Laryngoscope use in anesthesia: Safety and efficiency.
JUL 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Laryngoscopy Evolution
Laryngoscopy has undergone significant evolution since its inception in the early 19th century. The journey began with rudimentary devices designed for throat examinations and has progressed to sophisticated instruments crucial for airway management in anesthesia. This evolution has been driven by the need for improved safety and efficiency in medical procedures.
The first laryngoscope, developed by Benjamin Guy Babington in 1829, was a simple mirror device used to examine the larynx. However, it wasn't until 1854 that Manuel Garcia invented the first practical laryngoscope, using a dental mirror and a larger hand mirror to observe his own vocal cords. This breakthrough paved the way for further advancements in laryngoscopy.
In the early 20th century, Chevalier Jackson introduced the direct laryngoscope, which allowed for a more direct view of the larynx. This innovation significantly improved the ability to perform intubations and other airway procedures. The Macintosh laryngoscope, developed by Robert Macintosh in 1943, marked another milestone. Its curved blade design revolutionized laryngoscopy by providing a better view of the glottis and easing the intubation process.
The late 20th century saw the introduction of fiber-optic technology in laryngoscopy. Fiber-optic laryngoscopes offered improved illumination and visualization, particularly in difficult airway scenarios. This technology allowed for more precise and less traumatic intubations, enhancing patient safety.
The 21st century has brought about video laryngoscopy, a game-changing development in airway management. Video laryngoscopes incorporate small cameras at the tip of the blade, projecting a magnified view onto a screen. This technology has significantly improved the success rate of intubations, especially in challenging cases, and has become an essential tool in anesthesia practice.
Recent advancements include the development of disposable laryngoscope blades to reduce the risk of cross-contamination, and the integration of augmented reality technology to provide real-time guidance during intubation procedures. These innovations continue to enhance the safety and efficiency of laryngoscopy in anesthesia.
The evolution of laryngoscopy reflects a constant drive towards improving patient outcomes and practitioner capabilities. From simple mirrors to sophisticated video systems, each advancement has addressed specific challenges in airway management. This ongoing evolution underscores the critical role of laryngoscopy in anesthesia and the continuous efforts to enhance its safety and efficiency.
The first laryngoscope, developed by Benjamin Guy Babington in 1829, was a simple mirror device used to examine the larynx. However, it wasn't until 1854 that Manuel Garcia invented the first practical laryngoscope, using a dental mirror and a larger hand mirror to observe his own vocal cords. This breakthrough paved the way for further advancements in laryngoscopy.
In the early 20th century, Chevalier Jackson introduced the direct laryngoscope, which allowed for a more direct view of the larynx. This innovation significantly improved the ability to perform intubations and other airway procedures. The Macintosh laryngoscope, developed by Robert Macintosh in 1943, marked another milestone. Its curved blade design revolutionized laryngoscopy by providing a better view of the glottis and easing the intubation process.
The late 20th century saw the introduction of fiber-optic technology in laryngoscopy. Fiber-optic laryngoscopes offered improved illumination and visualization, particularly in difficult airway scenarios. This technology allowed for more precise and less traumatic intubations, enhancing patient safety.
The 21st century has brought about video laryngoscopy, a game-changing development in airway management. Video laryngoscopes incorporate small cameras at the tip of the blade, projecting a magnified view onto a screen. This technology has significantly improved the success rate of intubations, especially in challenging cases, and has become an essential tool in anesthesia practice.
Recent advancements include the development of disposable laryngoscope blades to reduce the risk of cross-contamination, and the integration of augmented reality technology to provide real-time guidance during intubation procedures. These innovations continue to enhance the safety and efficiency of laryngoscopy in anesthesia.
The evolution of laryngoscopy reflects a constant drive towards improving patient outcomes and practitioner capabilities. From simple mirrors to sophisticated video systems, each advancement has addressed specific challenges in airway management. This ongoing evolution underscores the critical role of laryngoscopy in anesthesia and the continuous efforts to enhance its safety and efficiency.
Anesthesia Market Needs
The anesthesia market has witnessed significant growth in recent years, driven by an increasing number of surgical procedures, advancements in healthcare infrastructure, and a growing aging population. The demand for safe and efficient anesthesia delivery methods, including laryngoscopes, has become paramount in ensuring optimal patient outcomes during surgical interventions.
Laryngoscopes play a crucial role in anesthesia administration, particularly during endotracheal intubation. The market need for improved laryngoscope technology stems from the challenges faced by anesthesiologists in managing difficult airways and reducing the risk of complications associated with intubation procedures. Healthcare providers are seeking innovative solutions that enhance visualization, increase first-attempt success rates, and minimize the potential for trauma to the patient's airway.
One of the primary market drivers is the growing emphasis on patient safety. Anesthesiologists and healthcare facilities are increasingly focused on reducing the incidence of adverse events related to airway management. This has led to a demand for laryngoscopes with advanced features such as video-assisted visualization, ergonomic designs, and real-time feedback mechanisms to improve intubation accuracy and reduce the risk of complications.
Efficiency in anesthesia delivery is another critical market need. With the increasing pressure on healthcare systems to optimize resource utilization and reduce procedure times, there is a strong demand for laryngoscopes that can facilitate rapid and successful intubations. This includes devices that offer improved maneuverability, enhanced blade designs, and intuitive user interfaces to streamline the intubation process and minimize the learning curve for healthcare professionals.
The market also shows a growing interest in disposable laryngoscopes, driven by concerns over cross-contamination and the need for cost-effective solutions in healthcare settings. Single-use devices eliminate the need for sterilization between procedures, potentially reducing the risk of hospital-acquired infections and streamlining workflow in busy operating rooms.
Furthermore, there is an increasing demand for laryngoscopes that can integrate with other anesthesia equipment and hospital information systems. This integration allows for better data management, real-time monitoring, and improved documentation of intubation procedures, contributing to overall patient care quality and regulatory compliance.
As the anesthesia market continues to evolve, there is a clear need for laryngoscopes that address both safety and efficiency concerns. Manufacturers are expected to focus on developing innovative technologies that not only improve visualization and ease of use but also contribute to better patient outcomes and operational efficiency in healthcare settings.
Laryngoscopes play a crucial role in anesthesia administration, particularly during endotracheal intubation. The market need for improved laryngoscope technology stems from the challenges faced by anesthesiologists in managing difficult airways and reducing the risk of complications associated with intubation procedures. Healthcare providers are seeking innovative solutions that enhance visualization, increase first-attempt success rates, and minimize the potential for trauma to the patient's airway.
One of the primary market drivers is the growing emphasis on patient safety. Anesthesiologists and healthcare facilities are increasingly focused on reducing the incidence of adverse events related to airway management. This has led to a demand for laryngoscopes with advanced features such as video-assisted visualization, ergonomic designs, and real-time feedback mechanisms to improve intubation accuracy and reduce the risk of complications.
Efficiency in anesthesia delivery is another critical market need. With the increasing pressure on healthcare systems to optimize resource utilization and reduce procedure times, there is a strong demand for laryngoscopes that can facilitate rapid and successful intubations. This includes devices that offer improved maneuverability, enhanced blade designs, and intuitive user interfaces to streamline the intubation process and minimize the learning curve for healthcare professionals.
The market also shows a growing interest in disposable laryngoscopes, driven by concerns over cross-contamination and the need for cost-effective solutions in healthcare settings. Single-use devices eliminate the need for sterilization between procedures, potentially reducing the risk of hospital-acquired infections and streamlining workflow in busy operating rooms.
Furthermore, there is an increasing demand for laryngoscopes that can integrate with other anesthesia equipment and hospital information systems. This integration allows for better data management, real-time monitoring, and improved documentation of intubation procedures, contributing to overall patient care quality and regulatory compliance.
As the anesthesia market continues to evolve, there is a clear need for laryngoscopes that address both safety and efficiency concerns. Manufacturers are expected to focus on developing innovative technologies that not only improve visualization and ease of use but also contribute to better patient outcomes and operational efficiency in healthcare settings.
Current Challenges
The use of laryngoscopes in anesthesia faces several significant challenges that impact both safety and efficiency. One of the primary concerns is the risk of airway trauma during intubation. Despite advancements in laryngoscope design, the potential for dental damage, soft tissue injury, and vocal cord trauma remains a persistent issue. This risk is particularly elevated in patients with difficult airways or anatomical abnormalities.
Another challenge is the learning curve associated with laryngoscope use. Proper technique requires extensive training and practice, which can lead to prolonged intubation times and increased risks for novice practitioners. This learning curve affects not only the efficiency of the procedure but also patient safety, as longer intubation times can result in oxygen desaturation and other complications.
The issue of cross-contamination and infection control presents an ongoing challenge in laryngoscope use. Despite sterilization protocols, ensuring complete decontamination of reusable laryngoscopes between patients remains difficult. This has led to an increased interest in single-use devices, which bring their own set of challenges related to cost and environmental impact.
Ergonomic considerations pose another significant challenge. Prolonged or repeated use of laryngoscopes can lead to hand and wrist fatigue for anesthesiologists, potentially affecting their performance and the safety of the procedure. This is particularly problematic during long surgeries or in high-volume settings where multiple intubations are performed in succession.
The limitations of traditional direct laryngoscopy in difficult airway scenarios continue to be a major challenge. While video laryngoscopes have addressed some of these issues, they introduce new challenges such as the need for different skill sets, higher costs, and potential over-reliance on technology.
Lastly, the challenge of integrating new technologies and techniques into established clinical practices presents ongoing difficulties. The adoption of advanced laryngoscope designs and adjunct devices often faces resistance due to factors such as cost, training requirements, and the inertia of established practices. This slow adoption can delay improvements in safety and efficiency that new technologies might offer.
Addressing these challenges requires a multifaceted approach, including ongoing research into laryngoscope design, improved training methodologies, and the development of clear guidelines for device selection and use in various clinical scenarios. The goal is to enhance both the safety and efficiency of laryngoscope use in anesthesia, ultimately improving patient outcomes and practitioner performance.
Another challenge is the learning curve associated with laryngoscope use. Proper technique requires extensive training and practice, which can lead to prolonged intubation times and increased risks for novice practitioners. This learning curve affects not only the efficiency of the procedure but also patient safety, as longer intubation times can result in oxygen desaturation and other complications.
The issue of cross-contamination and infection control presents an ongoing challenge in laryngoscope use. Despite sterilization protocols, ensuring complete decontamination of reusable laryngoscopes between patients remains difficult. This has led to an increased interest in single-use devices, which bring their own set of challenges related to cost and environmental impact.
Ergonomic considerations pose another significant challenge. Prolonged or repeated use of laryngoscopes can lead to hand and wrist fatigue for anesthesiologists, potentially affecting their performance and the safety of the procedure. This is particularly problematic during long surgeries or in high-volume settings where multiple intubations are performed in succession.
The limitations of traditional direct laryngoscopy in difficult airway scenarios continue to be a major challenge. While video laryngoscopes have addressed some of these issues, they introduce new challenges such as the need for different skill sets, higher costs, and potential over-reliance on technology.
Lastly, the challenge of integrating new technologies and techniques into established clinical practices presents ongoing difficulties. The adoption of advanced laryngoscope designs and adjunct devices often faces resistance due to factors such as cost, training requirements, and the inertia of established practices. This slow adoption can delay improvements in safety and efficiency that new technologies might offer.
Addressing these challenges requires a multifaceted approach, including ongoing research into laryngoscope design, improved training methodologies, and the development of clear guidelines for device selection and use in various clinical scenarios. The goal is to enhance both the safety and efficiency of laryngoscope use in anesthesia, ultimately improving patient outcomes and practitioner performance.
Safety Mechanisms
01 Enhanced visualization and illumination
Improved laryngoscope designs incorporate advanced lighting systems and camera technologies to enhance visualization of the airway during intubation. These features provide better illumination and image quality, allowing for more precise and efficient procedures while reducing the risk of injury to the patient.- Enhanced visualization and illumination: Improved laryngoscope designs incorporate advanced lighting systems and camera technologies to enhance visualization of the airway during intubation procedures. These features provide clearer views of the larynx and surrounding structures, potentially reducing the risk of injury and improving the success rate of intubation.
- Ergonomic design and handling: Laryngoscopes with ergonomic designs aim to improve user comfort and control during procedures. These designs may include adjustable handles, lightweight materials, and balanced weight distribution to reduce hand fatigue and enhance maneuverability, ultimately contributing to safer and more efficient intubations.
- Disposable and sterilizable components: To address infection control concerns, laryngoscopes may feature disposable blades or easily sterilizable components. This approach helps minimize the risk of cross-contamination between patients and ensures that each procedure is performed with clean, sterile equipment.
- Integration of monitoring and feedback systems: Advanced laryngoscopes may incorporate sensors and monitoring systems to provide real-time feedback on intubation depth, pressure applied, and other critical parameters. This integration can help prevent injuries and improve the overall safety and efficiency of the intubation process.
- Modular and adaptable designs: Laryngoscopes with modular designs allow for customization and adaptability to different patient anatomies and clinical scenarios. These designs may include interchangeable blades, adjustable angles, or expandable components, providing healthcare professionals with versatile tools to address various intubation challenges safely and efficiently.
02 Ergonomic design and handling
Laryngoscopes with ergonomic designs improve handling and maneuverability during intubation procedures. These designs reduce operator fatigue and increase control, leading to safer and more efficient intubations. Features may include adjustable handles, lightweight materials, and balanced weight distribution.Expand Specific Solutions03 Disposable and sterile components
The use of disposable or easily sterilizable components in laryngoscopes enhances safety by reducing the risk of cross-contamination between patients. These designs may include disposable blades, removable camera modules, or materials that can withstand rigorous sterilization processes.Expand Specific Solutions04 Integration of monitoring and feedback systems
Advanced laryngoscopes incorporate monitoring and feedback systems to provide real-time data during intubation procedures. These may include sensors for detecting proper tube placement, pressure monitors to prevent tissue damage, and alarms to alert the operator of potential issues, improving both safety and efficiency.Expand Specific Solutions05 Adaptable and multi-functional designs
Laryngoscopes with adaptable and multi-functional designs offer versatility in various clinical scenarios. These may include adjustable blade sizes, interchangeable components for different patient anatomies, or integration with other medical devices, enhancing both safety and efficiency across a range of intubation procedures.Expand Specific Solutions
Key Manufacturers
The laryngoscope market in anesthesia is in a mature stage, with a global market size estimated to reach $795 million by 2025. The technology has evolved significantly, moving from traditional direct laryngoscopes to advanced video laryngoscopes. Key players like Karl Storz SE & Co. KG, Covidien (part of Medtronic), and Verathon, Inc. are driving innovation in this space. Emerging companies such as Zhejiang Youyi Medical Equipment Co Ltd and Adroit Surgical LLC are introducing novel designs to enhance safety and efficiency. The competitive landscape is characterized by a focus on improving visualization, reducing intubation time, and minimizing complications, with ongoing research and development efforts at institutions like Zhejiang University and the Industrial Technology Research Institute.
Covidien AG
Technical Solution: Covidien AG has developed the McGrath MAC video laryngoscope, which incorporates a high-resolution camera and screen for improved visualization during intubation. The device features a unique single-use blade design that reduces the risk of cross-contamination[1]. The McGrath MAC also includes an anti-fog mechanism to maintain clear visibility in various clinical settings[2]. Covidien's technology allows for real-time image capture and recording, enabling post-procedure analysis and training opportunities[3]. The ergonomic design of the handle promotes ease of use and reduces operator fatigue during prolonged procedures[4].
Strengths: Enhanced visualization, reduced risk of cross-contamination, and improved ergonomics. Weaknesses: Higher initial cost compared to traditional laryngoscopes and potential reliance on battery power.
Karl Storz SE & Co. KG
Technical Solution: Karl Storz has introduced the C-MAC video laryngoscope system, which offers a range of blade sizes and styles to accommodate different patient anatomies. The system features a high-resolution camera and LED light source for optimal visualization[5]. Karl Storz's C-MAC pocket monitor provides a portable solution for emergency situations, allowing for quick deployment in various clinical settings[6]. The company has also developed a specialized difficult airway blade design to address challenging intubation scenarios[7]. Integration with hospital information systems allows for seamless documentation and data management[8].
Strengths: Versatility in blade options, portability for emergency use, and integration capabilities. Weaknesses: Potential for higher maintenance costs and the need for specialized training.
Innovative Designs
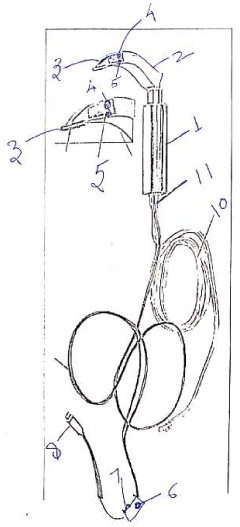
A video laryngoscope with oxygen delivery port for apneic oxygenation
PatentUndeterminedIN202111027640A
Innovation
- A cost-effective video laryngoscope with an integrated oxygen delivery port, a borescope camera, and a waterproof medical-grade stainless steel design, allowing for apneic oxygenation and eliminating the need for batteries, designed to resemble traditional laryngoscopes for ease of use, with a reusable and low-maintenance construction.
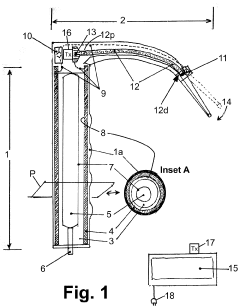
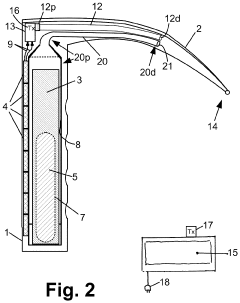
laryngoscope
PatentActiveUS20210059512A1
Innovation
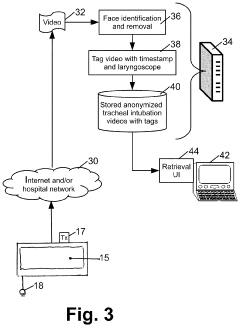
- A video laryngoscope design incorporating a chemiluminescent light source powered by photovoltaic cells, which eliminates the need for battery disposal and reduces interference with a separate video monitor, and an anonymization system for recording tracheal intubation procedures to provide evidence against malpractice claims.
Regulatory Framework
The regulatory framework surrounding laryngoscope use in anesthesia is a critical aspect of ensuring patient safety and maintaining high standards of care. In the United States, the Food and Drug Administration (FDA) classifies laryngoscopes as Class I medical devices, which are subject to general controls but typically exempt from premarket notification requirements. However, manufacturers must still adhere to Good Manufacturing Practices (GMP) and maintain quality control systems.
The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) plays a significant role in setting standards for healthcare facilities, including guidelines for the use and maintenance of laryngoscopes. These standards emphasize proper cleaning, disinfection, and sterilization procedures to prevent cross-contamination between patients.
Internationally, regulatory bodies such as the European Medicines Agency (EMA) and the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK have similar classifications for laryngoscopes. The European Union's Medical Device Regulation (MDR) requires manufacturers to demonstrate the safety and performance of their devices through clinical evaluation and post-market surveillance.
Professional organizations like the American Society of Anesthesiologists (ASA) and the European Society of Anaesthesiology (ESA) provide guidelines and recommendations for the safe use of laryngoscopes in clinical practice. These guidelines often address issues such as proper technique, maintenance, and infection control measures.
In recent years, there has been an increased focus on single-use laryngoscope blades to reduce the risk of cross-contamination. Regulatory bodies have responded by developing specific guidelines for the manufacture, testing, and disposal of these disposable devices.
The regulatory landscape also encompasses training and certification requirements for anesthesia providers. Many countries mandate specific training programs and ongoing education to ensure that healthcare professionals are competent in the use of laryngoscopes and other anesthesia equipment.
As technology advances, regulations are evolving to address new types of laryngoscopes, such as video laryngoscopes. These devices may be subject to additional regulatory scrutiny due to their more complex nature and the potential for software-related issues.
Compliance with these regulatory frameworks is essential for manufacturers, healthcare facilities, and practitioners to ensure the safe and efficient use of laryngoscopes in anesthesia practice. Regular audits and inspections by regulatory bodies help maintain adherence to these standards and promote continuous improvement in patient care and safety.
The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) plays a significant role in setting standards for healthcare facilities, including guidelines for the use and maintenance of laryngoscopes. These standards emphasize proper cleaning, disinfection, and sterilization procedures to prevent cross-contamination between patients.
Internationally, regulatory bodies such as the European Medicines Agency (EMA) and the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK have similar classifications for laryngoscopes. The European Union's Medical Device Regulation (MDR) requires manufacturers to demonstrate the safety and performance of their devices through clinical evaluation and post-market surveillance.
Professional organizations like the American Society of Anesthesiologists (ASA) and the European Society of Anaesthesiology (ESA) provide guidelines and recommendations for the safe use of laryngoscopes in clinical practice. These guidelines often address issues such as proper technique, maintenance, and infection control measures.
In recent years, there has been an increased focus on single-use laryngoscope blades to reduce the risk of cross-contamination. Regulatory bodies have responded by developing specific guidelines for the manufacture, testing, and disposal of these disposable devices.
The regulatory landscape also encompasses training and certification requirements for anesthesia providers. Many countries mandate specific training programs and ongoing education to ensure that healthcare professionals are competent in the use of laryngoscopes and other anesthesia equipment.
As technology advances, regulations are evolving to address new types of laryngoscopes, such as video laryngoscopes. These devices may be subject to additional regulatory scrutiny due to their more complex nature and the potential for software-related issues.
Compliance with these regulatory frameworks is essential for manufacturers, healthcare facilities, and practitioners to ensure the safe and efficient use of laryngoscopes in anesthesia practice. Regular audits and inspections by regulatory bodies help maintain adherence to these standards and promote continuous improvement in patient care and safety.
Training and Education
Training and education play a crucial role in ensuring the safe and efficient use of laryngoscopes in anesthesia practice. Proper training programs are essential for developing the skills and knowledge required to perform intubation procedures effectively while minimizing risks to patients.
Comprehensive training curricula typically include both theoretical and practical components. The theoretical aspect covers topics such as anatomy of the airway, principles of laryngoscopy, and potential complications. This foundational knowledge is essential for understanding the intricacies of the procedure and making informed decisions during challenging situations.
Practical training often begins with simulation-based learning, allowing trainees to practice laryngoscopy techniques in a controlled environment. Advanced simulation technologies, such as high-fidelity mannequins and virtual reality systems, provide realistic scenarios for skill development without risking patient safety. These tools enable learners to gain confidence and proficiency before performing procedures on actual patients.
Supervised clinical experience forms a critical part of the training process. Under the guidance of experienced anesthesiologists, trainees gradually progress from observing procedures to performing them independently. This hands-on experience is invaluable for developing the tactile skills and decision-making abilities necessary for successful laryngoscopy.
Continuing education is equally important for maintaining and updating skills throughout an anesthesiologist's career. Regular refresher courses, workshops, and conferences help practitioners stay current with the latest techniques, technologies, and safety protocols. These educational opportunities also provide a platform for sharing experiences and best practices among professionals.
The integration of new technologies, such as video laryngoscopes, into training programs has become increasingly important. These devices offer enhanced visualization of the airway and can be particularly useful in difficult intubation scenarios. Training on both traditional and video laryngoscopes ensures that anesthesiologists are prepared for a wide range of clinical situations.
Standardized assessment methods are crucial for evaluating the effectiveness of training programs and ensuring that practitioners meet the required competency levels. These assessments may include written exams, practical skills tests, and performance evaluations during simulated and real clinical scenarios.
In conclusion, comprehensive and ongoing training and education are fundamental to promoting safety and efficiency in laryngoscope use during anesthesia procedures. By combining theoretical knowledge, practical skills development, and continuous learning opportunities, anesthesiologists can enhance their proficiency and adapt to evolving techniques and technologies in this critical aspect of patient care.
Comprehensive training curricula typically include both theoretical and practical components. The theoretical aspect covers topics such as anatomy of the airway, principles of laryngoscopy, and potential complications. This foundational knowledge is essential for understanding the intricacies of the procedure and making informed decisions during challenging situations.
Practical training often begins with simulation-based learning, allowing trainees to practice laryngoscopy techniques in a controlled environment. Advanced simulation technologies, such as high-fidelity mannequins and virtual reality systems, provide realistic scenarios for skill development without risking patient safety. These tools enable learners to gain confidence and proficiency before performing procedures on actual patients.
Supervised clinical experience forms a critical part of the training process. Under the guidance of experienced anesthesiologists, trainees gradually progress from observing procedures to performing them independently. This hands-on experience is invaluable for developing the tactile skills and decision-making abilities necessary for successful laryngoscopy.
Continuing education is equally important for maintaining and updating skills throughout an anesthesiologist's career. Regular refresher courses, workshops, and conferences help practitioners stay current with the latest techniques, technologies, and safety protocols. These educational opportunities also provide a platform for sharing experiences and best practices among professionals.
The integration of new technologies, such as video laryngoscopes, into training programs has become increasingly important. These devices offer enhanced visualization of the airway and can be particularly useful in difficult intubation scenarios. Training on both traditional and video laryngoscopes ensures that anesthesiologists are prepared for a wide range of clinical situations.
Standardized assessment methods are crucial for evaluating the effectiveness of training programs and ensuring that practitioners meet the required competency levels. These assessments may include written exams, practical skills tests, and performance evaluations during simulated and real clinical scenarios.
In conclusion, comprehensive and ongoing training and education are fundamental to promoting safety and efficiency in laryngoscope use during anesthesia procedures. By combining theoretical knowledge, practical skills development, and continuous learning opportunities, anesthesiologists can enhance their proficiency and adapt to evolving techniques and technologies in this critical aspect of patient care.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!