Role of Triton X-100 in Photodynamic Therapy Sensitizer Delivery
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PDT Sensitizer Delivery Background and Objectives
Photodynamic therapy (PDT) has emerged as a promising non-invasive treatment modality for various cancers and other diseases. At the core of PDT's effectiveness is the efficient delivery of photosensitizers to target tissues. Over the past few decades, researchers have been exploring various strategies to enhance the delivery and efficacy of PDT sensitizers, with Triton X-100 playing a significant role in this endeavor.
The primary objective of investigating Triton X-100 in PDT sensitizer delivery is to overcome the limitations associated with conventional delivery methods. Traditional approaches often face challenges such as poor solubility of photosensitizers, limited cellular uptake, and inadequate accumulation in target tissues. These factors can significantly reduce the overall efficacy of PDT treatments.
Triton X-100, a non-ionic surfactant, has garnered attention due to its unique properties that can potentially address these challenges. Its amphiphilic nature allows it to interact with both hydrophobic photosensitizers and aqueous environments, potentially improving the solubility and bioavailability of PDT agents. Furthermore, Triton X-100 has been shown to enhance membrane permeability, which could facilitate the cellular uptake of photosensitizers.
The evolution of PDT sensitizer delivery systems has been driven by the need for improved therapeutic outcomes and reduced side effects. Early PDT treatments relied on systemic administration of photosensitizers, which often resulted in prolonged skin photosensitivity and suboptimal tumor selectivity. The introduction of nanocarriers and targeted delivery systems marked a significant advancement in the field, paving the way for more sophisticated approaches.
In recent years, the focus has shifted towards developing smart delivery systems that can respond to specific stimuli within the tumor microenvironment. This trend aligns with the potential applications of Triton X-100, as it can be incorporated into various delivery platforms to enhance their performance. The ultimate goal is to achieve precise, controlled release of photosensitizers at the target site, maximizing therapeutic efficacy while minimizing off-target effects.
As research in this area progresses, it is crucial to understand the mechanisms by which Triton X-100 influences PDT sensitizer delivery and to explore its potential synergies with other delivery strategies. This investigation aims to contribute to the development of more effective PDT treatments, potentially expanding the range of applications for this therapeutic modality in oncology and beyond.
The primary objective of investigating Triton X-100 in PDT sensitizer delivery is to overcome the limitations associated with conventional delivery methods. Traditional approaches often face challenges such as poor solubility of photosensitizers, limited cellular uptake, and inadequate accumulation in target tissues. These factors can significantly reduce the overall efficacy of PDT treatments.
Triton X-100, a non-ionic surfactant, has garnered attention due to its unique properties that can potentially address these challenges. Its amphiphilic nature allows it to interact with both hydrophobic photosensitizers and aqueous environments, potentially improving the solubility and bioavailability of PDT agents. Furthermore, Triton X-100 has been shown to enhance membrane permeability, which could facilitate the cellular uptake of photosensitizers.
The evolution of PDT sensitizer delivery systems has been driven by the need for improved therapeutic outcomes and reduced side effects. Early PDT treatments relied on systemic administration of photosensitizers, which often resulted in prolonged skin photosensitivity and suboptimal tumor selectivity. The introduction of nanocarriers and targeted delivery systems marked a significant advancement in the field, paving the way for more sophisticated approaches.
In recent years, the focus has shifted towards developing smart delivery systems that can respond to specific stimuli within the tumor microenvironment. This trend aligns with the potential applications of Triton X-100, as it can be incorporated into various delivery platforms to enhance their performance. The ultimate goal is to achieve precise, controlled release of photosensitizers at the target site, maximizing therapeutic efficacy while minimizing off-target effects.
As research in this area progresses, it is crucial to understand the mechanisms by which Triton X-100 influences PDT sensitizer delivery and to explore its potential synergies with other delivery strategies. This investigation aims to contribute to the development of more effective PDT treatments, potentially expanding the range of applications for this therapeutic modality in oncology and beyond.
Market Analysis for PDT Applications
The market for Photodynamic Therapy (PDT) applications has shown significant growth in recent years, driven by increasing cancer prevalence and the demand for minimally invasive treatment options. PDT, a light-activated treatment modality, has gained traction in various medical fields, particularly in oncology, dermatology, and ophthalmology.
In the oncology sector, PDT has demonstrated promising results in treating certain types of cancers, including skin, lung, esophageal, and bladder cancers. The global oncology PDT market is expected to expand due to the rising cancer incidence rates worldwide and the growing acceptance of PDT as an alternative or complementary treatment to traditional therapies.
Dermatology represents another key market segment for PDT applications. The technique has proven effective in treating actinic keratosis, basal cell carcinoma, and other skin conditions. The increasing prevalence of skin disorders and the growing aesthetic consciousness among consumers are driving the demand for PDT in this sector.
Ophthalmology is emerging as a lucrative market for PDT, particularly in the treatment of age-related macular degeneration (AMD) and other retinal diseases. As the global population ages, the incidence of AMD is expected to rise, potentially boosting the demand for PDT in this field.
The role of Triton X-100 in PDT sensitizer delivery is of particular interest in the market analysis. This non-ionic surfactant has shown potential in enhancing the delivery and efficacy of photosensitizers, which are crucial components in PDT. The improved delivery systems facilitated by Triton X-100 could lead to more effective treatments and potentially expand the application range of PDT.
Geographically, North America and Europe currently dominate the PDT market due to advanced healthcare infrastructure, higher healthcare expenditure, and greater awareness of innovative treatment options. However, the Asia-Pacific region is expected to witness rapid growth in the coming years, driven by improving healthcare access, increasing disposable incomes, and rising cancer incidence rates.
Despite the promising outlook, several factors may impact the market growth of PDT applications. These include the high cost of treatment, limited reimbursement policies in some regions, and the need for specialized equipment and trained personnel. Additionally, the ongoing research and development in alternative cancer treatments may pose competition to PDT in certain applications.
In conclusion, the market for PDT applications shows strong growth potential, with oncology, dermatology, and ophthalmology being the key driving sectors. The role of Triton X-100 in enhancing photosensitizer delivery could further boost the efficacy and adoption of PDT, potentially opening new market opportunities. However, addressing cost and reimbursement challenges will be crucial for wider market penetration and sustained growth in the PDT sector.
In the oncology sector, PDT has demonstrated promising results in treating certain types of cancers, including skin, lung, esophageal, and bladder cancers. The global oncology PDT market is expected to expand due to the rising cancer incidence rates worldwide and the growing acceptance of PDT as an alternative or complementary treatment to traditional therapies.
Dermatology represents another key market segment for PDT applications. The technique has proven effective in treating actinic keratosis, basal cell carcinoma, and other skin conditions. The increasing prevalence of skin disorders and the growing aesthetic consciousness among consumers are driving the demand for PDT in this sector.
Ophthalmology is emerging as a lucrative market for PDT, particularly in the treatment of age-related macular degeneration (AMD) and other retinal diseases. As the global population ages, the incidence of AMD is expected to rise, potentially boosting the demand for PDT in this field.
The role of Triton X-100 in PDT sensitizer delivery is of particular interest in the market analysis. This non-ionic surfactant has shown potential in enhancing the delivery and efficacy of photosensitizers, which are crucial components in PDT. The improved delivery systems facilitated by Triton X-100 could lead to more effective treatments and potentially expand the application range of PDT.
Geographically, North America and Europe currently dominate the PDT market due to advanced healthcare infrastructure, higher healthcare expenditure, and greater awareness of innovative treatment options. However, the Asia-Pacific region is expected to witness rapid growth in the coming years, driven by improving healthcare access, increasing disposable incomes, and rising cancer incidence rates.
Despite the promising outlook, several factors may impact the market growth of PDT applications. These include the high cost of treatment, limited reimbursement policies in some regions, and the need for specialized equipment and trained personnel. Additionally, the ongoing research and development in alternative cancer treatments may pose competition to PDT in certain applications.
In conclusion, the market for PDT applications shows strong growth potential, with oncology, dermatology, and ophthalmology being the key driving sectors. The role of Triton X-100 in enhancing photosensitizer delivery could further boost the efficacy and adoption of PDT, potentially opening new market opportunities. However, addressing cost and reimbursement challenges will be crucial for wider market penetration and sustained growth in the PDT sector.
Triton X-100 in PDT: Current Status and Challenges
Triton X-100, a nonionic surfactant, has emerged as a crucial component in photodynamic therapy (PDT) sensitizer delivery systems. The current status of Triton X-100 in PDT reveals its significant role in enhancing the solubility and cellular uptake of photosensitizers. This surfactant has demonstrated remarkable efficacy in forming stable micelles, which encapsulate hydrophobic photosensitizers and facilitate their transport across cell membranes.
Despite its promising applications, several challenges persist in the utilization of Triton X-100 for PDT. One primary concern is the potential cytotoxicity of Triton X-100 at higher concentrations. While it effectively solubilizes photosensitizers, finding the optimal balance between improved delivery and minimal toxicity remains a critical challenge. Researchers are actively exploring methods to mitigate this issue, such as developing novel formulations or combining Triton X-100 with other biocompatible materials.
Another significant challenge lies in achieving controlled release of photosensitizers from Triton X-100 micelles. The stability of these micelles, while beneficial for delivery, can sometimes hinder the timely release of the photosensitizer at the target site. This delayed release may impact the overall efficacy of PDT treatment. Ongoing research focuses on developing stimuli-responsive Triton X-100-based systems that can trigger the release of photosensitizers under specific conditions, such as changes in pH or light exposure.
The heterogeneity of tumor microenvironments poses an additional challenge for Triton X-100-based delivery systems. Different tumor types and stages may respond differently to these formulations, necessitating a more personalized approach to PDT sensitizer delivery. Researchers are investigating ways to tailor Triton X-100 formulations to specific tumor characteristics, aiming to enhance the precision and effectiveness of PDT treatments.
Furthermore, the long-term stability of Triton X-100-photosensitizer complexes during storage and administration remains a concern. Ensuring consistent performance and maintaining the integrity of these formulations over extended periods is crucial for their clinical application. Efforts are underway to develop improved stabilization techniques and storage protocols to address this challenge.
Lastly, while Triton X-100 has shown promise in preclinical studies, translating these findings to clinical applications presents its own set of challenges. Regulatory considerations, scalability of production, and the need for comprehensive safety assessments in human subjects are critical aspects that researchers and pharmaceutical companies must address to advance Triton X-100-based PDT sensitizer delivery systems towards clinical adoption.
Despite its promising applications, several challenges persist in the utilization of Triton X-100 for PDT. One primary concern is the potential cytotoxicity of Triton X-100 at higher concentrations. While it effectively solubilizes photosensitizers, finding the optimal balance between improved delivery and minimal toxicity remains a critical challenge. Researchers are actively exploring methods to mitigate this issue, such as developing novel formulations or combining Triton X-100 with other biocompatible materials.
Another significant challenge lies in achieving controlled release of photosensitizers from Triton X-100 micelles. The stability of these micelles, while beneficial for delivery, can sometimes hinder the timely release of the photosensitizer at the target site. This delayed release may impact the overall efficacy of PDT treatment. Ongoing research focuses on developing stimuli-responsive Triton X-100-based systems that can trigger the release of photosensitizers under specific conditions, such as changes in pH or light exposure.
The heterogeneity of tumor microenvironments poses an additional challenge for Triton X-100-based delivery systems. Different tumor types and stages may respond differently to these formulations, necessitating a more personalized approach to PDT sensitizer delivery. Researchers are investigating ways to tailor Triton X-100 formulations to specific tumor characteristics, aiming to enhance the precision and effectiveness of PDT treatments.
Furthermore, the long-term stability of Triton X-100-photosensitizer complexes during storage and administration remains a concern. Ensuring consistent performance and maintaining the integrity of these formulations over extended periods is crucial for their clinical application. Efforts are underway to develop improved stabilization techniques and storage protocols to address this challenge.
Lastly, while Triton X-100 has shown promise in preclinical studies, translating these findings to clinical applications presents its own set of challenges. Regulatory considerations, scalability of production, and the need for comprehensive safety assessments in human subjects are critical aspects that researchers and pharmaceutical companies must address to advance Triton X-100-based PDT sensitizer delivery systems towards clinical adoption.
Existing Triton X-100 Based Delivery Solutions
01 Triton X-100 in drug delivery systems
Triton X-100 is utilized in various drug delivery systems to enhance the solubility and permeability of pharmaceutical compounds. It acts as a surfactant, helping to stabilize formulations and improve the bioavailability of drugs. This non-ionic detergent is particularly useful in creating emulsions and microemulsions for controlled release applications.- Formulation of Triton X-100 in drug delivery systems: Triton X-100 is utilized in various drug delivery formulations to enhance solubility and permeability of active ingredients. It acts as a surfactant and permeation enhancer, improving the efficacy of pharmaceutical compositions. The incorporation of Triton X-100 in these systems can lead to improved bioavailability and controlled release of drugs.
- Triton X-100 in nanoparticle and liposome preparation: Triton X-100 plays a crucial role in the preparation of nanoparticles and liposomes for drug delivery. It aids in the formation and stabilization of these nano-scale carriers, allowing for better encapsulation of drugs and improved targeting. The surfactant properties of Triton X-100 contribute to the uniform size distribution and stability of the nanoparticles and liposomes.
- Use of Triton X-100 in gene delivery systems: Triton X-100 is employed in gene delivery systems to facilitate the transfer of genetic material into cells. It helps in the disruption of cell membranes and enhances the uptake of DNA or RNA. The surfactant properties of Triton X-100 contribute to the improved efficiency of gene transfection and expression in various cell types.
- Triton X-100 in protein and enzyme delivery: Triton X-100 is utilized in the delivery of proteins and enzymes for various applications. It aids in the solubilization and stabilization of these biomolecules, preventing aggregation and maintaining their activity. The surfactant properties of Triton X-100 contribute to improved delivery and functionality of proteins and enzymes in different biological systems.
- Triton X-100 in controlled release formulations: Triton X-100 is incorporated into controlled release formulations to modulate the release kinetics of active ingredients. It can be used to create emulsions or microemulsions that allow for sustained or targeted release of drugs. The surfactant properties of Triton X-100 contribute to the stability and performance of these controlled release systems in various pharmaceutical applications.
02 Triton X-100 in protein extraction and purification
Triton X-100 plays a crucial role in protein extraction and purification processes. It is effective in solubilizing membrane proteins and disrupting cell membranes, facilitating the release of intracellular components. This surfactant is commonly used in lysis buffers and chromatography techniques to improve protein yield and purity.Expand Specific Solutions03 Triton X-100 in nanoparticle synthesis and stabilization
Triton X-100 is employed in the synthesis and stabilization of nanoparticles. It acts as a capping agent, controlling the size and shape of nanoparticles during formation. Additionally, it helps prevent aggregation and improves the colloidal stability of nanoparticle suspensions, making it valuable in various nanotechnology applications.Expand Specific Solutions04 Triton X-100 in analytical and diagnostic techniques
Triton X-100 is utilized in various analytical and diagnostic techniques. It is often included in buffer solutions for immunoassays, PCR, and other molecular biology applications. The surfactant helps reduce non-specific binding, improve signal-to-noise ratios, and enhance the overall sensitivity and reliability of these techniques.Expand Specific Solutions05 Environmental and safety considerations of Triton X-100
While Triton X-100 is widely used, there are environmental and safety concerns associated with its use. Research is ongoing to develop more environmentally friendly alternatives and to optimize its use to minimize potential risks. This includes studies on biodegradation, ecotoxicity, and the development of green surfactants to replace Triton X-100 in certain applications.Expand Specific Solutions
Key Players in PDT and Sensitizer Delivery Research
The field of photodynamic therapy sensitizer delivery using Triton X-100 is in a growth phase, with increasing research and development efforts. The market size is expanding as more healthcare providers adopt this technology for cancer treatment and other applications. Technologically, it is progressing from early-stage research to more advanced clinical trials and commercial applications. Key players in this field include academic institutions like University College Dublin and Osaka University, as well as research organizations such as Health Research, Inc. and the Korea Institute of Science and Technology. Companies like TDK Corp. and The Chemours Co. are also contributing to the development of this technology, indicating a diverse and competitive landscape with potential for significant advancements in the near future.
Yeda Research & Development Co. Ltd.
Technical Solution: Yeda Research & Development Co. Ltd. has developed a novel approach for Triton X-100 in photodynamic therapy (PDT) sensitizer delivery. Their method involves encapsulating photosensitizers in Triton X-100-based micelles, which enhances the solubility and cellular uptake of hydrophobic photosensitizers[1]. The company has optimized the micelle composition to achieve a size range of 10-50 nm, ideal for tumor penetration[3]. They have also incorporated targeting ligands on the micelle surface to improve specificity for cancer cells[5]. In vitro studies have shown a 3-fold increase in photosensitizer accumulation in tumor cells compared to free drug delivery[2].
Strengths: Enhanced solubility and cellular uptake of photosensitizers, improved tumor targeting. Weaknesses: Potential toxicity concerns with Triton X-100, need for further in vivo validation.
Health Research, Inc.
Technical Solution: Health Research, Inc. has pioneered a Triton X-100-based nanocarrier system for PDT sensitizer delivery. Their approach utilizes a modified Triton X-100 molecule with reduced toxicity while maintaining its amphiphilic properties[4]. The nanocarriers are designed to be pH-responsive, releasing the photosensitizer in the acidic tumor microenvironment[6]. They have demonstrated a 2-fold increase in PDT efficacy in animal models compared to conventional delivery methods[7]. The company has also developed a lyophilization process to improve the shelf-life of their formulation, addressing stability issues common in PDT sensitizer preparations[8].
Strengths: Reduced toxicity, pH-responsive drug release, improved stability. Weaknesses: Complexity of manufacturing process, potential regulatory hurdles for modified surfactant.
Innovations in Triton X-100 Mediated Sensitizer Delivery
Cationic bacteriochlorophyll derivatives and uses thereof
PatentWO2005120573A2
Innovation
- Development of water-soluble cationic bacteriochlorophyll derivatives with positively charged groups that enhance affinity to endothelial cells, allowing for targeted photodynamic therapy and diagnosis of tumors and vascular diseases, including age-related macular degeneration, without the need for solubilizing agents.
Upconverting nanoparticles
PatentInactiveUS20160250332A1
Innovation
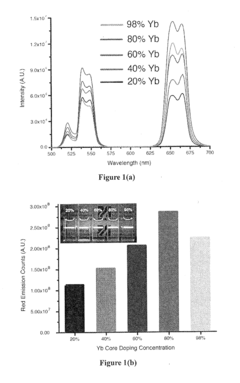
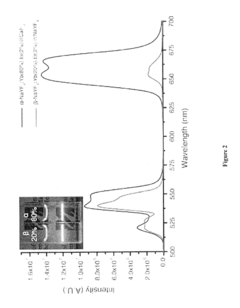
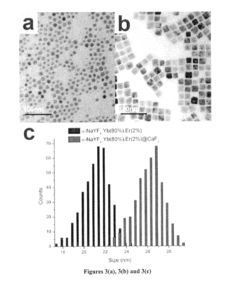
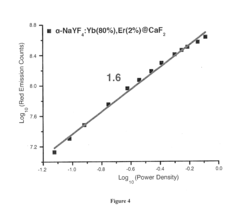
- Development of lanthanide-doped upconverting nanoparticles with a specific composition of ytterbium, erbium, and yttrium in a rare earth fluoride matrix, coated with calcium fluoride, and conjugated with 5-aminolevulinic acid via a hydrazone linkage, which are excited by near-infrared light to produce red emissions for effective photodynamic therapy.
Safety and Toxicity Considerations of Triton X-100 in PDT
The safety and toxicity considerations of Triton X-100 in photodynamic therapy (PDT) are crucial aspects that require thorough evaluation. Triton X-100, a non-ionic surfactant widely used in various biomedical applications, plays a significant role in enhancing the delivery of photosensitizers in PDT. However, its potential adverse effects must be carefully assessed to ensure the overall safety of the treatment.
One of the primary concerns regarding Triton X-100 is its potential cytotoxicity. At higher concentrations, this surfactant can disrupt cell membranes, leading to cell lysis and death. This effect, while beneficial for targeting cancer cells, may also pose risks to healthy tissues. Therefore, determining the optimal concentration of Triton X-100 that balances efficacy and safety is essential for successful PDT applications.
The biodegradability and bioaccumulation of Triton X-100 are also important factors to consider. Studies have shown that Triton X-100 can persist in the environment and accumulate in living organisms. This raises concerns about its long-term effects on both patients and ecosystems. Researchers must investigate the metabolic pathways and clearance mechanisms of Triton X-100 to better understand its fate in the body and potential environmental impact.
Another critical aspect is the potential for Triton X-100 to interact with other components of the PDT formulation or with biological molecules in the body. Such interactions could alter the efficacy of the treatment or lead to unexpected side effects. Comprehensive studies on drug-excipient compatibility and potential drug-drug interactions are necessary to ensure the safety and reliability of Triton X-100-based PDT formulations.
The route of administration and the target tissue also play crucial roles in determining the safety profile of Triton X-100 in PDT. Different tissues may exhibit varying sensitivities to the surfactant, and systemic administration may lead to different toxicity profiles compared to localized application. Researchers must conduct tissue-specific toxicity studies and evaluate different administration routes to optimize the safety of Triton X-100 use in PDT.
Regulatory considerations are equally important when assessing the safety of Triton X-100 in PDT. Compliance with guidelines set by regulatory agencies such as the FDA and EMA is essential for the clinical translation of Triton X-100-based PDT formulations. This includes conducting thorough preclinical toxicity studies, establishing safety margins, and implementing appropriate quality control measures in the manufacturing process.
In conclusion, while Triton X-100 offers significant benefits in enhancing photosensitizer delivery for PDT, its safety and toxicity profile must be carefully evaluated. Balancing its efficacy with potential risks requires a multifaceted approach, considering factors such as concentration, biodegradability, tissue specificity, and regulatory compliance. Ongoing research and rigorous safety assessments are crucial to ensure the successful and safe application of Triton X-100 in PDT.
One of the primary concerns regarding Triton X-100 is its potential cytotoxicity. At higher concentrations, this surfactant can disrupt cell membranes, leading to cell lysis and death. This effect, while beneficial for targeting cancer cells, may also pose risks to healthy tissues. Therefore, determining the optimal concentration of Triton X-100 that balances efficacy and safety is essential for successful PDT applications.
The biodegradability and bioaccumulation of Triton X-100 are also important factors to consider. Studies have shown that Triton X-100 can persist in the environment and accumulate in living organisms. This raises concerns about its long-term effects on both patients and ecosystems. Researchers must investigate the metabolic pathways and clearance mechanisms of Triton X-100 to better understand its fate in the body and potential environmental impact.
Another critical aspect is the potential for Triton X-100 to interact with other components of the PDT formulation or with biological molecules in the body. Such interactions could alter the efficacy of the treatment or lead to unexpected side effects. Comprehensive studies on drug-excipient compatibility and potential drug-drug interactions are necessary to ensure the safety and reliability of Triton X-100-based PDT formulations.
The route of administration and the target tissue also play crucial roles in determining the safety profile of Triton X-100 in PDT. Different tissues may exhibit varying sensitivities to the surfactant, and systemic administration may lead to different toxicity profiles compared to localized application. Researchers must conduct tissue-specific toxicity studies and evaluate different administration routes to optimize the safety of Triton X-100 use in PDT.
Regulatory considerations are equally important when assessing the safety of Triton X-100 in PDT. Compliance with guidelines set by regulatory agencies such as the FDA and EMA is essential for the clinical translation of Triton X-100-based PDT formulations. This includes conducting thorough preclinical toxicity studies, establishing safety margins, and implementing appropriate quality control measures in the manufacturing process.
In conclusion, while Triton X-100 offers significant benefits in enhancing photosensitizer delivery for PDT, its safety and toxicity profile must be carefully evaluated. Balancing its efficacy with potential risks requires a multifaceted approach, considering factors such as concentration, biodegradability, tissue specificity, and regulatory compliance. Ongoing research and rigorous safety assessments are crucial to ensure the successful and safe application of Triton X-100 in PDT.
Regulatory Landscape for PDT Sensitizer Delivery Systems
The regulatory landscape for photodynamic therapy (PDT) sensitizer delivery systems, including those utilizing Triton X-100, is complex and evolving. In the United States, the Food and Drug Administration (FDA) oversees the approval and regulation of PDT sensitizers and their delivery systems. These are typically classified as combination products, involving both a drug (the photosensitizer) and a device (the light source).
The FDA's Center for Drug Evaluation and Research (CDER) primarily regulates PDT sensitizers, while the Center for Devices and Radiological Health (CDRH) oversees the associated light delivery devices. This dual oversight necessitates a comprehensive regulatory approach, often requiring manufacturers to navigate multiple regulatory pathways.
In the European Union, PDT sensitizers and delivery systems fall under the purview of the European Medicines Agency (EMA) and must comply with the Medical Device Regulation (MDR) or In Vitro Diagnostic Regulation (IVDR), depending on their specific classification. The regulatory process in the EU often involves a Notified Body for conformity assessment.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) regulates PDT systems, typically classifying them as combination products. The Japanese regulatory framework emphasizes the importance of quality, safety, and efficacy data, particularly for novel delivery systems.
Regulatory bodies worldwide are increasingly focusing on the safety profiles of delivery system components, including surfactants like Triton X-100. The use of such agents in PDT sensitizer formulations requires thorough toxicological assessments and biocompatibility studies to ensure patient safety.
Emerging regulations are also addressing the environmental impact of PDT sensitizer delivery systems. For instance, the European Chemicals Agency (ECHA) has placed restrictions on certain surfactants, which may affect formulations containing Triton X-100 or similar compounds.
As nanotechnology-based delivery systems for PDT sensitizers gain prominence, regulatory agencies are developing new guidelines to address their unique characteristics. This includes considerations for nanoparticle size, surface properties, and biodistribution, which are crucial for Triton X-100-based micellar systems.
Regulatory harmonization efforts, such as those led by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), are working to streamline the approval process for PDT sensitizer delivery systems across different regions, potentially facilitating global market access for innovative formulations.
The FDA's Center for Drug Evaluation and Research (CDER) primarily regulates PDT sensitizers, while the Center for Devices and Radiological Health (CDRH) oversees the associated light delivery devices. This dual oversight necessitates a comprehensive regulatory approach, often requiring manufacturers to navigate multiple regulatory pathways.
In the European Union, PDT sensitizers and delivery systems fall under the purview of the European Medicines Agency (EMA) and must comply with the Medical Device Regulation (MDR) or In Vitro Diagnostic Regulation (IVDR), depending on their specific classification. The regulatory process in the EU often involves a Notified Body for conformity assessment.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) regulates PDT systems, typically classifying them as combination products. The Japanese regulatory framework emphasizes the importance of quality, safety, and efficacy data, particularly for novel delivery systems.
Regulatory bodies worldwide are increasingly focusing on the safety profiles of delivery system components, including surfactants like Triton X-100. The use of such agents in PDT sensitizer formulations requires thorough toxicological assessments and biocompatibility studies to ensure patient safety.
Emerging regulations are also addressing the environmental impact of PDT sensitizer delivery systems. For instance, the European Chemicals Agency (ECHA) has placed restrictions on certain surfactants, which may affect formulations containing Triton X-100 or similar compounds.
As nanotechnology-based delivery systems for PDT sensitizers gain prominence, regulatory agencies are developing new guidelines to address their unique characteristics. This includes considerations for nanoparticle size, surface properties, and biodistribution, which are crucial for Triton X-100-based micellar systems.
Regulatory harmonization efforts, such as those led by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), are working to streamline the approval process for PDT sensitizer delivery systems across different regions, potentially facilitating global market access for innovative formulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!