agencies, participation in industry forums, and regular updates to internal processes and documentation to maintain compliance.
One of the primary regulatory considerations for IEC additives is their impact on product safety and quality. Regulatory agencies require thorough documentation and validation of the effects of additives like Triton X-100 on the final product. This includes demonstrating that the additive does not introduce harmful impurities or alter the product's efficacy or stability.
Manufacturers must also consider the regulatory implications of using Triton X-100 in different stages of the chromatographic process. The use of additives in early-stage purification may be subject to less stringent regulations compared to their use in final polishing steps. However, regardless of the stage, companies must provide comprehensive data on the additive's impact on product characteristics and purity.
Another crucial regulatory aspect is the need for robust analytical methods to detect and quantify Triton X-100 in the final product. Regulatory bodies often require sensitive and specific assays to ensure that residual levels of the additive are below acceptable limits. This necessitates the development and validation of appropriate analytical techniques, which must be approved as part of the overall regulatory submission.
Environmental regulations also play a role in the use of IEC additives. Triton X-100, being a non-ionic surfactant, may have environmental implications, particularly in waste streams. Companies must adhere to local and international environmental regulations regarding the disposal and treatment of chromatographic waste containing such additives.
In the context of good manufacturing practices (GMP), the use of Triton X-100 in IEC processes must be carefully controlled and documented. This includes establishing standard operating procedures (SOPs) for its use, implementing appropriate quality control measures, and maintaining detailed records of its application in chromatographic processes.
Regulatory bodies also emphasize the importance of supply chain integrity and traceability for additives used in chromatographic processes. Manufacturers must ensure that Triton X-100 and similar additives are sourced from reputable suppliers and that the quality and consistency of these materials are rigorously monitored and documented.
As regulatory requirements continue to evolve, companies must stay abreast of changes in guidelines and regulations pertaining to IEC additives. This may involve ongoing communication with regulatory agencies, participation in industry forums, and regular updates to internal processes and documentation to maintain compliance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!