Laryngoscope translation: Facilitating adoption across diverse healthcare systems.
JUL 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Laryngoscope Evolution
The laryngoscope has undergone significant evolution since its inception in the early 19th century. Initially developed as a simple tool for examining the larynx, it has transformed into a sophisticated device crucial for airway management in various medical settings. The early designs, such as those by Manuel Garcia in 1854, were primarily used for vocal cord examination and relied on reflected sunlight for illumination.
As medical knowledge and technology advanced, so did the laryngoscope. The introduction of electric lighting in the late 19th century marked a pivotal moment, allowing for better visualization of the airway. This improvement led to the development of more specialized designs, such as the Macintosh and Miller blades in the 1940s, which remain widely used today.
The latter half of the 20th century saw further refinements in laryngoscope design, focusing on ergonomics, materials, and functionality. The introduction of disposable blades addressed concerns about cross-contamination, while advancements in fiber optics improved illumination quality. These developments enhanced the tool's effectiveness in various clinical scenarios, from routine intubations to emergency airway management.
The digital era brought about video laryngoscopy, a game-changing innovation that revolutionized airway management. By integrating small cameras and video screens, these devices provided a clearer view of the airway and facilitated easier intubation, especially in difficult cases. This technology has proven particularly valuable in teaching settings and for managing complex airways.
Recent years have seen a focus on portability and adaptability, with the development of compact, battery-powered devices suitable for use in diverse healthcare environments. These innovations have made advanced laryngoscopy techniques more accessible in resource-limited settings and emergency situations outside of traditional hospital environments.
The evolution of the laryngoscope reflects broader trends in medical technology, emphasizing improved patient outcomes, user-friendly designs, and adaptability to various healthcare contexts. As healthcare systems worldwide face different challenges and resource constraints, the need for laryngoscopes that can be easily adopted across diverse settings has become increasingly apparent. This has driven research into more versatile, cost-effective designs that maintain high performance standards while being suitable for a wide range of healthcare environments.
As medical knowledge and technology advanced, so did the laryngoscope. The introduction of electric lighting in the late 19th century marked a pivotal moment, allowing for better visualization of the airway. This improvement led to the development of more specialized designs, such as the Macintosh and Miller blades in the 1940s, which remain widely used today.
The latter half of the 20th century saw further refinements in laryngoscope design, focusing on ergonomics, materials, and functionality. The introduction of disposable blades addressed concerns about cross-contamination, while advancements in fiber optics improved illumination quality. These developments enhanced the tool's effectiveness in various clinical scenarios, from routine intubations to emergency airway management.
The digital era brought about video laryngoscopy, a game-changing innovation that revolutionized airway management. By integrating small cameras and video screens, these devices provided a clearer view of the airway and facilitated easier intubation, especially in difficult cases. This technology has proven particularly valuable in teaching settings and for managing complex airways.
Recent years have seen a focus on portability and adaptability, with the development of compact, battery-powered devices suitable for use in diverse healthcare environments. These innovations have made advanced laryngoscopy techniques more accessible in resource-limited settings and emergency situations outside of traditional hospital environments.
The evolution of the laryngoscope reflects broader trends in medical technology, emphasizing improved patient outcomes, user-friendly designs, and adaptability to various healthcare contexts. As healthcare systems worldwide face different challenges and resource constraints, the need for laryngoscopes that can be easily adopted across diverse settings has become increasingly apparent. This has driven research into more versatile, cost-effective designs that maintain high performance standards while being suitable for a wide range of healthcare environments.
Global Healthcare Needs
The global healthcare landscape is characterized by significant disparities in access to medical technology and expertise, particularly in critical care settings. Laryngoscopy, a crucial procedure for airway management, exemplifies these disparities. While advanced healthcare systems in developed nations often have access to state-of-the-art video laryngoscopes and well-trained personnel, many regions worldwide still rely on traditional direct laryngoscopy techniques or face shortages of skilled practitioners.
This disparity creates a pressing need for innovative solutions that can bridge the gap between diverse healthcare systems. The concept of laryngoscope translation addresses this need by focusing on developing technologies and methodologies that can facilitate the adoption and effective use of laryngoscopy across varied healthcare environments.
In low-resource settings, the challenges often include limited access to advanced equipment, inadequate training opportunities, and a lack of experienced mentors. These factors contribute to higher rates of complications during intubation procedures and potentially poorer patient outcomes. Conversely, in more advanced healthcare systems, the challenge lies in optimizing the use of sophisticated equipment and ensuring consistent performance across a wide range of clinical scenarios.
The global demand for improved laryngoscopy solutions is driven by several factors. First, the increasing prevalence of chronic respiratory diseases and the aging population in many countries are leading to a higher incidence of situations requiring airway management. Second, the ongoing global efforts to improve emergency and critical care services, particularly in developing nations, create a market for cost-effective and adaptable laryngoscopy technologies.
Furthermore, the COVID-19 pandemic has highlighted the critical importance of effective airway management techniques that minimize the risk of disease transmission to healthcare workers. This has accelerated the need for laryngoscopy solutions that can be quickly adopted and effectively used by healthcare providers with varying levels of experience.
The concept of laryngoscope translation also aligns with broader global health initiatives aimed at reducing healthcare disparities. By developing technologies and training methodologies that can be effectively implemented across diverse healthcare systems, there is potential to significantly improve patient outcomes and reduce the global burden of airway-related complications.
In conclusion, the global healthcare needs driving the development of laryngoscope translation solutions are multifaceted and urgent. They encompass technological, educational, and systemic challenges that must be addressed to ensure equitable access to safe and effective airway management techniques worldwide.
This disparity creates a pressing need for innovative solutions that can bridge the gap between diverse healthcare systems. The concept of laryngoscope translation addresses this need by focusing on developing technologies and methodologies that can facilitate the adoption and effective use of laryngoscopy across varied healthcare environments.
In low-resource settings, the challenges often include limited access to advanced equipment, inadequate training opportunities, and a lack of experienced mentors. These factors contribute to higher rates of complications during intubation procedures and potentially poorer patient outcomes. Conversely, in more advanced healthcare systems, the challenge lies in optimizing the use of sophisticated equipment and ensuring consistent performance across a wide range of clinical scenarios.
The global demand for improved laryngoscopy solutions is driven by several factors. First, the increasing prevalence of chronic respiratory diseases and the aging population in many countries are leading to a higher incidence of situations requiring airway management. Second, the ongoing global efforts to improve emergency and critical care services, particularly in developing nations, create a market for cost-effective and adaptable laryngoscopy technologies.
Furthermore, the COVID-19 pandemic has highlighted the critical importance of effective airway management techniques that minimize the risk of disease transmission to healthcare workers. This has accelerated the need for laryngoscopy solutions that can be quickly adopted and effectively used by healthcare providers with varying levels of experience.
The concept of laryngoscope translation also aligns with broader global health initiatives aimed at reducing healthcare disparities. By developing technologies and training methodologies that can be effectively implemented across diverse healthcare systems, there is potential to significantly improve patient outcomes and reduce the global burden of airway-related complications.
In conclusion, the global healthcare needs driving the development of laryngoscope translation solutions are multifaceted and urgent. They encompass technological, educational, and systemic challenges that must be addressed to ensure equitable access to safe and effective airway management techniques worldwide.
Technical Barriers
The adoption of laryngoscope translation across diverse healthcare systems faces several significant technical barriers. One of the primary challenges is the integration of translation technology with existing medical devices. Laryngoscopes are precision instruments designed for specific medical procedures, and incorporating translation capabilities without compromising their primary function requires careful engineering and design considerations.
Compatibility issues arise when attempting to interface translation systems with various laryngoscope models from different manufacturers. The lack of standardization in hardware interfaces and communication protocols across medical devices complicates the development of universal translation solutions. This fragmentation in the medical device ecosystem necessitates the creation of multiple adaptations or custom integrations, increasing development costs and time-to-market.
Another technical hurdle is the real-time processing of audio and visual data in a medical environment. Laryngoscope translation systems must operate with minimal latency to ensure effective communication during critical procedures. This demands high-performance computing capabilities in a compact form factor, which can be challenging to achieve within the constraints of medical device design and regulatory requirements.
The accuracy and reliability of translation in medical contexts pose additional technical challenges. Medical terminology is highly specialized and context-dependent, requiring advanced natural language processing algorithms and extensive medical knowledge bases. Ensuring the precision of translations, especially for rare or complex medical terms, is crucial to prevent miscommunication that could lead to serious medical errors.
Data privacy and security present further technical barriers. Medical information is highly sensitive, and any translation system must comply with stringent healthcare data protection regulations such as HIPAA in the United States or GDPR in Europe. Implementing robust encryption, secure data transmission, and access control mechanisms within the constraints of medical devices adds layers of complexity to the system architecture.
The variability in accents, dialects, and speech patterns across different healthcare professionals and patients introduces challenges for speech recognition systems. Developing algorithms that can accurately interpret diverse linguistic inputs while maintaining high performance in noisy clinical environments requires sophisticated audio processing and machine learning techniques.
Lastly, the power management and battery life of portable translation devices integrated with laryngoscopes present technical challenges. Balancing the need for extended operation times with the compact design requirements of medical instruments demands innovative energy-efficient hardware and software solutions. These technical barriers collectively underscore the complexity of implementing laryngoscope translation systems across diverse healthcare environments, necessitating multidisciplinary approaches to overcome them.
Compatibility issues arise when attempting to interface translation systems with various laryngoscope models from different manufacturers. The lack of standardization in hardware interfaces and communication protocols across medical devices complicates the development of universal translation solutions. This fragmentation in the medical device ecosystem necessitates the creation of multiple adaptations or custom integrations, increasing development costs and time-to-market.
Another technical hurdle is the real-time processing of audio and visual data in a medical environment. Laryngoscope translation systems must operate with minimal latency to ensure effective communication during critical procedures. This demands high-performance computing capabilities in a compact form factor, which can be challenging to achieve within the constraints of medical device design and regulatory requirements.
The accuracy and reliability of translation in medical contexts pose additional technical challenges. Medical terminology is highly specialized and context-dependent, requiring advanced natural language processing algorithms and extensive medical knowledge bases. Ensuring the precision of translations, especially for rare or complex medical terms, is crucial to prevent miscommunication that could lead to serious medical errors.
Data privacy and security present further technical barriers. Medical information is highly sensitive, and any translation system must comply with stringent healthcare data protection regulations such as HIPAA in the United States or GDPR in Europe. Implementing robust encryption, secure data transmission, and access control mechanisms within the constraints of medical devices adds layers of complexity to the system architecture.
The variability in accents, dialects, and speech patterns across different healthcare professionals and patients introduces challenges for speech recognition systems. Developing algorithms that can accurately interpret diverse linguistic inputs while maintaining high performance in noisy clinical environments requires sophisticated audio processing and machine learning techniques.
Lastly, the power management and battery life of portable translation devices integrated with laryngoscopes present technical challenges. Balancing the need for extended operation times with the compact design requirements of medical instruments demands innovative energy-efficient hardware and software solutions. These technical barriers collectively underscore the complexity of implementing laryngoscope translation systems across diverse healthcare environments, necessitating multidisciplinary approaches to overcome them.
Current Solutions
01 Translating laryngoscope blade
Laryngoscopes with translating blades allow for adjustable positioning during intubation. The blade can move forward, backward, or laterally to provide better visibility and access to the airway. This feature enhances the versatility of the laryngoscope and improves its effectiveness in various anatomical situations.- Adjustable laryngoscope designs: Laryngoscopes with adjustable features allow for better adaptation to different patient anatomies. These designs may include movable blades, adjustable angles, or flexible components that can be manipulated during intubation procedures. Such adaptability enhances the effectiveness of the laryngoscope in various clinical scenarios.
- Video-assisted laryngoscopy: Integration of video technology into laryngoscopes enables real-time visualization of the intubation process. These devices typically include a camera at the blade tip and a display screen, allowing for improved accuracy and potentially easier intubation, especially in difficult cases.
- Disposable laryngoscope components: Disposable parts for laryngoscopes, such as single-use blades or covers, address hygiene concerns and reduce the risk of cross-contamination between patients. These components are designed to be easily attached and removed, ensuring a clean instrument for each procedure.
- Illumination systems for laryngoscopes: Advanced lighting systems in laryngoscopes improve visibility during intubation. These may include LED lights, fiber optic illumination, or other technologies that provide bright, focused light to the laryngeal area, enhancing the practitioner's view of the airway.
- Ergonomic handle designs: Laryngoscope handles with improved ergonomics aim to reduce operator fatigue and enhance control during intubation procedures. These designs may feature contoured grips, lightweight materials, or balanced weight distribution to improve handling and precision.
02 Video-assisted laryngoscopy with translation
Video laryngoscopes incorporate cameras and screens to provide a clear view of the airway. Some designs feature translating mechanisms for the camera or screen, allowing for optimal positioning and improved visualization during intubation procedures. This combination of video technology and translational movement enhances the precision and ease of use.Expand Specific Solutions03 Motorized translation mechanisms
Advanced laryngoscopes utilize motorized translation mechanisms to adjust the position of the blade or other components. These systems allow for precise, controlled movements that can be operated remotely or through automated processes. Motorized translation enhances the accuracy and reduces the physical effort required during intubation.Expand Specific Solutions04 Multi-axis translation systems
Some laryngoscopes feature multi-axis translation systems that allow for movement in multiple directions. These systems provide greater flexibility in positioning the blade, camera, or other components. Multi-axis translation enables practitioners to navigate complex airway anatomies and adapt to various patient positions more effectively.Expand Specific Solutions05 Integrated sensor-guided translation
Advanced laryngoscope designs incorporate sensors to guide the translation of components. These sensors can detect anatomical landmarks or obstacles, providing feedback to assist in optimal positioning. Sensor-guided translation systems enhance the precision and safety of intubation procedures by offering real-time adjustments based on patient-specific data.Expand Specific Solutions
Market Leaders Analysis
The laryngoscope translation market is in a growth phase, driven by increasing demand for advanced medical devices across diverse healthcare systems globally. The market size is expanding, fueled by rising surgical procedures and the need for improved visualization during intubation. Technologically, the field is evolving rapidly, with companies like Zhejiang Youyi Medical Equipment Co Ltd and Verathon, Inc. leading innovation in visualized airway management tools. The competitive landscape is diverse, featuring established medical device manufacturers, specialized airway management companies, and emerging players leveraging AI and IoT technologies. While some solutions are reaching maturity, there's ongoing development in areas like AI-assisted translation and remote operation capabilities, indicating potential for further market expansion and technological advancement.
FUJIFILM Corp.
Technical Solution: FUJIFILM has developed the FUJIFILM UltimateVision laryngoscope system, which incorporates advanced imaging technology and a user-friendly interface to facilitate adoption across diverse healthcare systems. The system features a high-resolution camera and display, providing clear visualization of the airway during intubation procedures. To address language barriers, FUJIFILM has implemented a multi-language interface that supports over 30 languages, allowing healthcare professionals from various linguistic backgrounds to operate the device effectively[9]. The company has also developed a unique augmented reality (AR) feature that overlays translated instructions and anatomical labels directly onto the laryngoscope's display, enhancing understanding and reducing the need for separate translation tools[10]. To further facilitate adoption, FUJIFILM offers a cloud-based training platform that provides virtual simulations and step-by-step guides in multiple languages, enabling healthcare systems to efficiently train their staff on the use of the laryngoscope technology.
Strengths: Advanced imaging technology with AR-enhanced translations. Comprehensive multi-language support and cloud-based training platform. Weaknesses: AR features may require additional processing power and could potentially increase device cost.
Verathon, Inc.
Technical Solution: Verathon has developed the GlideScope video laryngoscope system, which incorporates advanced imaging technology to provide a clear view of the airway during intubation procedures. The system includes a high-resolution camera and display, allowing for real-time visualization of the larynx and vocal cords. To facilitate adoption across diverse healthcare systems, Verathon has implemented a multi-language user interface and voice guidance system, supporting over 20 languages[1]. This feature enables healthcare professionals from various linguistic backgrounds to operate the device effectively. Additionally, Verathon has integrated a cloud-based data management system that allows for seamless sharing of intubation data and images across different healthcare facilities, promoting collaboration and standardization of care[2].
Strengths: Advanced imaging technology, multi-language support, and cloud-based data sharing facilitate global adoption. Weaknesses: May require significant initial investment and training for healthcare systems transitioning from traditional laryngoscopes.
Key Patents Review
Multilingual communications device
PatentInactiveUSH2098H1
Innovation
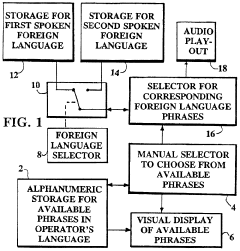
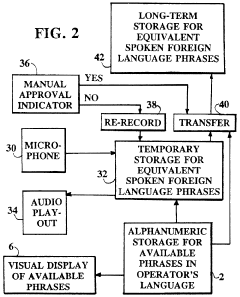
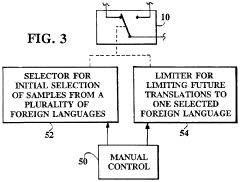
- A digital translating machine that stores pre-recorded audio translations of medical questions in multiple languages, allowing operators to select and play back sentences in the patient's chosen language, using a personal computer configuration with CD-ROM storage and audio playback capabilities, enabling language selection and efficient communication without literacy requirements.
Regulatory Compliance
Regulatory compliance is a critical aspect of introducing and adopting new medical devices, such as laryngoscopes, across diverse healthcare systems. The regulatory landscape for medical devices varies significantly between countries and regions, necessitating a comprehensive understanding of the requirements in each target market.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of medical devices. Laryngoscopes are typically classified as Class I or Class II devices, depending on their specific features and intended use. Manufacturers must comply with the FDA's Quality System Regulation (QSR) and obtain 510(k) clearance or premarket approval (PMA) before marketing their devices.
The European Union has implemented the Medical Device Regulation (MDR), which came into full effect in May 2021. This regulation harmonizes standards across EU member states and introduces more stringent requirements for medical device manufacturers. Laryngoscopes must bear the CE mark to indicate compliance with the MDR and be registered in the European Database on Medical Devices (EUDAMED).
In Asia, regulatory frameworks vary widely. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) requires manufacturers to obtain marketing authorization through a rigorous review process. China's National Medical Products Administration (NMPA) has its own set of regulations, including the need for clinical trials conducted in China for certain devices.
Emerging markets often present unique regulatory challenges. Some countries may have less developed regulatory frameworks, while others may rely on approvals from established regulatory bodies like the FDA or EU notified bodies. This can create a complex landscape for manufacturers seeking to enter multiple markets simultaneously.
To facilitate adoption across diverse healthcare systems, manufacturers must develop a comprehensive regulatory strategy. This includes conducting thorough market research to understand the specific requirements in each target region, engaging with local regulatory experts, and establishing a robust quality management system that can adapt to various regulatory frameworks.
Harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), aim to streamline regulatory processes globally. However, significant differences remain, and manufacturers must be prepared to navigate these complexities. Staying informed about regulatory changes and maintaining open communication with regulatory bodies is essential for successful market entry and sustained compliance.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of medical devices. Laryngoscopes are typically classified as Class I or Class II devices, depending on their specific features and intended use. Manufacturers must comply with the FDA's Quality System Regulation (QSR) and obtain 510(k) clearance or premarket approval (PMA) before marketing their devices.
The European Union has implemented the Medical Device Regulation (MDR), which came into full effect in May 2021. This regulation harmonizes standards across EU member states and introduces more stringent requirements for medical device manufacturers. Laryngoscopes must bear the CE mark to indicate compliance with the MDR and be registered in the European Database on Medical Devices (EUDAMED).
In Asia, regulatory frameworks vary widely. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) requires manufacturers to obtain marketing authorization through a rigorous review process. China's National Medical Products Administration (NMPA) has its own set of regulations, including the need for clinical trials conducted in China for certain devices.
Emerging markets often present unique regulatory challenges. Some countries may have less developed regulatory frameworks, while others may rely on approvals from established regulatory bodies like the FDA or EU notified bodies. This can create a complex landscape for manufacturers seeking to enter multiple markets simultaneously.
To facilitate adoption across diverse healthcare systems, manufacturers must develop a comprehensive regulatory strategy. This includes conducting thorough market research to understand the specific requirements in each target region, engaging with local regulatory experts, and establishing a robust quality management system that can adapt to various regulatory frameworks.
Harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), aim to streamline regulatory processes globally. However, significant differences remain, and manufacturers must be prepared to navigate these complexities. Staying informed about regulatory changes and maintaining open communication with regulatory bodies is essential for successful market entry and sustained compliance.
Cross-Cultural Design
Cross-cultural design is a critical aspect of facilitating the adoption of laryngoscopes across diverse healthcare systems. The design process must consider the unique cultural, linguistic, and operational contexts of different healthcare environments to ensure seamless integration and effective use of the device.
One key consideration in cross-cultural design is the adaptation of user interfaces and controls to accommodate various language preferences and literacy levels. This may involve implementing multilingual interfaces, intuitive iconography, and culturally appropriate color schemes. For instance, in some cultures, certain colors may have specific connotations that could affect the perception and acceptance of the device.
Ergonomics play a crucial role in cross-cultural design, as physical characteristics and preferences can vary significantly across populations. Designers must account for differences in hand size, grip strength, and preferred handling techniques when developing laryngoscope handles and blades. This may necessitate the creation of modular or adjustable components to ensure comfort and efficiency for a wide range of users.
Training materials and documentation must also be tailored to different cultural contexts. This includes not only translation into local languages but also adaptation of instructional methods, visual aids, and case studies to align with local medical practices and cultural norms. Interactive digital training platforms can be developed to provide customized learning experiences that resonate with healthcare professionals from diverse backgrounds.
The integration of laryngoscopes into existing healthcare workflows and systems is another critical aspect of cross-cultural design. Designers must consider variations in medical protocols, equipment storage practices, and sterilization procedures across different healthcare settings. This may involve creating adaptable storage solutions, developing compatible charging systems, and ensuring compatibility with local power supplies and medical equipment standards.
Feedback mechanisms and user support systems should be designed with cultural sensitivity in mind. This includes establishing culturally appropriate channels for reporting issues, seeking assistance, and providing suggestions for improvement. Localized support teams and region-specific online resources can help address unique challenges and concerns that may arise in different healthcare environments.
By prioritizing cross-cultural design principles, manufacturers can enhance the global adoption and effectiveness of laryngoscopes, ultimately improving patient care outcomes across diverse healthcare systems. This approach not only facilitates easier integration of the technology but also demonstrates respect for cultural diversity and promotes a more inclusive healthcare ecosystem.
One key consideration in cross-cultural design is the adaptation of user interfaces and controls to accommodate various language preferences and literacy levels. This may involve implementing multilingual interfaces, intuitive iconography, and culturally appropriate color schemes. For instance, in some cultures, certain colors may have specific connotations that could affect the perception and acceptance of the device.
Ergonomics play a crucial role in cross-cultural design, as physical characteristics and preferences can vary significantly across populations. Designers must account for differences in hand size, grip strength, and preferred handling techniques when developing laryngoscope handles and blades. This may necessitate the creation of modular or adjustable components to ensure comfort and efficiency for a wide range of users.
Training materials and documentation must also be tailored to different cultural contexts. This includes not only translation into local languages but also adaptation of instructional methods, visual aids, and case studies to align with local medical practices and cultural norms. Interactive digital training platforms can be developed to provide customized learning experiences that resonate with healthcare professionals from diverse backgrounds.
The integration of laryngoscopes into existing healthcare workflows and systems is another critical aspect of cross-cultural design. Designers must consider variations in medical protocols, equipment storage practices, and sterilization procedures across different healthcare settings. This may involve creating adaptable storage solutions, developing compatible charging systems, and ensuring compatibility with local power supplies and medical equipment standards.
Feedback mechanisms and user support systems should be designed with cultural sensitivity in mind. This includes establishing culturally appropriate channels for reporting issues, seeking assistance, and providing suggestions for improvement. Localized support teams and region-specific online resources can help address unique challenges and concerns that may arise in different healthcare environments.
By prioritizing cross-cultural design principles, manufacturers can enhance the global adoption and effectiveness of laryngoscopes, ultimately improving patient care outcomes across diverse healthcare systems. This approach not only facilitates easier integration of the technology but also demonstrates respect for cultural diversity and promotes a more inclusive healthcare ecosystem.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!