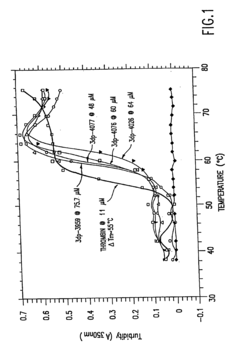
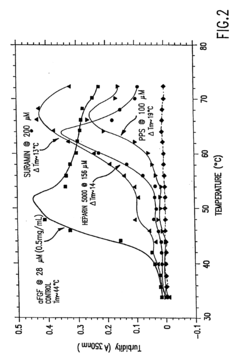
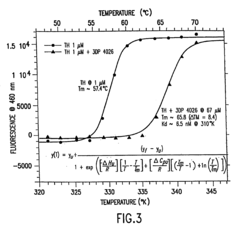
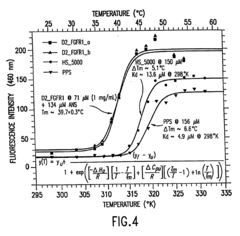
Investigation of Glycerol's Role in Protein Folding Mechanisms
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycerol and Protein Folding: Background and Objectives
Protein folding is a fundamental process in molecular biology, crucial for the proper functioning of cells and organisms. Over the past decades, researchers have been investigating various factors that influence this complex mechanism. Among these factors, glycerol has emerged as a significant player in protein folding dynamics. This technical report aims to explore the role of glycerol in protein folding mechanisms, providing a comprehensive overview of the field's development and setting clear objectives for future research.
The study of protein folding dates back to the 1960s when Christian Anfinsen demonstrated that the amino acid sequence of a protein contains all the information necessary for it to fold into its native, functional state. Since then, our understanding of protein folding has evolved significantly, incorporating concepts such as energy landscapes, folding funnels, and the importance of molecular chaperones.
Glycerol, a simple polyol compound, has been known to affect protein stability and folding for many years. Initially, it was primarily used as a cryoprotectant in protein storage and crystallization. However, its role in protein folding mechanisms has gained increasing attention in recent years, as researchers have observed its ability to modulate protein conformations and influence folding pathways.
The evolution of technology has played a crucial role in advancing our understanding of glycerol's impact on protein folding. High-resolution structural techniques, such as X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy, have allowed researchers to visualize protein structures in the presence of glycerol. Additionally, advanced computational methods, including molecular dynamics simulations, have provided insights into the molecular interactions between glycerol and proteins during the folding process.
The primary objective of this investigation is to elucidate the mechanisms by which glycerol influences protein folding. This includes understanding how glycerol affects the thermodynamics and kinetics of folding, its impact on intermediate states, and its role in preventing protein aggregation. Furthermore, we aim to explore the potential applications of these findings in biotechnology, pharmaceuticals, and medicine.
Another key goal is to identify the molecular basis of glycerol's effects on protein folding. This involves studying the interactions between glycerol and various amino acid residues, as well as its impact on water structure and hydrophobic interactions within proteins. By understanding these fundamental aspects, we can potentially develop strategies to manipulate protein folding processes for various applications.
Lastly, this investigation seeks to establish a framework for using glycerol as a tool in protein folding studies. This includes developing standardized protocols for incorporating glycerol in experimental setups, as well as guidelines for interpreting results in the context of glycerol's presence. Such a framework would facilitate more consistent and comparable research across different laboratories and protein systems.
The study of protein folding dates back to the 1960s when Christian Anfinsen demonstrated that the amino acid sequence of a protein contains all the information necessary for it to fold into its native, functional state. Since then, our understanding of protein folding has evolved significantly, incorporating concepts such as energy landscapes, folding funnels, and the importance of molecular chaperones.
Glycerol, a simple polyol compound, has been known to affect protein stability and folding for many years. Initially, it was primarily used as a cryoprotectant in protein storage and crystallization. However, its role in protein folding mechanisms has gained increasing attention in recent years, as researchers have observed its ability to modulate protein conformations and influence folding pathways.
The evolution of technology has played a crucial role in advancing our understanding of glycerol's impact on protein folding. High-resolution structural techniques, such as X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy, have allowed researchers to visualize protein structures in the presence of glycerol. Additionally, advanced computational methods, including molecular dynamics simulations, have provided insights into the molecular interactions between glycerol and proteins during the folding process.
The primary objective of this investigation is to elucidate the mechanisms by which glycerol influences protein folding. This includes understanding how glycerol affects the thermodynamics and kinetics of folding, its impact on intermediate states, and its role in preventing protein aggregation. Furthermore, we aim to explore the potential applications of these findings in biotechnology, pharmaceuticals, and medicine.
Another key goal is to identify the molecular basis of glycerol's effects on protein folding. This involves studying the interactions between glycerol and various amino acid residues, as well as its impact on water structure and hydrophobic interactions within proteins. By understanding these fundamental aspects, we can potentially develop strategies to manipulate protein folding processes for various applications.
Lastly, this investigation seeks to establish a framework for using glycerol as a tool in protein folding studies. This includes developing standardized protocols for incorporating glycerol in experimental setups, as well as guidelines for interpreting results in the context of glycerol's presence. Such a framework would facilitate more consistent and comparable research across different laboratories and protein systems.
Market Demand for Protein Folding Research
The market demand for protein folding research has been steadily increasing in recent years, driven by its critical importance in various fields, including pharmaceuticals, biotechnology, and materials science. The global protein engineering market, which heavily relies on protein folding research, was valued at $2.2 billion in 2020 and is projected to reach $3.8 billion by 2027, growing at a CAGR of 12.4% during this period.
Pharmaceutical companies are particularly interested in protein folding research due to its potential in drug discovery and development. Understanding protein folding mechanisms can lead to more effective and targeted therapies for diseases such as Alzheimer's, Parkinson's, and various cancers. The global pharmaceutical market, valued at $1.27 trillion in 2020, is expected to grow significantly, with protein-based drugs playing an increasingly important role.
Biotechnology firms are also driving demand for protein folding research. The ability to engineer proteins with specific functions has applications in industrial enzymes, biofuels, and sustainable materials production. The global industrial enzymes market, a key beneficiary of protein folding research, is expected to reach $7.1 billion by 2025, growing at a CAGR of 6.5%.
Academic institutions and research organizations continue to invest heavily in protein folding research, contributing to the overall market demand. Government funding for basic science research, including protein folding studies, has seen an uptick in many countries, recognizing its potential impact on healthcare and biotechnology advancements.
The food and beverage industry is another sector showing increased interest in protein folding research. As plant-based protein alternatives gain popularity, understanding protein folding mechanisms becomes crucial for developing products with improved texture and nutritional profiles. The global plant-based protein market is projected to reach $15.6 billion by 2026, growing at a CAGR of 7.2%.
Emerging technologies such as artificial intelligence and machine learning are creating new opportunities in protein folding research. Companies like DeepMind have demonstrated the potential of AI in predicting protein structures, which could accelerate drug discovery and protein engineering processes. This technological convergence is expected to further boost market demand for protein folding research in the coming years.
The COVID-19 pandemic has also highlighted the importance of protein folding research in developing vaccines and antiviral therapies. This has led to increased funding and interest in the field, which is likely to have a lasting impact on market demand even beyond the immediate crisis.
Pharmaceutical companies are particularly interested in protein folding research due to its potential in drug discovery and development. Understanding protein folding mechanisms can lead to more effective and targeted therapies for diseases such as Alzheimer's, Parkinson's, and various cancers. The global pharmaceutical market, valued at $1.27 trillion in 2020, is expected to grow significantly, with protein-based drugs playing an increasingly important role.
Biotechnology firms are also driving demand for protein folding research. The ability to engineer proteins with specific functions has applications in industrial enzymes, biofuels, and sustainable materials production. The global industrial enzymes market, a key beneficiary of protein folding research, is expected to reach $7.1 billion by 2025, growing at a CAGR of 6.5%.
Academic institutions and research organizations continue to invest heavily in protein folding research, contributing to the overall market demand. Government funding for basic science research, including protein folding studies, has seen an uptick in many countries, recognizing its potential impact on healthcare and biotechnology advancements.
The food and beverage industry is another sector showing increased interest in protein folding research. As plant-based protein alternatives gain popularity, understanding protein folding mechanisms becomes crucial for developing products with improved texture and nutritional profiles. The global plant-based protein market is projected to reach $15.6 billion by 2026, growing at a CAGR of 7.2%.
Emerging technologies such as artificial intelligence and machine learning are creating new opportunities in protein folding research. Companies like DeepMind have demonstrated the potential of AI in predicting protein structures, which could accelerate drug discovery and protein engineering processes. This technological convergence is expected to further boost market demand for protein folding research in the coming years.
The COVID-19 pandemic has also highlighted the importance of protein folding research in developing vaccines and antiviral therapies. This has led to increased funding and interest in the field, which is likely to have a lasting impact on market demand even beyond the immediate crisis.
Current Understanding and Challenges in Glycerol-Protein Interactions
The current understanding of glycerol's role in protein folding mechanisms has advanced significantly in recent years, yet several challenges remain. Glycerol, a simple polyol, has been recognized as an important osmolyte that can influence protein stability and folding dynamics. Research has shown that glycerol can act as a chemical chaperone, assisting in the correct folding of proteins and preventing aggregation.
One of the key findings is that glycerol preferentially interacts with the protein backbone, stabilizing the native state of proteins. This interaction is believed to occur through a mechanism known as preferential exclusion, where glycerol is excluded from the protein surface, leading to a preferential hydration of the protein. This effect increases the free energy of the denatured state more than that of the native state, thereby shifting the equilibrium towards the folded conformation.
Studies have also revealed that glycerol can slow down protein folding kinetics. This deceleration is thought to be due to the increased viscosity of the solution, which reduces the rate of conformational changes necessary for folding. However, this slower folding process may actually enhance the overall yield of correctly folded proteins by allowing more time for proper interactions to form.
Despite these insights, several challenges persist in fully understanding glycerol-protein interactions. One major difficulty lies in elucidating the precise molecular mechanisms by which glycerol influences protein folding at different stages of the process. The complex interplay between glycerol, water, and various protein conformations during folding remains incompletely understood.
Another challenge is quantifying the concentration-dependent effects of glycerol on protein stability and folding. While it is known that glycerol's impact varies with concentration, developing accurate models to predict these effects across different protein types and environmental conditions is still an ongoing effort.
Furthermore, the role of glycerol in modulating protein-protein interactions and its impact on the formation of functional protein complexes is an area that requires further investigation. Understanding these aspects is crucial for applications in biotechnology and pharmaceutical industries, where protein stability and proper folding are critical.
Lastly, there is a need to develop more sophisticated experimental techniques and computational models to study glycerol-protein interactions at a molecular level. Advanced spectroscopic methods, high-resolution structural studies, and molecular dynamics simulations are being employed to address this challenge, but integrating these diverse approaches to form a cohesive understanding remains a significant hurdle in the field.
One of the key findings is that glycerol preferentially interacts with the protein backbone, stabilizing the native state of proteins. This interaction is believed to occur through a mechanism known as preferential exclusion, where glycerol is excluded from the protein surface, leading to a preferential hydration of the protein. This effect increases the free energy of the denatured state more than that of the native state, thereby shifting the equilibrium towards the folded conformation.
Studies have also revealed that glycerol can slow down protein folding kinetics. This deceleration is thought to be due to the increased viscosity of the solution, which reduces the rate of conformational changes necessary for folding. However, this slower folding process may actually enhance the overall yield of correctly folded proteins by allowing more time for proper interactions to form.
Despite these insights, several challenges persist in fully understanding glycerol-protein interactions. One major difficulty lies in elucidating the precise molecular mechanisms by which glycerol influences protein folding at different stages of the process. The complex interplay between glycerol, water, and various protein conformations during folding remains incompletely understood.
Another challenge is quantifying the concentration-dependent effects of glycerol on protein stability and folding. While it is known that glycerol's impact varies with concentration, developing accurate models to predict these effects across different protein types and environmental conditions is still an ongoing effort.
Furthermore, the role of glycerol in modulating protein-protein interactions and its impact on the formation of functional protein complexes is an area that requires further investigation. Understanding these aspects is crucial for applications in biotechnology and pharmaceutical industries, where protein stability and proper folding are critical.
Lastly, there is a need to develop more sophisticated experimental techniques and computational models to study glycerol-protein interactions at a molecular level. Advanced spectroscopic methods, high-resolution structural studies, and molecular dynamics simulations are being employed to address this challenge, but integrating these diverse approaches to form a cohesive understanding remains a significant hurdle in the field.
Existing Methodologies for Studying Glycerol's Effects
01 Glycerol as a stabilizing agent for protein folding
Glycerol is used as a stabilizing agent in protein folding processes. It helps maintain the native structure of proteins, prevents aggregation, and enhances the efficiency of protein folding. The addition of glycerol to protein solutions can improve their stability during various experimental procedures and storage conditions.- Glycerol as a protein stabilizer: Glycerol is used as a stabilizing agent in protein folding processes. It helps maintain the native structure of proteins, preventing denaturation and aggregation. This property makes glycerol valuable in various biotechnological applications, including the production and storage of therapeutic proteins.
- Glycerol in protein crystallization: Glycerol plays a role in protein crystallization techniques. It can be used as a cryoprotectant during the freezing of protein crystals for X-ray diffraction studies. Additionally, glycerol can influence the crystal formation process, potentially improving the quality of protein crystals obtained for structural analysis.
- Glycerol's effect on protein conformation: The presence of glycerol can influence protein conformation and folding pathways. It affects the hydration shell around proteins, potentially altering their three-dimensional structure. This property is utilized in studying protein folding mechanisms and in the development of protein-based therapeutics.
- Glycerol in protein formulations: Glycerol is commonly used in protein formulations to enhance stability and prevent aggregation. It acts as a cosolvent, modifying the protein's environment and influencing its folding behavior. This application is particularly important in the pharmaceutical industry for the development of stable protein-based drugs.
- Glycerol's role in protein refolding: Glycerol can assist in the refolding of denatured proteins. It helps create an environment conducive to proper protein folding, potentially increasing the yield of correctly folded proteins. This property is valuable in biotechnological processes where protein refolding is necessary, such as in the production of recombinant proteins.
02 Glycerol in protein crystallization and structure determination
Glycerol plays a role in protein crystallization techniques and structure determination methods. It can be used as a cryoprotectant during X-ray crystallography experiments and helps in obtaining high-quality protein crystals. The presence of glycerol can also affect the crystal packing and diffraction quality of protein samples.Expand Specific Solutions03 Glycerol's impact on protein-ligand interactions
The presence of glycerol can influence protein-ligand interactions and binding studies. It may affect the binding affinity and kinetics of protein-ligand complexes. Researchers consider the impact of glycerol when designing and interpreting protein-ligand interaction experiments, especially in drug discovery and development processes.Expand Specific Solutions04 Glycerol in protein formulations and storage
Glycerol is used in protein formulations to enhance stability during storage and prevent denaturation. It acts as a cryoprotectant and helps maintain protein structure during freeze-thaw cycles. The addition of glycerol to protein solutions can extend shelf life and preserve biological activity of therapeutic proteins and enzymes.Expand Specific Solutions05 Glycerol's role in membrane protein folding and stability
Glycerol has a specific impact on the folding and stability of membrane proteins. It can help maintain the native conformation of membrane proteins during extraction, purification, and reconstitution processes. The presence of glycerol in membrane protein studies can affect their functional properties and structural integrity.Expand Specific Solutions
Key Players in Protein Folding Research
The investigation of glycerol's role in protein folding mechanisms is currently in a nascent stage of development, with a growing market potential as the importance of protein folding in various diseases becomes more apparent. The field is characterized by a mix of academic institutions and biotechnology companies, indicating an emerging competitive landscape. Key players like The Scripps Research Institute, Massachusetts Institute of Technology, and Life Technologies Corp. are at the forefront of research, leveraging their expertise in biochemistry and molecular biology. The technology is still in the early stages of maturity, with most efforts focused on fundamental research rather than commercial applications. As the understanding of protein folding mechanisms deepens, we can expect increased interest from pharmaceutical companies like AbbVie and Genentech, potentially leading to a more competitive and commercially-oriented market in the future.
The Scripps Research Institute
Technical Solution: The Scripps Research Institute has developed advanced techniques for investigating glycerol's role in protein folding mechanisms. They utilize nuclear magnetic resonance (NMR) spectroscopy and molecular dynamics simulations to study the effects of glycerol on protein structure and stability[1]. Their research has shown that glycerol acts as an osmolyte, stabilizing protein conformations by preferential exclusion from the protein surface[2]. This mechanism helps prevent protein aggregation and misfolding. The institute has also explored the use of glycerol in combination with other osmolytes to enhance protein stability in various biotechnological applications[3].
Strengths: Cutting-edge research techniques, comprehensive understanding of glycerol's molecular interactions. Weaknesses: Potential limitations in translating findings to large-scale industrial applications.
Massachusetts Institute of Technology
Technical Solution: MIT has developed innovative approaches to study glycerol's role in protein folding. They employ single-molecule fluorescence resonance energy transfer (FRET) techniques to observe protein folding in real-time under various glycerol concentrations[4]. Their research has revealed that glycerol can slow down protein folding kinetics while increasing the overall stability of the folded state[5]. MIT researchers have also investigated the molecular mechanisms by which glycerol affects the hydration shell around proteins, providing insights into its role as a chemical chaperone[6]. Additionally, they have explored the use of glycerol in preserving protein function during freeze-drying processes, which has significant implications for biopharmaceutical storage and transport[7].
Strengths: Advanced single-molecule techniques, multidisciplinary approach combining physics and biology. Weaknesses: May focus more on fundamental research rather than immediate industrial applications.
Critical Insights into Glycerol-Mediated Protein Folding
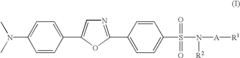
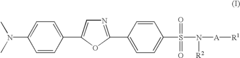
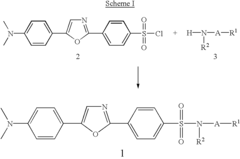
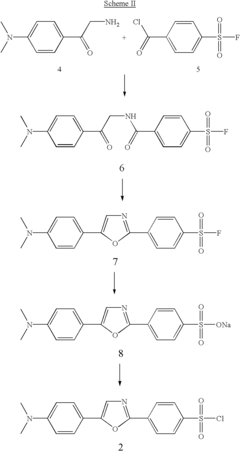
Substituted diphenyloxazoles, the synthesis thereof, and the use thereof as fluorescence probes
PatentInactiveUS6815445B2
Innovation
- Development of new derivatives of 2-(4'-sulfamoylphenyl)-5-(4'-dimethylaminophenyl)oxazoles with improved solubility and emission spectra, allowing for their use as fluorescence probes that emit at longer wavelengths, reducing background interference and enhancing sensitivity in thermal shift assays and high-throughput screening.
Microplate thermal shift assay apparatus for ligand development and multi-variable protein chemistry optimization
PatentInactiveUS6849458B2
Innovation
- A multi-variable method for simultaneously heating multiple samples in microplate thermal shift assays to generate thermal denaturation curves, allowing for the ranking of molecules and biochemical conditions based on their effect on protein stability and ligand binding, applicable to all receptor types, including reversibly folding proteins.
Biophysical Techniques for Protein Folding Analysis
Biophysical techniques play a crucial role in unraveling the complex mechanisms of protein folding, particularly in the context of investigating glycerol's influence on this process. These techniques provide valuable insights into the structural changes, kinetics, and thermodynamics of protein folding, enabling researchers to elucidate the intricate interplay between proteins and their environment.
Circular dichroism (CD) spectroscopy is a widely used technique for studying protein secondary structure and folding dynamics. By measuring the differential absorption of left- and right-handed circularly polarized light, CD spectroscopy can detect changes in protein conformation induced by glycerol. This method is particularly useful for monitoring the formation of α-helices and β-sheets during the folding process, allowing researchers to assess how glycerol affects the stability and kinetics of these structural elements.
Fluorescence spectroscopy offers another powerful tool for investigating protein folding mechanisms in the presence of glycerol. This technique exploits the intrinsic fluorescence of aromatic amino acids, such as tryptophan, to probe local environmental changes during folding. By monitoring shifts in fluorescence intensity and emission wavelength, researchers can gain insights into the solvent accessibility of specific protein regions and track the formation of tertiary structure in real-time.
Nuclear magnetic resonance (NMR) spectroscopy provides atomic-level resolution of protein structure and dynamics. In the context of glycerol-induced folding, NMR can reveal detailed information about local conformational changes, hydrogen bonding patterns, and the formation of specific structural motifs. Moreover, NMR relaxation experiments can elucidate the dynamics of protein folding intermediates and characterize the energy landscape of the folding process in the presence of glycerol.
Fourier-transform infrared (FTIR) spectroscopy is particularly useful for studying protein secondary structure and aggregation. By analyzing the absorption of infrared radiation by peptide bonds, FTIR can provide information about the overall secondary structure content of proteins and detect changes induced by glycerol. This technique is especially valuable for monitoring β-sheet formation and protein aggregation, which are critical aspects of protein folding and misfolding processes.
Small-angle X-ray scattering (SAXS) and small-angle neutron scattering (SANS) techniques offer insights into the overall shape and size of proteins during folding. These methods can reveal how glycerol affects the compactness and globularity of protein conformations, providing valuable information about the folding pathway and potential intermediate states. By combining SAXS or SANS with other biophysical techniques, researchers can build a comprehensive picture of protein folding mechanisms in the presence of glycerol.
Single-molecule techniques, such as single-molecule Förster resonance energy transfer (smFRET) and optical tweezers, have revolutionized the study of protein folding by allowing researchers to observe individual molecules as they fold. These methods can provide unique insights into the heterogeneity of folding pathways and the influence of glycerol on folding kinetics at the single-molecule level, revealing details that may be obscured in ensemble measurements.
Circular dichroism (CD) spectroscopy is a widely used technique for studying protein secondary structure and folding dynamics. By measuring the differential absorption of left- and right-handed circularly polarized light, CD spectroscopy can detect changes in protein conformation induced by glycerol. This method is particularly useful for monitoring the formation of α-helices and β-sheets during the folding process, allowing researchers to assess how glycerol affects the stability and kinetics of these structural elements.
Fluorescence spectroscopy offers another powerful tool for investigating protein folding mechanisms in the presence of glycerol. This technique exploits the intrinsic fluorescence of aromatic amino acids, such as tryptophan, to probe local environmental changes during folding. By monitoring shifts in fluorescence intensity and emission wavelength, researchers can gain insights into the solvent accessibility of specific protein regions and track the formation of tertiary structure in real-time.
Nuclear magnetic resonance (NMR) spectroscopy provides atomic-level resolution of protein structure and dynamics. In the context of glycerol-induced folding, NMR can reveal detailed information about local conformational changes, hydrogen bonding patterns, and the formation of specific structural motifs. Moreover, NMR relaxation experiments can elucidate the dynamics of protein folding intermediates and characterize the energy landscape of the folding process in the presence of glycerol.
Fourier-transform infrared (FTIR) spectroscopy is particularly useful for studying protein secondary structure and aggregation. By analyzing the absorption of infrared radiation by peptide bonds, FTIR can provide information about the overall secondary structure content of proteins and detect changes induced by glycerol. This technique is especially valuable for monitoring β-sheet formation and protein aggregation, which are critical aspects of protein folding and misfolding processes.
Small-angle X-ray scattering (SAXS) and small-angle neutron scattering (SANS) techniques offer insights into the overall shape and size of proteins during folding. These methods can reveal how glycerol affects the compactness and globularity of protein conformations, providing valuable information about the folding pathway and potential intermediate states. By combining SAXS or SANS with other biophysical techniques, researchers can build a comprehensive picture of protein folding mechanisms in the presence of glycerol.
Single-molecule techniques, such as single-molecule Förster resonance energy transfer (smFRET) and optical tweezers, have revolutionized the study of protein folding by allowing researchers to observe individual molecules as they fold. These methods can provide unique insights into the heterogeneity of folding pathways and the influence of glycerol on folding kinetics at the single-molecule level, revealing details that may be obscured in ensemble measurements.
Implications for Pharmaceutical and Biotechnology Industries
The investigation of glycerol's role in protein folding mechanisms has significant implications for the pharmaceutical and biotechnology industries. This research opens up new avenues for drug development, protein-based therapeutics, and industrial enzyme production.
In the pharmaceutical sector, understanding glycerol's impact on protein folding can lead to improved formulation strategies for biopharmaceuticals. Many protein-based drugs, such as monoclonal antibodies and recombinant proteins, are sensitive to their environment during production, storage, and administration. By leveraging glycerol's stabilizing properties, pharmaceutical companies can potentially enhance the shelf-life and efficacy of these products.
For the biotechnology industry, this research can revolutionize the production of industrial enzymes. Many industrial processes rely on enzymes for catalyzing reactions, and maintaining enzyme stability is crucial for efficiency and cost-effectiveness. Incorporating glycerol into enzyme formulations or production processes could lead to more robust and long-lasting enzymatic products, benefiting industries ranging from food processing to biofuel production.
The findings from glycerol-protein folding studies also have implications for the development of novel protein engineering techniques. Biotechnology companies could use this knowledge to design proteins with enhanced stability or specific folding characteristics, opening up possibilities for creating new biomaterials or improving existing ones.
In the field of personalized medicine, understanding protein folding mechanisms in the presence of glycerol could aid in developing targeted therapies for diseases caused by protein misfolding, such as Alzheimer's or Parkinson's. This research might lead to new approaches for preventing or reversing protein aggregation, potentially resulting in groundbreaking treatments.
Moreover, the insights gained from this investigation could impact the production of biosimilars – generic versions of biologic drugs. As the biosimilar market continues to grow, manufacturers could utilize glycerol-related strategies to improve the stability and comparability of their products to the original biologics.
Lastly, this research could influence the development of new analytical tools and techniques for assessing protein stability and quality in both pharmaceutical and biotechnology settings. Improved methods for monitoring protein folding and stability could enhance quality control processes, leading to more consistent and reliable products across these industries.
In the pharmaceutical sector, understanding glycerol's impact on protein folding can lead to improved formulation strategies for biopharmaceuticals. Many protein-based drugs, such as monoclonal antibodies and recombinant proteins, are sensitive to their environment during production, storage, and administration. By leveraging glycerol's stabilizing properties, pharmaceutical companies can potentially enhance the shelf-life and efficacy of these products.
For the biotechnology industry, this research can revolutionize the production of industrial enzymes. Many industrial processes rely on enzymes for catalyzing reactions, and maintaining enzyme stability is crucial for efficiency and cost-effectiveness. Incorporating glycerol into enzyme formulations or production processes could lead to more robust and long-lasting enzymatic products, benefiting industries ranging from food processing to biofuel production.
The findings from glycerol-protein folding studies also have implications for the development of novel protein engineering techniques. Biotechnology companies could use this knowledge to design proteins with enhanced stability or specific folding characteristics, opening up possibilities for creating new biomaterials or improving existing ones.
In the field of personalized medicine, understanding protein folding mechanisms in the presence of glycerol could aid in developing targeted therapies for diseases caused by protein misfolding, such as Alzheimer's or Parkinson's. This research might lead to new approaches for preventing or reversing protein aggregation, potentially resulting in groundbreaking treatments.
Moreover, the insights gained from this investigation could impact the production of biosimilars – generic versions of biologic drugs. As the biosimilar market continues to grow, manufacturers could utilize glycerol-related strategies to improve the stability and comparability of their products to the original biologics.
Lastly, this research could influence the development of new analytical tools and techniques for assessing protein stability and quality in both pharmaceutical and biotechnology settings. Improved methods for monitoring protein folding and stability could enhance quality control processes, leading to more consistent and reliable products across these industries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!