Laryngoscope precision-engineering: Overcoming production barriers.
JUL 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Laryngoscope Evolution
The laryngoscope has undergone significant evolution since its inception in the early 19th century. Initially developed as a simple tool for examining the larynx, it has transformed into a sophisticated medical device crucial for airway management and intubation procedures.
The earliest laryngoscopes were rudimentary instruments consisting of mirrors and light sources. In 1844, Manuel Garcia invented the first indirect laryngoscope, using a dental mirror to reflect sunlight onto the larynx. This breakthrough allowed for the first visualization of vocal cords in a living person.
The late 19th and early 20th centuries saw rapid advancements in laryngoscope design. In 1895, Alfred Kirstein developed the first direct laryngoscope, enabling a more straightforward view of the larynx. This innovation paved the way for modern laryngoscopy techniques.
The introduction of electric lighting in the early 1900s revolutionized laryngoscope design. Battery-powered light sources replaced mirrors and external illumination, significantly improving visibility during procedures. This advancement led to the development of the Macintosh laryngoscope in 1943, which remains a standard design in modern medical practice.
The latter half of the 20th century witnessed further refinements in laryngoscope technology. The integration of fiber optics in the 1960s enhanced illumination and reduced the risk of electrical hazards. This period also saw the development of disposable laryngoscope blades, addressing concerns about cross-contamination and sterilization.
In recent decades, video laryngoscopy has emerged as a game-changing technology. By incorporating miniature cameras and high-resolution displays, these devices provide superior visualization of the airway, particularly in difficult intubation scenarios. This innovation has significantly improved success rates and reduced complications in airway management procedures.
The 21st century has brought about further advancements in laryngoscope design, focusing on ergonomics, materials science, and digital integration. Modern laryngoscopes often feature lightweight, durable materials such as reinforced plastics and advanced alloys. Additionally, the integration of digital technologies has enabled real-time data capture, analysis, and sharing, enhancing both clinical practice and medical education.
As precision engineering techniques continue to evolve, laryngoscopes are becoming increasingly sophisticated. Current research focuses on developing smart laryngoscopes with sensors for pressure and positioning, as well as augmented reality interfaces to assist in complex procedures. These innovations aim to further improve patient outcomes and expand the capabilities of airway management techniques.
The earliest laryngoscopes were rudimentary instruments consisting of mirrors and light sources. In 1844, Manuel Garcia invented the first indirect laryngoscope, using a dental mirror to reflect sunlight onto the larynx. This breakthrough allowed for the first visualization of vocal cords in a living person.
The late 19th and early 20th centuries saw rapid advancements in laryngoscope design. In 1895, Alfred Kirstein developed the first direct laryngoscope, enabling a more straightforward view of the larynx. This innovation paved the way for modern laryngoscopy techniques.
The introduction of electric lighting in the early 1900s revolutionized laryngoscope design. Battery-powered light sources replaced mirrors and external illumination, significantly improving visibility during procedures. This advancement led to the development of the Macintosh laryngoscope in 1943, which remains a standard design in modern medical practice.
The latter half of the 20th century witnessed further refinements in laryngoscope technology. The integration of fiber optics in the 1960s enhanced illumination and reduced the risk of electrical hazards. This period also saw the development of disposable laryngoscope blades, addressing concerns about cross-contamination and sterilization.
In recent decades, video laryngoscopy has emerged as a game-changing technology. By incorporating miniature cameras and high-resolution displays, these devices provide superior visualization of the airway, particularly in difficult intubation scenarios. This innovation has significantly improved success rates and reduced complications in airway management procedures.
The 21st century has brought about further advancements in laryngoscope design, focusing on ergonomics, materials science, and digital integration. Modern laryngoscopes often feature lightweight, durable materials such as reinforced plastics and advanced alloys. Additionally, the integration of digital technologies has enabled real-time data capture, analysis, and sharing, enhancing both clinical practice and medical education.
As precision engineering techniques continue to evolve, laryngoscopes are becoming increasingly sophisticated. Current research focuses on developing smart laryngoscopes with sensors for pressure and positioning, as well as augmented reality interfaces to assist in complex procedures. These innovations aim to further improve patient outcomes and expand the capabilities of airway management techniques.
Market Analysis
The laryngoscope market has experienced steady growth in recent years, driven by increasing demand for minimally invasive surgical procedures and advancements in medical technology. The global laryngoscope market size was valued at approximately $200 million in 2020 and is projected to reach $300 million by 2027, growing at a CAGR of around 6% during the forecast period.
The rising prevalence of respiratory diseases, such as chronic obstructive pulmonary disease (COPD) and asthma, has significantly contributed to the market expansion. Additionally, the growing geriatric population, who are more susceptible to respiratory issues, has further fueled the demand for laryngoscopes in both diagnostic and therapeutic applications.
In terms of product types, video laryngoscopes have gained substantial traction in recent years due to their superior visualization capabilities and ease of use. This segment is expected to witness the highest growth rate in the coming years, as healthcare facilities increasingly adopt advanced technologies to improve patient outcomes and reduce procedure times.
Geographically, North America holds the largest market share, attributed to the presence of well-established healthcare infrastructure and high adoption rates of advanced medical devices. However, the Asia-Pacific region is anticipated to exhibit the fastest growth, driven by improving healthcare facilities, increasing healthcare expenditure, and rising awareness about minimally invasive procedures in emerging economies like China and India.
The COVID-19 pandemic has had a mixed impact on the laryngoscope market. While elective procedures were postponed during the initial phases of the outbreak, the demand for laryngoscopes in emergency and critical care settings increased significantly. The pandemic has also accelerated the adoption of single-use laryngoscopes to minimize the risk of cross-contamination and reduce the burden of sterilization processes.
Key market players are focusing on product innovations and strategic collaborations to gain a competitive edge. For instance, the development of laryngoscopes with integrated cameras and LED lighting systems has improved visualization during intubation procedures. Moreover, the integration of artificial intelligence and machine learning technologies in laryngoscopes is expected to revolutionize the market in the coming years.
Despite the positive market outlook, challenges such as the high cost of advanced laryngoscopes and the lack of skilled professionals in developing regions may hinder market growth to some extent. However, ongoing research and development activities aimed at overcoming production barriers and improving the precision engineering of laryngoscopes are expected to create new opportunities for market expansion in the near future.
The rising prevalence of respiratory diseases, such as chronic obstructive pulmonary disease (COPD) and asthma, has significantly contributed to the market expansion. Additionally, the growing geriatric population, who are more susceptible to respiratory issues, has further fueled the demand for laryngoscopes in both diagnostic and therapeutic applications.
In terms of product types, video laryngoscopes have gained substantial traction in recent years due to their superior visualization capabilities and ease of use. This segment is expected to witness the highest growth rate in the coming years, as healthcare facilities increasingly adopt advanced technologies to improve patient outcomes and reduce procedure times.
Geographically, North America holds the largest market share, attributed to the presence of well-established healthcare infrastructure and high adoption rates of advanced medical devices. However, the Asia-Pacific region is anticipated to exhibit the fastest growth, driven by improving healthcare facilities, increasing healthcare expenditure, and rising awareness about minimally invasive procedures in emerging economies like China and India.
The COVID-19 pandemic has had a mixed impact on the laryngoscope market. While elective procedures were postponed during the initial phases of the outbreak, the demand for laryngoscopes in emergency and critical care settings increased significantly. The pandemic has also accelerated the adoption of single-use laryngoscopes to minimize the risk of cross-contamination and reduce the burden of sterilization processes.
Key market players are focusing on product innovations and strategic collaborations to gain a competitive edge. For instance, the development of laryngoscopes with integrated cameras and LED lighting systems has improved visualization during intubation procedures. Moreover, the integration of artificial intelligence and machine learning technologies in laryngoscopes is expected to revolutionize the market in the coming years.
Despite the positive market outlook, challenges such as the high cost of advanced laryngoscopes and the lack of skilled professionals in developing regions may hinder market growth to some extent. However, ongoing research and development activities aimed at overcoming production barriers and improving the precision engineering of laryngoscopes are expected to create new opportunities for market expansion in the near future.
Technical Challenges
The precision engineering of laryngoscopes faces several significant technical challenges that hinder production efficiency and product quality. One of the primary obstacles is the miniaturization of components while maintaining structural integrity. As laryngoscopes require intricate designs to navigate the delicate anatomy of the throat, manufacturers struggle to produce ultra-thin yet durable materials that can withstand repeated sterilization processes without compromising functionality.
Another critical challenge lies in the integration of advanced imaging technologies within the confined space of the laryngoscope blade. High-resolution cameras and fiber optic systems must be seamlessly incorporated without increasing the overall dimensions of the device. This necessitates innovative approaches to component placement and wiring, often pushing the boundaries of current manufacturing capabilities.
The demand for enhanced ergonomics presents additional hurdles in laryngoscope production. Achieving the optimal balance between comfort for the practitioner and effectiveness for the patient requires precise weight distribution and carefully contoured surfaces. These design requirements often conflict with the need for robust construction, creating a complex engineering problem that impacts production processes.
Furthermore, the development of reliable articulation mechanisms for video laryngoscopes poses significant technical difficulties. These mechanisms must provide smooth, controlled movement while being compact enough to fit within the device's slim profile. The precision required in manufacturing these components often leads to high rejection rates and increased production costs.
Ensuring consistent optical quality across mass-produced laryngoscopes is another major challenge. The lenses and light guides must meet exacting standards to provide clear, undistorted views of the larynx. Achieving this level of optical precision at scale requires advanced manufacturing techniques and rigorous quality control measures, which can be difficult to implement efficiently.
Lastly, the integration of smart features, such as real-time data transmission and analysis capabilities, introduces new complexities to laryngoscope production. Incorporating sensors, microprocessors, and wireless communication modules while maintaining the device's core functionality and meeting stringent medical device regulations presents a multifaceted engineering challenge that impacts the entire production pipeline.
Another critical challenge lies in the integration of advanced imaging technologies within the confined space of the laryngoscope blade. High-resolution cameras and fiber optic systems must be seamlessly incorporated without increasing the overall dimensions of the device. This necessitates innovative approaches to component placement and wiring, often pushing the boundaries of current manufacturing capabilities.
The demand for enhanced ergonomics presents additional hurdles in laryngoscope production. Achieving the optimal balance between comfort for the practitioner and effectiveness for the patient requires precise weight distribution and carefully contoured surfaces. These design requirements often conflict with the need for robust construction, creating a complex engineering problem that impacts production processes.
Furthermore, the development of reliable articulation mechanisms for video laryngoscopes poses significant technical difficulties. These mechanisms must provide smooth, controlled movement while being compact enough to fit within the device's slim profile. The precision required in manufacturing these components often leads to high rejection rates and increased production costs.
Ensuring consistent optical quality across mass-produced laryngoscopes is another major challenge. The lenses and light guides must meet exacting standards to provide clear, undistorted views of the larynx. Achieving this level of optical precision at scale requires advanced manufacturing techniques and rigorous quality control measures, which can be difficult to implement efficiently.
Lastly, the integration of smart features, such as real-time data transmission and analysis capabilities, introduces new complexities to laryngoscope production. Incorporating sensors, microprocessors, and wireless communication modules while maintaining the device's core functionality and meeting stringent medical device regulations presents a multifaceted engineering challenge that impacts the entire production pipeline.
Current Solutions
01 Advanced imaging and visualization techniques
Modern laryngoscopes incorporate advanced imaging technologies such as high-resolution cameras, enhanced lighting systems, and digital displays to improve visualization of the larynx and surrounding structures. These features allow for more precise identification of anatomical landmarks and potential abnormalities, leading to more accurate intubation procedures.- Advanced imaging and visualization systems: Modern laryngoscopes incorporate advanced imaging technologies such as high-resolution cameras and video systems to provide clearer, more detailed views of the larynx and surrounding structures. These systems often include features like image enhancement, zoom capabilities, and real-time display, allowing for more precise examination and intervention during procedures.
- Improved blade design and materials: Innovations in laryngoscope blade design focus on enhancing precision through optimized shape, size, and material selection. Advanced materials such as reinforced plastics or lightweight metals are used to create blades that are both durable and flexible, allowing for better maneuverability and reduced patient discomfort during intubation procedures.
- Integration of sensor technologies: Laryngoscopes are being equipped with various sensors to improve precision and provide additional data during procedures. These may include pressure sensors to monitor force applied during intubation, position sensors for accurate blade placement, or even biosensors to detect physiological parameters, enhancing overall procedural safety and effectiveness.
- Ergonomic design and control mechanisms: Emphasis on ergonomic design and improved control mechanisms aims to enhance the precision of laryngoscope use. This includes features such as adjustable handles, intuitive control interfaces, and balanced weight distribution, allowing healthcare professionals to manipulate the device with greater accuracy and reduced fatigue during prolonged procedures.
- Augmented reality and guidance systems: Cutting-edge laryngoscopes incorporate augmented reality (AR) and guidance systems to assist in precise navigation and placement. These technologies can overlay real-time imaging with pre-operative data, provide visual cues for optimal intubation paths, or offer computer-assisted guidance, significantly enhancing the accuracy and success rate of laryngoscopic procedures.
02 Ergonomic design and handling improvements
Laryngoscopes are being designed with improved ergonomics to enhance user comfort and control during procedures. This includes optimized handle shapes, balanced weight distribution, and intuitive control interfaces. These design improvements contribute to increased stability and precision during laryngoscopy, reducing the risk of patient injury and improving overall procedural outcomes.Expand Specific Solutions03 Integration of sensor technologies
Incorporation of various sensors into laryngoscopes, such as pressure sensors, position sensors, and force feedback mechanisms, provides real-time data to the operator. This information helps in guiding the insertion process, avoiding excessive force application, and ensuring optimal blade positioning for improved precision and patient safety during intubation procedures.Expand Specific Solutions04 Articulating and adjustable blade designs
Development of laryngoscope blades with articulating or adjustable components allows for better adaptation to individual patient anatomy. These designs enable fine-tuning of the blade angle and shape during the procedure, facilitating more precise navigation of the airway and improving the success rate of intubation attempts, particularly in challenging cases.Expand Specific Solutions05 Integration with AI and image processing
Incorporation of artificial intelligence and advanced image processing algorithms in laryngoscope systems enhances the interpretation of visual data. These technologies can assist in real-time identification of anatomical structures, provide guidance for optimal device positioning, and even predict potential complications, thereby increasing the precision and safety of laryngoscopy procedures.Expand Specific Solutions
Industry Leaders
The laryngoscope precision-engineering market is in a growth phase, driven by increasing demand for advanced medical devices in airway management. The global market size is expanding, with projections indicating significant growth in the coming years. Technologically, the field is advancing rapidly, with companies like Ambu A/S, Verathon, Inc., and Zhejiang Youyi Medical Equipment Co Ltd leading innovation. These firms are developing more precise, user-friendly, and cost-effective laryngoscopes. Established players such as Covidien AG (part of Medtronic) and HOYA Corp. are also contributing to technological advancements, while emerging companies are introducing novel solutions to overcome production barriers and improve patient outcomes.
Zhejiang Youyi Medical Equipment Co Ltd
Technical Solution: Zhejiang Youyi Medical Equipment Co Ltd has developed a range of video laryngoscopes that focus on cost-effective solutions for emerging markets. Their devices typically feature a simplified design with a focus on durability and ease of maintenance. The company has addressed production barriers by implementing modular assembly techniques, allowing for more efficient manufacturing processes. Zhejiang Youyi's laryngoscopes often incorporate locally sourced components, which helps to reduce production costs and improve supply chain resilience[10]. While specific technical details are limited in publicly available sources, the company's approach appears to prioritize accessibility and affordability in laryngoscope design.
Strengths: Cost-effective solutions suitable for resource-constrained settings, and potentially easier maintenance due to simplified design. Weaknesses: May lack some advanced features found in premium devices, and limited international presence compared to global market leaders.
Covidien AG
Technical Solution: Covidien AG, now part of Medtronic, has developed the McGrath MAC video laryngoscope, which features a unique articulating blade design. This design allows for easy adjustment of the blade angle during intubation, providing flexibility in difficult airway scenarios. The McGrath MAC incorporates a high-resolution camera and screen integrated into the handle, offering a compact and portable solution. Covidien's precision engineering focuses on creating an ultra-thin blade profile, which facilitates easier insertion and maneuverability in the patient's airway[4]. The device also utilizes advanced image processing algorithms to enhance visualization in low-light conditions, addressing one of the key challenges in laryngoscopy[5].
Strengths: Compact design, versatility in various clinical settings, and improved maneuverability. Weaknesses: Learning curve for practitioners transitioning from traditional laryngoscopes, and potential issues with screen glare in certain lighting conditions.
Key Innovations
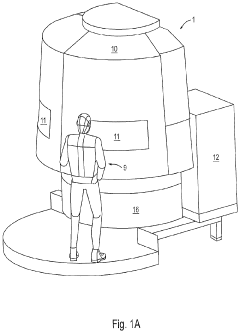
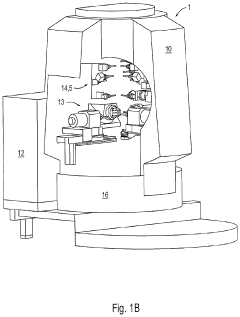
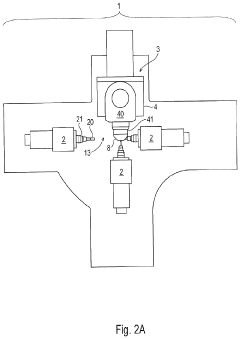
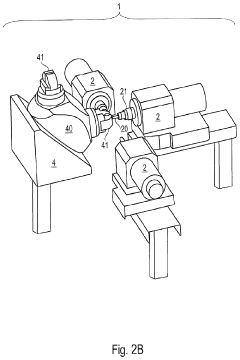
Machine Concept for the Mass Production of High-Precision Workpieces
PatentPendingUS20230398651A1
Innovation
- The machine concept optimizes machining units and technological sequences relative to workpiece characteristics, allowing for flexible expansion by adding machining centers or transferring production to a rotary transfer machine while maintaining validated production conditions, thereby minimizing resource usage and environmental impact.
Regulatory Compliance
Regulatory compliance is a critical aspect of laryngoscope precision-engineering, particularly in overcoming production barriers. The medical device industry is heavily regulated to ensure patient safety and product efficacy. For laryngoscopes, compliance with regulatory standards is essential throughout the entire product lifecycle, from design and manufacturing to distribution and post-market surveillance.
In the United States, the Food and Drug Administration (FDA) classifies laryngoscopes as Class I medical devices, which are subject to general controls. Manufacturers must comply with the FDA's Quality System Regulation (QSR), which outlines requirements for design controls, manufacturing processes, and quality assurance. Additionally, laryngoscopes must meet the standards set by the International Electrotechnical Commission (IEC) for medical electrical equipment safety.
The European Union's Medical Device Regulation (MDR) imposes stringent requirements on laryngoscope manufacturers. The MDR mandates comprehensive clinical evaluation, post-market surveillance, and unique device identification (UDI) systems. Compliance with these regulations often necessitates significant investments in quality management systems and documentation processes.
In Asia, regulatory frameworks vary by country. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) requires rigorous pre-market approval processes, while China's National Medical Products Administration (NMPA) has implemented a classification-based system similar to the FDA's approach.
Overcoming production barriers in laryngoscope precision-engineering often involves addressing regulatory challenges. Manufacturers must implement robust quality management systems that ensure consistent product quality and traceability. This includes establishing validated processes for sterilization, packaging, and labeling to meet regulatory requirements across different markets.
Regulatory compliance also extends to material selection and biocompatibility testing. Laryngoscopes must be manufactured using materials that meet international standards for biocompatibility, such as ISO 10993. This often requires extensive testing and documentation to demonstrate the safety of materials in contact with patients.
As technology advances, regulatory bodies are adapting their frameworks to address new challenges. For instance, the integration of digital technologies in laryngoscopes has led to increased scrutiny of software validation and cybersecurity measures. Manufacturers must now consider data protection regulations and implement appropriate safeguards to protect patient information.
To overcome production barriers related to regulatory compliance, companies are increasingly adopting risk-based approaches to quality management. This involves identifying and mitigating potential risks throughout the product lifecycle, aligning with regulatory expectations for proactive quality assurance.
In the United States, the Food and Drug Administration (FDA) classifies laryngoscopes as Class I medical devices, which are subject to general controls. Manufacturers must comply with the FDA's Quality System Regulation (QSR), which outlines requirements for design controls, manufacturing processes, and quality assurance. Additionally, laryngoscopes must meet the standards set by the International Electrotechnical Commission (IEC) for medical electrical equipment safety.
The European Union's Medical Device Regulation (MDR) imposes stringent requirements on laryngoscope manufacturers. The MDR mandates comprehensive clinical evaluation, post-market surveillance, and unique device identification (UDI) systems. Compliance with these regulations often necessitates significant investments in quality management systems and documentation processes.
In Asia, regulatory frameworks vary by country. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) requires rigorous pre-market approval processes, while China's National Medical Products Administration (NMPA) has implemented a classification-based system similar to the FDA's approach.
Overcoming production barriers in laryngoscope precision-engineering often involves addressing regulatory challenges. Manufacturers must implement robust quality management systems that ensure consistent product quality and traceability. This includes establishing validated processes for sterilization, packaging, and labeling to meet regulatory requirements across different markets.
Regulatory compliance also extends to material selection and biocompatibility testing. Laryngoscopes must be manufactured using materials that meet international standards for biocompatibility, such as ISO 10993. This often requires extensive testing and documentation to demonstrate the safety of materials in contact with patients.
As technology advances, regulatory bodies are adapting their frameworks to address new challenges. For instance, the integration of digital technologies in laryngoscopes has led to increased scrutiny of software validation and cybersecurity measures. Manufacturers must now consider data protection regulations and implement appropriate safeguards to protect patient information.
To overcome production barriers related to regulatory compliance, companies are increasingly adopting risk-based approaches to quality management. This involves identifying and mitigating potential risks throughout the product lifecycle, aligning with regulatory expectations for proactive quality assurance.
Material Advancements
Material advancements have played a crucial role in overcoming production barriers in laryngoscope precision-engineering. The development of new materials has significantly improved the performance, durability, and functionality of laryngoscopes, addressing many of the challenges faced in their manufacturing process.
One of the most significant advancements has been the introduction of high-performance polymers. These materials offer a unique combination of strength, flexibility, and lightweight properties, making them ideal for laryngoscope blades. Polymers such as polyetheretherketone (PEEK) and polyetherimide (PEI) have gained popularity due to their excellent mechanical properties and resistance to sterilization processes. These materials have enabled the production of thinner, more maneuverable blades without compromising structural integrity.
Advancements in metal alloys have also contributed to improved laryngoscope design. Titanium alloys, known for their high strength-to-weight ratio and corrosion resistance, have been increasingly used in laryngoscope handles and components. These alloys provide enhanced durability and longevity, addressing the issue of wear and tear in frequently used medical devices.
The integration of advanced composite materials has further revolutionized laryngoscope production. Carbon fiber reinforced polymers (CFRP) have been utilized to create ultra-lightweight yet rigid structures. This has allowed for the development of laryngoscopes that are easier to manipulate during intubation procedures while maintaining the necessary strength to withstand repeated use and sterilization cycles.
Innovations in surface coating technologies have also addressed production barriers. The application of anti-microbial coatings on laryngoscope surfaces has enhanced infection control measures, a critical aspect in medical device manufacturing. Additionally, low-friction coatings have been developed to improve the ease of intubation and reduce the risk of tissue damage during procedures.
Nanotechnology has opened up new possibilities in material engineering for laryngoscopes. Nanocomposites and nanostructured materials have been explored to enhance the optical properties of laryngoscope blades, improving visibility during intubation. These materials can also provide superior scratch resistance and durability, extending the lifespan of the devices.
The development of biocompatible materials has addressed concerns related to patient safety and allergic reactions. Hypoallergenic materials and those with reduced potential for causing tissue irritation have been incorporated into laryngoscope designs, improving patient outcomes and reducing the risk of complications during use.
These material advancements have not only overcome production barriers but have also driven innovation in laryngoscope design and functionality. As research in materials science continues to progress, it is expected that future developments will further enhance the precision, efficiency, and safety of laryngoscopes, ultimately improving patient care in critical medical procedures.
One of the most significant advancements has been the introduction of high-performance polymers. These materials offer a unique combination of strength, flexibility, and lightweight properties, making them ideal for laryngoscope blades. Polymers such as polyetheretherketone (PEEK) and polyetherimide (PEI) have gained popularity due to their excellent mechanical properties and resistance to sterilization processes. These materials have enabled the production of thinner, more maneuverable blades without compromising structural integrity.
Advancements in metal alloys have also contributed to improved laryngoscope design. Titanium alloys, known for their high strength-to-weight ratio and corrosion resistance, have been increasingly used in laryngoscope handles and components. These alloys provide enhanced durability and longevity, addressing the issue of wear and tear in frequently used medical devices.
The integration of advanced composite materials has further revolutionized laryngoscope production. Carbon fiber reinforced polymers (CFRP) have been utilized to create ultra-lightweight yet rigid structures. This has allowed for the development of laryngoscopes that are easier to manipulate during intubation procedures while maintaining the necessary strength to withstand repeated use and sterilization cycles.
Innovations in surface coating technologies have also addressed production barriers. The application of anti-microbial coatings on laryngoscope surfaces has enhanced infection control measures, a critical aspect in medical device manufacturing. Additionally, low-friction coatings have been developed to improve the ease of intubation and reduce the risk of tissue damage during procedures.
Nanotechnology has opened up new possibilities in material engineering for laryngoscopes. Nanocomposites and nanostructured materials have been explored to enhance the optical properties of laryngoscope blades, improving visibility during intubation. These materials can also provide superior scratch resistance and durability, extending the lifespan of the devices.
The development of biocompatible materials has addressed concerns related to patient safety and allergic reactions. Hypoallergenic materials and those with reduced potential for causing tissue irritation have been incorporated into laryngoscope designs, improving patient outcomes and reducing the risk of complications during use.
These material advancements have not only overcome production barriers but have also driven innovation in laryngoscope design and functionality. As research in materials science continues to progress, it is expected that future developments will further enhance the precision, efficiency, and safety of laryngoscopes, ultimately improving patient care in critical medical procedures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!