Influence of Solenoid Valve Technologies on Breakthrough Medical Innovations
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Solenoid Valve Evolution in Medical Field
The evolution of solenoid valve technologies in the medical field has been marked by significant advancements and innovations over the past few decades. Initially developed for industrial applications, solenoid valves have found their way into various medical devices, revolutionizing patient care and treatment methodologies.
In the early stages, solenoid valves in medical applications were primarily used in basic fluid control systems for equipment such as dialysis machines and anesthesia delivery systems. These early valves were relatively large, had limited precision, and often suffered from reliability issues. However, as miniaturization techniques improved, solenoid valves became smaller, more efficient, and increasingly reliable, opening up new possibilities in medical device design.
The 1980s and 1990s saw a rapid expansion of solenoid valve applications in the medical field. Improved materials and manufacturing processes led to the development of valves capable of handling a wider range of fluids and gases, including corrosive substances and ultra-pure materials. This expansion allowed for the integration of solenoid valves into more sophisticated medical equipment, such as automated drug delivery systems and advanced respiratory support devices.
A significant milestone in the evolution of medical solenoid valves came with the advent of proportional control technology. Unlike traditional on-off valves, proportional solenoid valves allowed for precise control of flow rates, enabling more accurate dosing in drug delivery systems and finer control in critical care equipment. This development was particularly impactful in the field of anesthesiology, where precise control of gas mixtures is crucial.
The turn of the millennium brought about further miniaturization and integration of solenoid valves in medical devices. Microfluidic applications began to emerge, with solenoid valves playing a crucial role in lab-on-a-chip devices and point-of-care diagnostic tools. These miniature valves, some barely larger than a grain of rice, enabled the manipulation of minute fluid volumes, revolutionizing diagnostic capabilities and opening new avenues for personalized medicine.
Recent years have seen the incorporation of smart technologies into solenoid valve design. The integration of sensors and microcontrollers has led to the development of self-diagnostic and self-calibrating valves, enhancing reliability and reducing maintenance requirements in critical medical applications. Additionally, the use of advanced materials such as shape memory alloys and piezoelectric actuators has further improved valve performance and energy efficiency.
Looking ahead, the evolution of solenoid valves in the medical field is likely to continue along the paths of miniaturization, integration, and increased intelligence. Emerging technologies such as 3D printing and nanotechnology are expected to play significant roles in future valve designs, potentially leading to even more compact, efficient, and versatile solutions for medical applications.
In the early stages, solenoid valves in medical applications were primarily used in basic fluid control systems for equipment such as dialysis machines and anesthesia delivery systems. These early valves were relatively large, had limited precision, and often suffered from reliability issues. However, as miniaturization techniques improved, solenoid valves became smaller, more efficient, and increasingly reliable, opening up new possibilities in medical device design.
The 1980s and 1990s saw a rapid expansion of solenoid valve applications in the medical field. Improved materials and manufacturing processes led to the development of valves capable of handling a wider range of fluids and gases, including corrosive substances and ultra-pure materials. This expansion allowed for the integration of solenoid valves into more sophisticated medical equipment, such as automated drug delivery systems and advanced respiratory support devices.
A significant milestone in the evolution of medical solenoid valves came with the advent of proportional control technology. Unlike traditional on-off valves, proportional solenoid valves allowed for precise control of flow rates, enabling more accurate dosing in drug delivery systems and finer control in critical care equipment. This development was particularly impactful in the field of anesthesiology, where precise control of gas mixtures is crucial.
The turn of the millennium brought about further miniaturization and integration of solenoid valves in medical devices. Microfluidic applications began to emerge, with solenoid valves playing a crucial role in lab-on-a-chip devices and point-of-care diagnostic tools. These miniature valves, some barely larger than a grain of rice, enabled the manipulation of minute fluid volumes, revolutionizing diagnostic capabilities and opening new avenues for personalized medicine.
Recent years have seen the incorporation of smart technologies into solenoid valve design. The integration of sensors and microcontrollers has led to the development of self-diagnostic and self-calibrating valves, enhancing reliability and reducing maintenance requirements in critical medical applications. Additionally, the use of advanced materials such as shape memory alloys and piezoelectric actuators has further improved valve performance and energy efficiency.
Looking ahead, the evolution of solenoid valves in the medical field is likely to continue along the paths of miniaturization, integration, and increased intelligence. Emerging technologies such as 3D printing and nanotechnology are expected to play significant roles in future valve designs, potentially leading to even more compact, efficient, and versatile solutions for medical applications.
Medical Market Demand for Advanced Valve Systems
The medical market's demand for advanced valve systems has been steadily increasing, driven by the need for more precise, reliable, and efficient medical devices. Solenoid valve technologies, in particular, have emerged as a critical component in various medical applications, contributing significantly to breakthrough innovations in the field.
In recent years, the global medical device market has experienced substantial growth, with a significant portion attributed to advanced valve systems. These systems play a crucial role in a wide range of medical equipment, including ventilators, anesthesia machines, infusion pumps, and diagnostic devices. The demand for such advanced valve systems is primarily fueled by the growing prevalence of chronic diseases, an aging population, and the increasing adoption of minimally invasive surgical procedures.
One of the key drivers of market demand is the need for miniaturization and improved precision in medical devices. Solenoid valves offer compact designs and precise control, making them ideal for integration into portable and wearable medical devices. This trend aligns with the shift towards personalized medicine and home healthcare, where patients require reliable and easy-to-use medical equipment for self-administration of treatments.
The COVID-19 pandemic has further accelerated the demand for advanced valve systems, particularly in respiratory care equipment. The sudden surge in the need for ventilators and oxygen therapy devices highlighted the critical role of reliable valve technologies in life-saving medical equipment. This has led to increased investment in research and development of more advanced and versatile valve systems capable of meeting diverse medical requirements.
Another significant factor driving market demand is the increasing focus on patient safety and infection control. Advanced valve systems with improved sealing capabilities and materials that are resistant to contamination are highly sought after in the medical industry. These features are essential in preventing cross-contamination and ensuring the sterility of medical procedures, particularly in critical care settings.
The market also shows a growing demand for smart valve systems that can integrate with digital health platforms and Internet of Things (IoT) technologies. These advanced systems enable real-time monitoring, data collection, and remote control of medical devices, enhancing patient care and treatment outcomes. The ability to gather and analyze data from valve operations contributes to predictive maintenance and optimization of medical equipment performance.
As healthcare facilities worldwide strive to improve operational efficiency and reduce costs, there is an increasing demand for valve systems that offer long-term reliability and low maintenance requirements. This trend favors the development of solenoid valves with extended lifecycles and self-diagnostic capabilities, reducing the need for frequent replacements and minimizing downtime in critical medical applications.
In recent years, the global medical device market has experienced substantial growth, with a significant portion attributed to advanced valve systems. These systems play a crucial role in a wide range of medical equipment, including ventilators, anesthesia machines, infusion pumps, and diagnostic devices. The demand for such advanced valve systems is primarily fueled by the growing prevalence of chronic diseases, an aging population, and the increasing adoption of minimally invasive surgical procedures.
One of the key drivers of market demand is the need for miniaturization and improved precision in medical devices. Solenoid valves offer compact designs and precise control, making them ideal for integration into portable and wearable medical devices. This trend aligns with the shift towards personalized medicine and home healthcare, where patients require reliable and easy-to-use medical equipment for self-administration of treatments.
The COVID-19 pandemic has further accelerated the demand for advanced valve systems, particularly in respiratory care equipment. The sudden surge in the need for ventilators and oxygen therapy devices highlighted the critical role of reliable valve technologies in life-saving medical equipment. This has led to increased investment in research and development of more advanced and versatile valve systems capable of meeting diverse medical requirements.
Another significant factor driving market demand is the increasing focus on patient safety and infection control. Advanced valve systems with improved sealing capabilities and materials that are resistant to contamination are highly sought after in the medical industry. These features are essential in preventing cross-contamination and ensuring the sterility of medical procedures, particularly in critical care settings.
The market also shows a growing demand for smart valve systems that can integrate with digital health platforms and Internet of Things (IoT) technologies. These advanced systems enable real-time monitoring, data collection, and remote control of medical devices, enhancing patient care and treatment outcomes. The ability to gather and analyze data from valve operations contributes to predictive maintenance and optimization of medical equipment performance.
As healthcare facilities worldwide strive to improve operational efficiency and reduce costs, there is an increasing demand for valve systems that offer long-term reliability and low maintenance requirements. This trend favors the development of solenoid valves with extended lifecycles and self-diagnostic capabilities, reducing the need for frequent replacements and minimizing downtime in critical medical applications.
Current Challenges in Solenoid Valve Applications
Despite the widespread use of solenoid valves in medical applications, several challenges persist in their implementation, hindering the full realization of their potential in breakthrough medical innovations. One of the primary concerns is the issue of miniaturization. As medical devices become increasingly compact and portable, there is a growing demand for smaller solenoid valves that can maintain high performance in confined spaces. This miniaturization process often leads to compromises in valve efficiency and reliability, presenting a significant challenge for engineers and designers.
Another critical challenge lies in the power consumption of solenoid valves. In many medical devices, especially portable and implantable ones, energy efficiency is paramount. Traditional solenoid valves often require substantial power to operate, which can drain batteries quickly and limit the operational time of medical devices. Developing low-power solenoid valves that can maintain precise control and rapid response times remains a key area of focus for researchers and manufacturers.
The need for improved response times and precision control presents another hurdle in solenoid valve applications. In critical medical procedures and diagnostic equipment, even millisecond delays or minor inaccuracies in fluid control can have significant consequences. Enhancing the speed and accuracy of solenoid valve operations without compromising reliability is a complex engineering challenge that requires innovative solutions.
Biocompatibility and sterilization compatibility are also major concerns in medical applications of solenoid valves. Materials used in valve construction must not only withstand repeated sterilization processes but also be inert to biological fluids and tissues. Finding materials that meet these stringent requirements while maintaining the valve's functional properties is an ongoing challenge in the industry.
Reliability and longevity of solenoid valves in medical applications pose another significant challenge. Medical devices often require components that can operate flawlessly for extended periods without maintenance. Improving the durability of solenoid valves, particularly in environments with frequent cycling or exposure to corrosive fluids, is crucial for their successful integration into long-term medical solutions.
Lastly, the integration of smart technologies and IoT capabilities into solenoid valves presents both opportunities and challenges. While these advancements can greatly enhance the functionality and monitoring capabilities of medical devices, they also introduce complexities in terms of data security, connectivity, and system integration. Balancing these technological advancements with the need for simplicity and reliability in medical applications remains a significant challenge for developers and manufacturers in the field.
Another critical challenge lies in the power consumption of solenoid valves. In many medical devices, especially portable and implantable ones, energy efficiency is paramount. Traditional solenoid valves often require substantial power to operate, which can drain batteries quickly and limit the operational time of medical devices. Developing low-power solenoid valves that can maintain precise control and rapid response times remains a key area of focus for researchers and manufacturers.
The need for improved response times and precision control presents another hurdle in solenoid valve applications. In critical medical procedures and diagnostic equipment, even millisecond delays or minor inaccuracies in fluid control can have significant consequences. Enhancing the speed and accuracy of solenoid valve operations without compromising reliability is a complex engineering challenge that requires innovative solutions.
Biocompatibility and sterilization compatibility are also major concerns in medical applications of solenoid valves. Materials used in valve construction must not only withstand repeated sterilization processes but also be inert to biological fluids and tissues. Finding materials that meet these stringent requirements while maintaining the valve's functional properties is an ongoing challenge in the industry.
Reliability and longevity of solenoid valves in medical applications pose another significant challenge. Medical devices often require components that can operate flawlessly for extended periods without maintenance. Improving the durability of solenoid valves, particularly in environments with frequent cycling or exposure to corrosive fluids, is crucial for their successful integration into long-term medical solutions.
Lastly, the integration of smart technologies and IoT capabilities into solenoid valves presents both opportunities and challenges. While these advancements can greatly enhance the functionality and monitoring capabilities of medical devices, they also introduce complexities in terms of data security, connectivity, and system integration. Balancing these technological advancements with the need for simplicity and reliability in medical applications remains a significant challenge for developers and manufacturers in the field.
Cutting-edge Solenoid Valve Solutions
01 Improved solenoid valve designs
Advanced solenoid valve designs focus on enhancing performance, reliability, and efficiency. These improvements include optimized magnetic circuits, innovative armature configurations, and precision-engineered valve seats. Such designs aim to reduce power consumption, increase flow rates, and improve response times, making them suitable for a wide range of applications in various industries.- Improved solenoid valve designs: Advanced solenoid valve designs focus on enhancing performance, reliability, and efficiency. These improvements include optimized magnetic circuits, innovative armature configurations, and precision-engineered valve seats. Such designs aim to reduce power consumption, increase flow rates, and improve response times, making them suitable for a wide range of applications in various industries.
- Smart solenoid valve technologies: Integration of smart technologies in solenoid valves enables advanced control, monitoring, and diagnostics. These valves incorporate sensors, microprocessors, and communication interfaces to provide real-time data on valve status, flow rates, and system conditions. Smart solenoid valves offer improved accuracy, remote operation capabilities, and predictive maintenance features, enhancing overall system efficiency and reliability.
- Energy-efficient solenoid valve solutions: Energy-efficient solenoid valve technologies focus on reducing power consumption while maintaining optimal performance. These solutions incorporate low-power coils, energy-saving control algorithms, and innovative materials to minimize heat generation and improve overall efficiency. Such valves are particularly beneficial in battery-powered systems and applications requiring continuous operation.
- Miniaturization of solenoid valves: Advancements in miniaturization techniques allow for the development of compact solenoid valves without compromising performance. These miniaturized valves utilize precision manufacturing processes, advanced materials, and optimized designs to achieve reduced size and weight. Compact solenoid valves are ideal for space-constrained applications in industries such as medical devices, automotive, and portable equipment.
- Multi-functional solenoid valve systems: Multi-functional solenoid valve systems integrate multiple valve functions into a single unit, offering space-saving and cost-effective solutions. These systems may combine different valve types, flow control mechanisms, and auxiliary functions such as pressure regulation or flow measurement. By consolidating multiple functions, these valve systems simplify installation, reduce potential leak points, and improve overall system reliability.
02 Smart solenoid valve technologies
Integration of smart technologies in solenoid valves enables advanced control and monitoring capabilities. These valves incorporate sensors, microcontrollers, and communication interfaces to provide real-time data on valve status, flow rates, and system conditions. Smart solenoid valves offer features such as self-diagnostics, predictive maintenance, and remote operation, enhancing overall system efficiency and reliability.Expand Specific Solutions03 Energy-efficient solenoid valve solutions
Development of energy-efficient solenoid valve technologies focuses on reducing power consumption without compromising performance. These solutions incorporate low-power coils, energy-saving control algorithms, and innovative materials to minimize heat generation and improve overall efficiency. Energy-efficient solenoid valves are particularly valuable in battery-powered systems and applications requiring continuous operation.Expand Specific Solutions04 Miniaturization of solenoid valves
Advancements in miniaturization techniques enable the development of compact and lightweight solenoid valves. These miniaturized valves maintain high performance while reducing overall size and weight, making them ideal for space-constrained applications. Miniaturization efforts focus on optimizing component design, integrating multiple functions, and utilizing advanced manufacturing processes to achieve smaller form factors.Expand Specific Solutions05 Specialized solenoid valve applications
Solenoid valve technologies are being adapted for specialized applications across various industries. These include high-pressure valves for hydraulic systems, corrosion-resistant valves for chemical processing, and high-temperature valves for automotive and aerospace applications. Specialized solenoid valves are designed to meet specific performance requirements and environmental conditions, expanding their use in challenging operational environments.Expand Specific Solutions
Key Players in Medical Solenoid Valve Industry
The solenoid valve technology market in medical innovations is in a growth phase, driven by increasing demand for precision fluid control in medical devices. The global market size is projected to expand significantly, with key players like Robert Bosch GmbH, Baxter International, and Medtronic leading innovation. These companies are investing heavily in R&D to develop advanced solenoid valves for applications such as drug delivery systems, diagnostic equipment, and life support machines. The technology is maturing rapidly, with companies like BorgWarner and Parker-Hannifin contributing to improved miniaturization, energy efficiency, and reliability. Emerging players like Cardiovascular Systems and Voyager Therapeutics are also making strides in specialized medical applications, indicating a competitive and dynamic market landscape.
Robert Bosch GmbH
Technical Solution: Bosch has developed advanced solenoid valve technologies for medical applications, focusing on miniaturization and precision control. Their latest innovations include microfluidic solenoid valves for lab-on-a-chip devices, enabling precise fluid control in diagnostic equipment[1]. These valves utilize advanced materials and manufacturing techniques to achieve sub-millimeter sizes while maintaining high reliability and accuracy. Bosch's solenoid valves incorporate smart sensing capabilities, allowing real-time monitoring of valve performance and fluid flow rates[3]. This technology has been crucial in developing portable medical devices and improving point-of-care diagnostics.
Strengths: High precision, miniaturization, and smart sensing capabilities. Weaknesses: Potentially higher cost compared to traditional valves, may require specialized maintenance.
Baxter International, Inc.
Technical Solution: Baxter has leveraged solenoid valve technology to enhance their infusion pumps and dialysis machines. Their latest innovation is a multi-channel infusion system that uses an array of miniature solenoid valves to precisely control the flow of multiple medications simultaneously[7]. This system allows for complex drug delivery protocols and reduces the risk of medication errors. Baxter has also developed a novel solenoid valve design for their hemodialysis machines, which improves fluid balance control and reduces the risk of air embolism. The valve incorporates self-cleaning mechanisms to prevent clogging and ensure long-term reliability in challenging clinical environments[8].
Strengths: Multi-channel capabilities, focus on safety and reliability in critical care applications. Weaknesses: Complexity of systems may require specialized training for healthcare providers.
Innovative Solenoid Valve Patents and Research
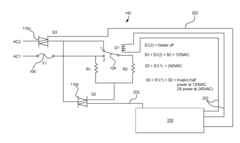
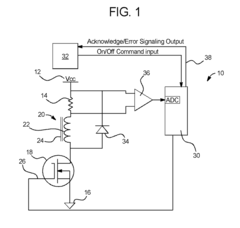
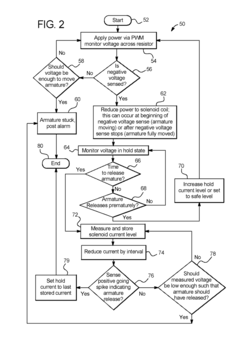
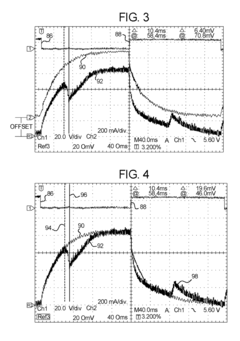
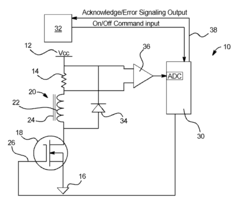
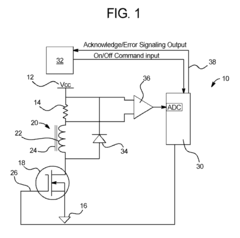
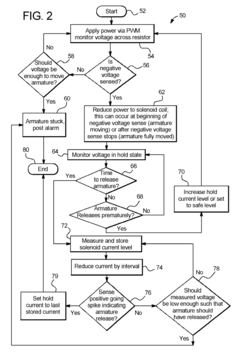
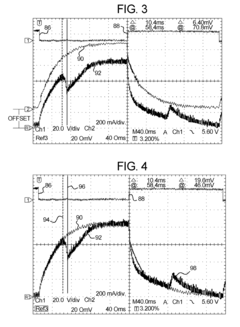
Dialysis machine having multi-input voltage capable heater
PatentActiveUS8160433B2
Innovation
- The implementation of a solenoid system with solenoid coil current sensing for feedback, reducing power dissipation and noise, and a dual supply line voltage fluid heating system that automatically configures for varying AC line voltages, using microcontrollers and optically-isolated serial buses for efficient operation.
Dialysis machine having multiple line voltage heater
PatentInactiveUS8027572B2
Innovation
- The implementation of a solenoid system using solenoid coil current sensing for feedback to reduce power dissipation and optimize hold current levels, combined with a dual supply line voltage fluid heating system that automatically configures for different AC line voltages and reduces heat generation, thereby improving power efficiency and reliability.
Regulatory Framework for Medical Valve Devices
The regulatory framework for medical valve devices plays a crucial role in ensuring patient safety and product efficacy. In the United States, the Food and Drug Administration (FDA) oversees the regulation of medical devices, including solenoid valves used in medical applications. These devices are typically classified as Class II medical devices, requiring premarket notification (510(k)) before they can be marketed.
The FDA's regulatory process for medical valve devices involves several key components. Manufacturers must demonstrate that their devices are substantially equivalent to a legally marketed predicate device in terms of safety and effectiveness. This process includes submitting detailed technical documentation, performance data, and clinical evidence when necessary.
Quality System Regulation (QSR) compliance is another critical aspect of the regulatory framework. Manufacturers must establish and maintain quality management systems that meet the requirements outlined in 21 CFR Part 820. This includes design controls, process validation, and risk management procedures specific to medical valve devices.
Post-market surveillance is an ongoing requirement for manufacturers of medical valve devices. They must monitor and report adverse events, implement corrective and preventive actions when necessary, and conduct periodic safety reviews to ensure continued compliance with regulatory standards.
Internationally, the regulatory landscape for medical valve devices varies. The European Union's Medical Device Regulation (MDR) provides a comprehensive framework for medical devices, including solenoid valves. Manufacturers seeking to market their products in the EU must obtain CE marking, which involves a conformity assessment process and compliance with essential requirements.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates medical devices, including valve technologies. The regulatory process involves premarket approval and post-market surveillance, with specific requirements for foreign manufacturers.
Emerging markets, such as China and India, are developing their regulatory frameworks for medical devices. These countries are increasingly aligning their regulations with international standards while addressing local market needs and healthcare priorities.
As solenoid valve technologies continue to advance and find new applications in medical innovations, regulatory bodies are adapting their frameworks to keep pace. This includes addressing emerging technologies like smart valves, miniaturized devices, and those incorporating artificial intelligence or machine learning algorithms.
The FDA's regulatory process for medical valve devices involves several key components. Manufacturers must demonstrate that their devices are substantially equivalent to a legally marketed predicate device in terms of safety and effectiveness. This process includes submitting detailed technical documentation, performance data, and clinical evidence when necessary.
Quality System Regulation (QSR) compliance is another critical aspect of the regulatory framework. Manufacturers must establish and maintain quality management systems that meet the requirements outlined in 21 CFR Part 820. This includes design controls, process validation, and risk management procedures specific to medical valve devices.
Post-market surveillance is an ongoing requirement for manufacturers of medical valve devices. They must monitor and report adverse events, implement corrective and preventive actions when necessary, and conduct periodic safety reviews to ensure continued compliance with regulatory standards.
Internationally, the regulatory landscape for medical valve devices varies. The European Union's Medical Device Regulation (MDR) provides a comprehensive framework for medical devices, including solenoid valves. Manufacturers seeking to market their products in the EU must obtain CE marking, which involves a conformity assessment process and compliance with essential requirements.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates medical devices, including valve technologies. The regulatory process involves premarket approval and post-market surveillance, with specific requirements for foreign manufacturers.
Emerging markets, such as China and India, are developing their regulatory frameworks for medical devices. These countries are increasingly aligning their regulations with international standards while addressing local market needs and healthcare priorities.
As solenoid valve technologies continue to advance and find new applications in medical innovations, regulatory bodies are adapting their frameworks to keep pace. This includes addressing emerging technologies like smart valves, miniaturized devices, and those incorporating artificial intelligence or machine learning algorithms.
Environmental Impact of Valve Manufacturing
The manufacturing of solenoid valves, while crucial for medical innovations, carries significant environmental implications. The production process involves various materials and energy-intensive steps that contribute to environmental concerns. Primarily, the manufacturing of valve components requires metals like brass, stainless steel, and aluminum, which are extracted through mining operations. These activities often lead to habitat destruction, soil erosion, and water pollution in mining areas.
The machining and fabrication of valve parts consume substantial energy, contributing to greenhouse gas emissions. Precision engineering processes, such as CNC machining and metal forming, require high-powered equipment that relies heavily on electricity, often sourced from fossil fuels. Additionally, the use of cutting fluids and lubricants in these processes can result in hazardous waste that requires careful disposal to prevent soil and water contamination.
Surface treatments and coatings applied to valve components for corrosion resistance and improved performance also pose environmental challenges. Processes like electroplating and anodizing involve chemicals that can be harmful if not properly managed. The disposal of these chemicals and the treatment of wastewater from these processes are critical environmental considerations in valve manufacturing.
The production of electronic components for solenoid actuation introduces another layer of environmental impact. The electronics industry is known for its use of rare earth elements and toxic materials in circuit boards and semiconductors. The extraction and processing of these materials can lead to significant environmental degradation and pollution if not carefully regulated.
Packaging and transportation of finished valves also contribute to the environmental footprint. The use of plastic packaging materials and the carbon emissions from shipping add to the overall environmental impact of valve production and distribution.
However, it's important to note that advancements in manufacturing technologies are helping to mitigate some of these environmental concerns. Lean manufacturing principles, energy-efficient machinery, and improved waste management systems are being adopted by many valve manufacturers to reduce their environmental impact. Additionally, the development of more durable and efficient valve designs can lead to longer product lifespans and reduced need for replacements, indirectly benefiting the environment.
As the medical industry continues to rely on solenoid valve technologies for breakthrough innovations, there is a growing emphasis on sustainable manufacturing practices. This includes the use of recycled materials, implementation of closed-loop manufacturing systems, and the adoption of renewable energy sources in production facilities. These efforts aim to balance the critical need for medical advancements with responsible environmental stewardship in valve manufacturing.
The machining and fabrication of valve parts consume substantial energy, contributing to greenhouse gas emissions. Precision engineering processes, such as CNC machining and metal forming, require high-powered equipment that relies heavily on electricity, often sourced from fossil fuels. Additionally, the use of cutting fluids and lubricants in these processes can result in hazardous waste that requires careful disposal to prevent soil and water contamination.
Surface treatments and coatings applied to valve components for corrosion resistance and improved performance also pose environmental challenges. Processes like electroplating and anodizing involve chemicals that can be harmful if not properly managed. The disposal of these chemicals and the treatment of wastewater from these processes are critical environmental considerations in valve manufacturing.
The production of electronic components for solenoid actuation introduces another layer of environmental impact. The electronics industry is known for its use of rare earth elements and toxic materials in circuit boards and semiconductors. The extraction and processing of these materials can lead to significant environmental degradation and pollution if not carefully regulated.
Packaging and transportation of finished valves also contribute to the environmental footprint. The use of plastic packaging materials and the carbon emissions from shipping add to the overall environmental impact of valve production and distribution.
However, it's important to note that advancements in manufacturing technologies are helping to mitigate some of these environmental concerns. Lean manufacturing principles, energy-efficient machinery, and improved waste management systems are being adopted by many valve manufacturers to reduce their environmental impact. Additionally, the development of more durable and efficient valve designs can lead to longer product lifespans and reduced need for replacements, indirectly benefiting the environment.
As the medical industry continues to rely on solenoid valve technologies for breakthrough innovations, there is a growing emphasis on sustainable manufacturing practices. This includes the use of recycled materials, implementation of closed-loop manufacturing systems, and the adoption of renewable energy sources in production facilities. These efforts aim to balance the critical need for medical advancements with responsible environmental stewardship in valve manufacturing.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!