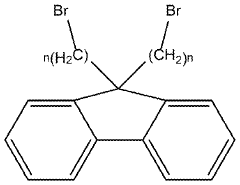
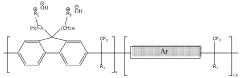
Ionomer Chemistry Advances For High-Conductivity AEM Membranes
AUG 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ionomer Chemistry Background and Objectives
Anion Exchange Membrane (AEM) technology has emerged as a promising alternative to traditional proton exchange membranes in electrochemical devices over the past three decades. The evolution of ionomer chemistry for AEM development has progressed through several distinct phases, beginning with the adaptation of hydrocarbon polymers in the 1990s, followed by the introduction of quaternary ammonium functionalized polymers in the early 2000s, and more recently advancing toward multi-cation and heterocyclic chemistry approaches.
The fundamental challenge in AEM development lies in achieving the delicate balance between high ionic conductivity and chemical stability under alkaline conditions. Early AEM materials suffered from significant degradation via Hofmann elimination and nucleophilic substitution reactions, limiting their practical application despite promising theoretical advantages over proton exchange membranes.
Recent technological breakthroughs have focused on novel ionomer chemistries that can withstand high pH environments while maintaining sufficient hydroxide ion conductivity. The development of sterically hindered cations, such as tris(2,4,6-trimethoxyphenyl)phosphonium and N-spirocyclic quaternary ammonium groups, has significantly improved alkaline stability. Concurrently, advances in polymer backbone design have enhanced mechanical durability and water management capabilities.
The primary objective of current research in ionomer chemistry for AEMs is to achieve hydroxide conductivity exceeding 100 mS/cm under practical operating conditions while maintaining chemical stability for over 10,000 hours of operation. This represents approximately a five-fold improvement over the best commercially available membranes today. Secondary objectives include reducing membrane swelling, improving mechanical properties at elevated temperatures, and developing scalable synthesis methods suitable for industrial production.
Global research trends indicate a shift toward multi-functional ionomers that incorporate both cationic groups for ion conduction and additional functional moieties for enhanced stability or self-healing properties. Computational chemistry and high-throughput experimental approaches are increasingly being employed to accelerate the discovery of novel ionomer structures with optimized properties.
The technological trajectory suggests that breakthrough innovations in AEM chemistry could enable a new generation of electrochemical devices, including alkaline fuel cells, electrolyzers, and flow batteries with significantly improved efficiency and reduced costs. These advances would directly support broader energy transition goals by enabling more efficient hydrogen production, utilization, and storage systems that do not rely on precious metal catalysts.
The fundamental challenge in AEM development lies in achieving the delicate balance between high ionic conductivity and chemical stability under alkaline conditions. Early AEM materials suffered from significant degradation via Hofmann elimination and nucleophilic substitution reactions, limiting their practical application despite promising theoretical advantages over proton exchange membranes.
Recent technological breakthroughs have focused on novel ionomer chemistries that can withstand high pH environments while maintaining sufficient hydroxide ion conductivity. The development of sterically hindered cations, such as tris(2,4,6-trimethoxyphenyl)phosphonium and N-spirocyclic quaternary ammonium groups, has significantly improved alkaline stability. Concurrently, advances in polymer backbone design have enhanced mechanical durability and water management capabilities.
The primary objective of current research in ionomer chemistry for AEMs is to achieve hydroxide conductivity exceeding 100 mS/cm under practical operating conditions while maintaining chemical stability for over 10,000 hours of operation. This represents approximately a five-fold improvement over the best commercially available membranes today. Secondary objectives include reducing membrane swelling, improving mechanical properties at elevated temperatures, and developing scalable synthesis methods suitable for industrial production.
Global research trends indicate a shift toward multi-functional ionomers that incorporate both cationic groups for ion conduction and additional functional moieties for enhanced stability or self-healing properties. Computational chemistry and high-throughput experimental approaches are increasingly being employed to accelerate the discovery of novel ionomer structures with optimized properties.
The technological trajectory suggests that breakthrough innovations in AEM chemistry could enable a new generation of electrochemical devices, including alkaline fuel cells, electrolyzers, and flow batteries with significantly improved efficiency and reduced costs. These advances would directly support broader energy transition goals by enabling more efficient hydrogen production, utilization, and storage systems that do not rely on precious metal catalysts.
Market Analysis for AEM Membrane Applications
The global market for anion exchange membrane (AEM) technology is experiencing significant growth, driven by increasing demand for clean energy solutions and sustainable industrial processes. The AEM market was valued at approximately $120 million in 2022 and is projected to reach $320 million by 2028, representing a compound annual growth rate of 17.8%. This growth trajectory is primarily fueled by applications in fuel cells, water electrolysis, and industrial separation processes.
In the energy sector, AEM fuel cells are gaining traction as viable alternatives to proton exchange membrane (PEM) fuel cells due to their potential cost advantages and reduced dependence on precious metal catalysts. The market for AEM fuel cells is expected to grow from $45 million in 2022 to $150 million by 2028, with automotive and stationary power generation applications leading this expansion.
Water electrolysis represents another significant market segment for AEM technology, particularly as green hydrogen production becomes increasingly central to global decarbonization strategies. The AEM electrolyzer market is currently smaller than its PEM counterpart but is growing at a faster rate of 22% annually due to technological advancements in ionomer chemistry that are improving conductivity and durability.
Industrial applications, including wastewater treatment, desalination, and chemical processing, constitute approximately 30% of the current AEM market. These sectors value the selective ion transport capabilities of high-conductivity AEM membranes for efficient separation processes and are expected to maintain steady growth at 15% annually through 2028.
Geographically, North America and Europe currently dominate the AEM market with combined market share of 65%, largely due to aggressive clean energy policies and substantial R&D investments. However, the Asia-Pacific region is emerging as the fastest-growing market with 25% annual growth, driven by China's ambitious hydrogen economy initiatives and Japan's fuel cell deployment programs.
Market adoption barriers include the relatively higher cost of advanced ionomers, durability concerns in real-world applications, and competition from established technologies. However, recent breakthroughs in quaternary ammonium functionalized polymers and metal-organic framework (MOF) composite membranes are addressing these challenges and expanding the potential application landscape.
The competitive landscape features established membrane manufacturers expanding into AEM technology alongside specialized startups focused exclusively on high-conductivity AEM development. Strategic partnerships between material science companies and end-users are increasingly common, accelerating commercialization timelines and market penetration for advanced ionomer chemistries.
In the energy sector, AEM fuel cells are gaining traction as viable alternatives to proton exchange membrane (PEM) fuel cells due to their potential cost advantages and reduced dependence on precious metal catalysts. The market for AEM fuel cells is expected to grow from $45 million in 2022 to $150 million by 2028, with automotive and stationary power generation applications leading this expansion.
Water electrolysis represents another significant market segment for AEM technology, particularly as green hydrogen production becomes increasingly central to global decarbonization strategies. The AEM electrolyzer market is currently smaller than its PEM counterpart but is growing at a faster rate of 22% annually due to technological advancements in ionomer chemistry that are improving conductivity and durability.
Industrial applications, including wastewater treatment, desalination, and chemical processing, constitute approximately 30% of the current AEM market. These sectors value the selective ion transport capabilities of high-conductivity AEM membranes for efficient separation processes and are expected to maintain steady growth at 15% annually through 2028.
Geographically, North America and Europe currently dominate the AEM market with combined market share of 65%, largely due to aggressive clean energy policies and substantial R&D investments. However, the Asia-Pacific region is emerging as the fastest-growing market with 25% annual growth, driven by China's ambitious hydrogen economy initiatives and Japan's fuel cell deployment programs.
Market adoption barriers include the relatively higher cost of advanced ionomers, durability concerns in real-world applications, and competition from established technologies. However, recent breakthroughs in quaternary ammonium functionalized polymers and metal-organic framework (MOF) composite membranes are addressing these challenges and expanding the potential application landscape.
The competitive landscape features established membrane manufacturers expanding into AEM technology alongside specialized startups focused exclusively on high-conductivity AEM development. Strategic partnerships between material science companies and end-users are increasingly common, accelerating commercialization timelines and market penetration for advanced ionomer chemistries.
Current Challenges in High-Conductivity AEM Development
Despite significant advancements in anion exchange membrane (AEM) technology, several critical challenges continue to impede the development of high-conductivity AEMs suitable for commercial applications. The most persistent obstacle remains the inherent trade-off between ion conductivity and mechanical stability. As hydroxide conductivity increases, typically achieved through higher ion exchange capacity (IEC), membrane swelling becomes excessive, compromising dimensional stability and mechanical integrity under operating conditions.
Chemical degradation presents another formidable challenge. The hydroxide ions that enable conductivity simultaneously attack the cationic functional groups responsible for anion transport. This degradation is particularly severe at elevated temperatures, limiting operational lifetimes and practical applications. Current state-of-the-art quaternary ammonium functionalities still suffer from nucleophilic displacement and Hofmann elimination reactions in alkaline environments.
Water management within AEMs remains problematic. Unlike proton exchange membranes where water facilitates proton hopping mechanisms, hydroxide transport requires more complex hydration structures. Achieving optimal hydration levels that balance conductivity needs without causing excessive swelling continues to challenge membrane designers. This is particularly evident in fuel cell applications where water generation and consumption occur simultaneously at different electrodes.
Synthetic scalability represents a significant barrier to commercialization. Many high-performance ionomers reported in academic literature involve multi-step syntheses with expensive reagents and environmentally problematic solvents. These complex procedures often yield materials that cannot be manufactured at scale with consistent properties, creating a gap between laboratory demonstrations and industrial implementation.
Interface engineering between the membrane and catalyst layers presents additional complications. Poor interfacial contact leads to increased resistance and performance losses in devices. Current ionomer chemistries often exhibit incompatibility with electrode structures, resulting in delamination and performance degradation over time.
Temperature stability limitations further restrict AEM applications. While higher operating temperatures would benefit reaction kinetics and system efficiency, most current AEM chemistries show accelerated degradation above 60°C. This temperature ceiling significantly constrains system design and operational parameters.
Finally, the fundamental understanding of structure-property relationships in AEM systems remains incomplete. The complex interplay between polymer architecture, functional group distribution, morphology development, and resulting transport properties is not fully characterized. This knowledge gap hampers rational design approaches and necessitates extensive empirical testing of new materials.
Chemical degradation presents another formidable challenge. The hydroxide ions that enable conductivity simultaneously attack the cationic functional groups responsible for anion transport. This degradation is particularly severe at elevated temperatures, limiting operational lifetimes and practical applications. Current state-of-the-art quaternary ammonium functionalities still suffer from nucleophilic displacement and Hofmann elimination reactions in alkaline environments.
Water management within AEMs remains problematic. Unlike proton exchange membranes where water facilitates proton hopping mechanisms, hydroxide transport requires more complex hydration structures. Achieving optimal hydration levels that balance conductivity needs without causing excessive swelling continues to challenge membrane designers. This is particularly evident in fuel cell applications where water generation and consumption occur simultaneously at different electrodes.
Synthetic scalability represents a significant barrier to commercialization. Many high-performance ionomers reported in academic literature involve multi-step syntheses with expensive reagents and environmentally problematic solvents. These complex procedures often yield materials that cannot be manufactured at scale with consistent properties, creating a gap between laboratory demonstrations and industrial implementation.
Interface engineering between the membrane and catalyst layers presents additional complications. Poor interfacial contact leads to increased resistance and performance losses in devices. Current ionomer chemistries often exhibit incompatibility with electrode structures, resulting in delamination and performance degradation over time.
Temperature stability limitations further restrict AEM applications. While higher operating temperatures would benefit reaction kinetics and system efficiency, most current AEM chemistries show accelerated degradation above 60°C. This temperature ceiling significantly constrains system design and operational parameters.
Finally, the fundamental understanding of structure-property relationships in AEM systems remains incomplete. The complex interplay between polymer architecture, functional group distribution, morphology development, and resulting transport properties is not fully characterized. This knowledge gap hampers rational design approaches and necessitates extensive empirical testing of new materials.
Current Ionomer Design Strategies for Conductivity Enhancement
01 Quaternary ammonium functionalized ionomers
Quaternary ammonium functional groups are incorporated into polymer backbones to create anion exchange membranes with enhanced conductivity. These ionomers facilitate hydroxide ion transport through the membrane while maintaining mechanical stability. The quaternary ammonium groups provide fixed positive charges that enable anion conduction, making them suitable for applications in fuel cells and electrolyzers. Various polymer architectures including block copolymers and crosslinked networks can be used as the backbone for these functional groups.- Quaternary ammonium functionalized ionomers for AEM conductivity: Quaternary ammonium functional groups incorporated into polymer backbones are widely used in anion exchange membranes (AEMs) to enhance ionic conductivity. These positively charged groups facilitate hydroxide ion transport through the membrane. The polymer architecture can be optimized by controlling the density and distribution of these functional groups to achieve higher conductivity while maintaining mechanical stability. Various synthetic approaches include direct polymerization of functionalized monomers or post-polymerization modification of base polymers.
- Crosslinking strategies to improve AEM stability and conductivity: Crosslinking techniques are employed to enhance the mechanical and chemical stability of AEMs while maintaining high ionic conductivity. These methods involve creating covalent bonds between polymer chains to form three-dimensional networks that resist swelling and degradation in alkaline environments. Controlled crosslinking density is crucial as excessive crosslinking can restrict ion mobility and reduce conductivity. Various crosslinking agents and methods, including thermal, chemical, and radiation-induced approaches, can be tailored to optimize the balance between stability and conductivity.
- Block copolymer architectures for phase-separated ion channels: Block copolymer architectures enable the formation of nanophase-separated domains that create efficient ion transport channels in AEMs. By combining hydrophobic blocks that provide mechanical stability with hydrophilic ionic blocks that facilitate ion conduction, these materials self-assemble into ordered structures. The morphology of these phase-separated domains significantly impacts ionic conductivity, with continuous hydrophilic channels offering optimal pathways for anion transport. Controlling block length ratios and processing conditions allows for tuning the membrane morphology to maximize conductivity.
- Composite and reinforced AEM structures for enhanced performance: Composite and reinforced AEM structures incorporate additional components such as inorganic fillers, porous supports, or fiber reinforcements to enhance mechanical properties and conductivity. These hybrid materials combine the benefits of different components to overcome limitations of single-component membranes. Inorganic fillers like metal oxides can improve water retention and create additional ion transport pathways, while reinforcing structures provide dimensional stability under operating conditions. The interface between components plays a critical role in determining overall membrane performance.
- Side-chain engineering for optimized ion transport: Side-chain engineering involves modifying the length, flexibility, and chemical composition of pendant groups attached to the polymer backbone to optimize ion transport properties. Longer side chains can increase the separation between the backbone and ionic groups, providing greater mobility for ion transport. The flexibility of these side chains affects water uptake and ion channel formation. Strategic placement of functional groups along these side chains can create favorable microenvironments for anion conduction, while maintaining the overall structural integrity of the membrane.
02 Polymer backbone modifications for stability
Modifications to the polymer backbone structure can significantly improve the chemical and thermal stability of AEM membranes. These modifications include the use of fluorinated polymers, aromatic hydrocarbon backbones, and crosslinking strategies that enhance resistance to degradation in alkaline environments. Stable backbones are crucial for maintaining long-term conductivity and mechanical integrity under operating conditions. Engineering the backbone chemistry also allows for controlling water uptake and swelling behavior, which directly impacts ionic conductivity.Expand Specific Solutions03 Composite and hybrid membrane structures
Composite and hybrid membrane structures combine different materials to optimize both conductivity and mechanical properties. These structures may include inorganic-organic hybrids, reinforced membranes with nanofillers, or multilayer architectures. The composite approach allows for independent optimization of different membrane properties, such as incorporating hydrophilic channels for ion transport while maintaining overall membrane stability. Nanoparticles or fibers can be added to enhance mechanical strength without compromising conductivity.Expand Specific Solutions04 Side-chain engineering for ion conductivity
Engineering the side chains of ionomers can significantly enhance ion conductivity in AEM membranes. This approach involves optimizing the length, flexibility, and hydrophilicity of side chains that connect the ionic functional groups to the polymer backbone. Longer and more flexible side chains can facilitate better ion mobility and create more efficient ion transport pathways. The spatial distribution of ionic groups can be controlled through side-chain design to create interconnected ion-conducting channels while maintaining dimensional stability.Expand Specific Solutions05 Hydration management strategies
Effective water management is crucial for optimizing AEM membrane conductivity. Ionomers can be designed with specific hydrophilic-hydrophobic balances to control water uptake and retention. Strategies include incorporating hydrophilic domains for water retention, designing phase-separated morphologies, and using additives that help maintain appropriate hydration levels. The right hydration level is essential as too little water reduces ion mobility while excessive water causes dimensional instability and mechanical weakening. Controlling the membrane microstructure can create efficient water channels that support ion transport.Expand Specific Solutions
Leading Companies and Research Institutions in AEM Technology
The anion exchange membrane (AEM) technology for high-conductivity membranes is currently in a growth phase, with increasing market adoption driven by clean energy demands. The global market for ionomer chemistry in fuel cells and electrolyzers is expanding rapidly, projected to reach significant scale as hydrogen technologies mature. Technologically, the field shows moderate maturity with several key players making substantial advances. Companies like 3M Innovative Properties, PolyFuel, and Solvay Specialty Polymers lead commercial development with established polymer expertise, while academic institutions including Beijing University of Chemical Technology and Cornell University contribute fundamental research breakthroughs. Automotive manufacturers such as Hyundai, Kia, and GM Global Technology Operations are increasingly investing in this technology for next-generation fuel cell vehicles, indicating growing industrial confidence in AEM technology's commercial viability.
Solvay Specialty Polymers Italy SpA
Technical Solution: Solvay has developed a proprietary platform of fluorinated ionomer chemistries for high-performance AEM membranes. Their approach centers on partially fluorinated aromatic polymer backbones with pendant quaternary ammonium groups, combining the chemical resistance of fluoropolymers with the high ion-exchange capacity needed for efficient hydroxide transport. Solvay's AQUIVION® technology has been adapted for anion exchange applications through careful molecular engineering of the side chains and ionic groups. Their membranes feature a multi-block architecture with hydrophilic domains for ion conduction and hydrophobic domains for mechanical stability, creating well-defined nanophase separation. The company has optimized the degree of functionalization and crosslinking to achieve hydroxide conductivities of 90-110 mS/cm at operating temperatures while maintaining dimensional stability. Solvay's manufacturing process allows for precise control of membrane thickness (10-50 μm) and uniform distribution of functional groups, addressing key challenges in AEM commercialization. Their latest generation membranes demonstrate significantly improved resistance to degradation under alkaline conditions through the incorporation of sterically protected quaternary ammonium groups.
Strengths: Excellent chemical and thermal stability due to fluorinated backbone; scalable manufacturing processes already established for related membrane technologies; balanced mechanical properties and conductivity. Weaknesses: Higher production costs associated with fluorinated materials; potential environmental concerns with fluoropolymer production; slightly lower peak conductivity compared to some academic research membranes.
GM Global Technology Operations LLC
Technical Solution: GM has developed a comprehensive approach to high-conductivity AEM membranes focused on automotive fuel cell applications. Their technology centers on hydrocarbon-based polymer architectures with strategically positioned quaternary ammonium functional groups designed to maximize hydroxide conductivity while withstanding the demanding conditions of vehicle operation. GM's proprietary membrane formulation incorporates a multi-block copolymer structure with precisely engineered hydrophilic/hydrophobic phase separation to create efficient ion transport channels while maintaining mechanical robustness. Their research has yielded membranes with hydroxide conductivities exceeding 80 mS/cm at 80°C and exceptional durability under start-stop cycling conditions typical in automotive applications. GM has pioneered advanced manufacturing techniques for these membranes, including solution casting methods that enable production of ultra-thin (15-25 μm) membranes with uniform properties. Their latest innovations include the incorporation of nano-reinforcement materials and specialized crosslinking agents that significantly improve mechanical stability without compromising ionic conductivity, addressing a key challenge in AEM commercialization for transportation applications.
Strengths: Optimized for automotive durability requirements; excellent balance of conductivity and mechanical properties; scalable manufacturing processes developed specifically for high-volume production. Weaknesses: Potentially lower peak conductivity compared to some laboratory-scale membranes; specific design constraints related to automotive applications may limit broader applicability; proprietary nature of technology limits academic collaboration.
Key Patents and Breakthroughs in AEM Membrane Chemistry
Anion exchange membrane
PatentInactiveUS8436057B1
Innovation
- An anion exchange membrane comprising a sulfonated fluoropolymer support covalently bonded to bicyclic proazaphosphatrane or tricyclic azaphosphatrane cations, which enhances mechanical and thermal stability while maintaining high electrical conductivity, allowing for efficient hydroxide ion transport in strongly basic conditions.
Anion exchange polymers and anion exchange membranes
PatentWO2023049459A1
Innovation
- Development of an anion conducting co-polymer with a poly(phenylene) backbone and aromatic compound linkages, incorporating quaternary ammonium or n-methyl piperidine functional groups, and a porous scaffold support for reinforcement, allowing for thin, mechanically stable, and chemically robust anion exchange membranes.
Sustainability Aspects of Advanced Ionomer Materials
The advancement of ionomer chemistry for anion exchange membranes (AEMs) presents significant sustainability implications that extend beyond performance metrics. As global energy demands continue to rise, the development of sustainable materials for clean energy technologies becomes increasingly critical. Advanced ionomer materials offer promising environmental benefits through their application in fuel cells, electrolyzers, and other electrochemical devices that can reduce dependence on fossil fuels.
The life cycle assessment of these novel ionomer materials reveals substantial advantages over traditional petroleum-based alternatives. By incorporating bio-derived components and reducing reliance on fluorinated compounds, next-generation AEM ionomers can significantly decrease the carbon footprint associated with membrane production. Research indicates that bio-based ionomers can achieve up to 40% reduction in greenhouse gas emissions during manufacturing compared to conventional materials.
Water consumption represents another crucial sustainability factor in ionomer development. High-conductivity AEM membranes typically require less water for optimal performance than their proton exchange counterparts, potentially reducing water usage in large-scale energy applications. Additionally, emerging synthesis routes for advanced ionomers increasingly employ green chemistry principles, minimizing hazardous waste generation and utilizing environmentally benign solvents.
End-of-life considerations for ionomer materials have gained attention as circular economy principles become more prominent in materials science. Recent innovations focus on designing degradable linkages within ionomer structures that facilitate recycling or biodegradation while maintaining performance during operational lifetime. This approach addresses the persistent challenge of polymer waste management that has historically plagued membrane technologies.
Resource efficiency in ionomer synthesis has improved through catalyst optimization and process intensification. Modern synthetic pathways achieve higher atom economy, reducing raw material requirements and associated extraction impacts. Furthermore, the elimination of precious metal catalysts in favor of earth-abundant alternatives enhances the long-term sustainability of production processes.
The durability enhancement of advanced ionomer materials contributes significantly to sustainability by extending operational lifetimes of electrochemical devices. Longer-lasting membranes reduce replacement frequency and associated material consumption, creating cascading sustainability benefits throughout the technology lifecycle. Research indicates that next-generation ionomers can potentially double the service life of current commercial membranes under comparable operating conditions.
Regulatory frameworks increasingly influence ionomer development trajectories, with restrictions on persistent chemicals driving innovation toward more environmentally compatible alternatives. This regulatory pressure has accelerated research into non-fluorinated chemistries and stimulated exploration of novel molecular architectures that maintain performance while reducing environmental persistence.
The life cycle assessment of these novel ionomer materials reveals substantial advantages over traditional petroleum-based alternatives. By incorporating bio-derived components and reducing reliance on fluorinated compounds, next-generation AEM ionomers can significantly decrease the carbon footprint associated with membrane production. Research indicates that bio-based ionomers can achieve up to 40% reduction in greenhouse gas emissions during manufacturing compared to conventional materials.
Water consumption represents another crucial sustainability factor in ionomer development. High-conductivity AEM membranes typically require less water for optimal performance than their proton exchange counterparts, potentially reducing water usage in large-scale energy applications. Additionally, emerging synthesis routes for advanced ionomers increasingly employ green chemistry principles, minimizing hazardous waste generation and utilizing environmentally benign solvents.
End-of-life considerations for ionomer materials have gained attention as circular economy principles become more prominent in materials science. Recent innovations focus on designing degradable linkages within ionomer structures that facilitate recycling or biodegradation while maintaining performance during operational lifetime. This approach addresses the persistent challenge of polymer waste management that has historically plagued membrane technologies.
Resource efficiency in ionomer synthesis has improved through catalyst optimization and process intensification. Modern synthetic pathways achieve higher atom economy, reducing raw material requirements and associated extraction impacts. Furthermore, the elimination of precious metal catalysts in favor of earth-abundant alternatives enhances the long-term sustainability of production processes.
The durability enhancement of advanced ionomer materials contributes significantly to sustainability by extending operational lifetimes of electrochemical devices. Longer-lasting membranes reduce replacement frequency and associated material consumption, creating cascading sustainability benefits throughout the technology lifecycle. Research indicates that next-generation ionomers can potentially double the service life of current commercial membranes under comparable operating conditions.
Regulatory frameworks increasingly influence ionomer development trajectories, with restrictions on persistent chemicals driving innovation toward more environmentally compatible alternatives. This regulatory pressure has accelerated research into non-fluorinated chemistries and stimulated exploration of novel molecular architectures that maintain performance while reducing environmental persistence.
Manufacturing Scalability of High-Performance AEM Membranes
The scalability of manufacturing processes for high-performance anion exchange membranes (AEMs) represents a critical bottleneck in the widespread commercialization of AEM-based technologies. Current laboratory-scale synthesis methods for advanced ionomers with high conductivity often involve complex multi-step reactions that are difficult to translate to industrial production scales.
Traditional membrane manufacturing techniques such as solution casting and phase inversion have been adapted for AEM production, but maintaining consistent ionomer chemistry distribution and mechanical properties becomes increasingly challenging at larger scales. The precision required for controlling crosslinking density and functional group placement—critical factors for high conductivity—often diminishes during scale-up operations.
Roll-to-roll processing shows promise for continuous AEM production, but requires significant optimization when incorporating advanced ionomers. The rheological properties of high-conductivity ionomer solutions often differ substantially from conventional polymers, necessitating specialized equipment modifications and process parameters. Several membrane manufacturers have reported difficulties in achieving uniform thickness and homogeneous ion-exchange capacity across large membrane areas.
Solvent selection presents another manufacturing challenge, as many high-performance ionomers require environmentally problematic solvents. Regulatory pressures and sustainability concerns are driving research toward greener processing methods, though these often result in compromised membrane performance. Recent advances in solvent-free melt processing techniques offer potential alternatives but remain limited to specific ionomer chemistries.
Quality control methodologies for large-scale AEM production require further development. Current analytical techniques for characterizing ion conductivity and chemical stability are primarily designed for small samples and cannot easily be adapted for in-line manufacturing inspection. This creates significant challenges in ensuring batch-to-batch consistency and long-term performance reliability.
Economic considerations also impact manufacturing scalability. The complex synthesis routes for advanced ionomers with quaternary ammonium, phosphonium, or imidazolium functional groups involve expensive reagents and catalysts. Cost-effective alternatives often sacrifice conductivity or durability, creating a challenging balance between performance and manufacturability. Recent techno-economic analyses suggest that production volumes exceeding 10,000 m²/year would be necessary to achieve competitive pricing for high-performance AEMs.
Collaborative efforts between academic researchers and industrial partners have begun addressing these challenges through the development of simplified ionomer chemistries that maintain high conductivity while being amenable to large-scale production. These approaches focus on reducing synthetic steps, improving functional group stability during processing, and developing specialized equipment for precise control of membrane morphology during manufacturing.
Traditional membrane manufacturing techniques such as solution casting and phase inversion have been adapted for AEM production, but maintaining consistent ionomer chemistry distribution and mechanical properties becomes increasingly challenging at larger scales. The precision required for controlling crosslinking density and functional group placement—critical factors for high conductivity—often diminishes during scale-up operations.
Roll-to-roll processing shows promise for continuous AEM production, but requires significant optimization when incorporating advanced ionomers. The rheological properties of high-conductivity ionomer solutions often differ substantially from conventional polymers, necessitating specialized equipment modifications and process parameters. Several membrane manufacturers have reported difficulties in achieving uniform thickness and homogeneous ion-exchange capacity across large membrane areas.
Solvent selection presents another manufacturing challenge, as many high-performance ionomers require environmentally problematic solvents. Regulatory pressures and sustainability concerns are driving research toward greener processing methods, though these often result in compromised membrane performance. Recent advances in solvent-free melt processing techniques offer potential alternatives but remain limited to specific ionomer chemistries.
Quality control methodologies for large-scale AEM production require further development. Current analytical techniques for characterizing ion conductivity and chemical stability are primarily designed for small samples and cannot easily be adapted for in-line manufacturing inspection. This creates significant challenges in ensuring batch-to-batch consistency and long-term performance reliability.
Economic considerations also impact manufacturing scalability. The complex synthesis routes for advanced ionomers with quaternary ammonium, phosphonium, or imidazolium functional groups involve expensive reagents and catalysts. Cost-effective alternatives often sacrifice conductivity or durability, creating a challenging balance between performance and manufacturability. Recent techno-economic analyses suggest that production volumes exceeding 10,000 m²/year would be necessary to achieve competitive pricing for high-performance AEMs.
Collaborative efforts between academic researchers and industrial partners have begun addressing these challenges through the development of simplified ionomer chemistries that maintain high conductivity while being amenable to large-scale production. These approaches focus on reducing synthetic steps, improving functional group stability during processing, and developing specialized equipment for precise control of membrane morphology during manufacturing.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!