LDPE in Medical Devices: Flexible and Sterile Solutions
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LDPE in Medical Devices: Evolution and Objectives
Low-density polyethylene (LDPE) has been a cornerstone material in medical device manufacturing since its introduction in the 1930s. Its journey in the medical field began with simple applications such as disposable syringes and has evolved to become an integral component in complex medical devices and equipment.
The evolution of LDPE in medical devices has been driven by the increasing demand for flexible, durable, and sterile materials in healthcare settings. Initially, LDPE was primarily used for packaging and basic disposable items. However, as medical technology advanced, so did the applications of LDPE. The material's properties, including its flexibility, chemical resistance, and ease of sterilization, made it an ideal candidate for more sophisticated medical applications.
Throughout the decades, researchers and manufacturers have continuously refined LDPE formulations to enhance its performance in medical environments. This ongoing development has led to the creation of specialized grades of LDPE tailored specifically for medical use, offering improved barrier properties, enhanced clarity, and better resistance to sterilization processes.
The objectives for LDPE in medical devices have expanded significantly over time. Today, the primary goals include developing LDPE variants that can withstand advanced sterilization techniques, such as gamma irradiation and ethylene oxide treatment, without compromising their physical or chemical properties. There is also a focus on creating LDPE formulations that offer improved biocompatibility and reduced risk of leaching, ensuring the safety of patients and healthcare professionals.
Another key objective is to enhance the material's sustainability profile. As environmental concerns grow, there is an increasing push to develop LDPE grades that are recyclable or biodegradable, without sacrificing the performance and safety standards required for medical applications. This aligns with the broader trend towards more environmentally friendly practices in the healthcare industry.
Looking ahead, the future objectives for LDPE in medical devices include further miniaturization capabilities to support the development of smaller, more precise medical instruments. There is also a growing interest in exploring the potential of LDPE in advanced drug delivery systems and smart medical devices, where the material's properties could be leveraged to create innovative solutions for patient care.
The evolution of LDPE in medical devices has been driven by the increasing demand for flexible, durable, and sterile materials in healthcare settings. Initially, LDPE was primarily used for packaging and basic disposable items. However, as medical technology advanced, so did the applications of LDPE. The material's properties, including its flexibility, chemical resistance, and ease of sterilization, made it an ideal candidate for more sophisticated medical applications.
Throughout the decades, researchers and manufacturers have continuously refined LDPE formulations to enhance its performance in medical environments. This ongoing development has led to the creation of specialized grades of LDPE tailored specifically for medical use, offering improved barrier properties, enhanced clarity, and better resistance to sterilization processes.
The objectives for LDPE in medical devices have expanded significantly over time. Today, the primary goals include developing LDPE variants that can withstand advanced sterilization techniques, such as gamma irradiation and ethylene oxide treatment, without compromising their physical or chemical properties. There is also a focus on creating LDPE formulations that offer improved biocompatibility and reduced risk of leaching, ensuring the safety of patients and healthcare professionals.
Another key objective is to enhance the material's sustainability profile. As environmental concerns grow, there is an increasing push to develop LDPE grades that are recyclable or biodegradable, without sacrificing the performance and safety standards required for medical applications. This aligns with the broader trend towards more environmentally friendly practices in the healthcare industry.
Looking ahead, the future objectives for LDPE in medical devices include further miniaturization capabilities to support the development of smaller, more precise medical instruments. There is also a growing interest in exploring the potential of LDPE in advanced drug delivery systems and smart medical devices, where the material's properties could be leveraged to create innovative solutions for patient care.
Market Demand for LDPE-based Medical Solutions
The market demand for LDPE-based medical solutions has been steadily increasing, driven by the growing healthcare industry and the unique properties of Low-Density Polyethylene (LDPE) that make it ideal for various medical applications. LDPE's flexibility, durability, and ability to maintain sterility have positioned it as a crucial material in the production of medical devices and packaging.
In the medical device sector, LDPE is widely used in the manufacture of tubing, catheters, and other disposable medical equipment. The global medical tubing market, where LDPE plays a significant role, has been experiencing robust growth. This growth is attributed to the rising prevalence of chronic diseases, increasing surgical procedures, and the expanding geriatric population worldwide.
The pharmaceutical packaging industry has also been a major driver of LDPE demand. LDPE is extensively used in the production of bottles, containers, and flexible packaging for medications and medical supplies. The pharmaceutical packaging market has been expanding due to the increasing production of generic drugs, the growth of the biopharmaceutical sector, and the rising demand for innovative drug delivery systems.
Another factor contributing to the market demand for LDPE-based medical solutions is the growing emphasis on infection control and prevention in healthcare settings. LDPE's resistance to chemicals and its ability to maintain sterility make it an excellent choice for packaging medical instruments and supplies. This has become particularly important in light of recent global health crises, which have heightened awareness of the need for effective sterilization and packaging solutions.
The COVID-19 pandemic has further accelerated the demand for LDPE in medical applications. The sudden surge in demand for personal protective equipment (PPE), testing kits, and other medical supplies has led to increased production of LDPE-based products. This trend is expected to continue even post-pandemic, as healthcare systems worldwide focus on improving their preparedness for future health emergencies.
Emerging markets, particularly in Asia-Pacific and Latin America, are presenting significant growth opportunities for LDPE-based medical solutions. The rapid expansion of healthcare infrastructure, increasing healthcare expenditure, and growing awareness about hygiene and infection control in these regions are driving the demand for high-quality medical devices and packaging solutions.
However, the market for LDPE-based medical solutions also faces challenges. Environmental concerns related to plastic waste have led to increased scrutiny of single-use plastics, including those used in medical applications. This has spurred research into biodegradable alternatives and recycling technologies for medical plastics. Despite these challenges, the unique properties of LDPE and its established role in ensuring patient safety continue to sustain its demand in the medical sector.
In the medical device sector, LDPE is widely used in the manufacture of tubing, catheters, and other disposable medical equipment. The global medical tubing market, where LDPE plays a significant role, has been experiencing robust growth. This growth is attributed to the rising prevalence of chronic diseases, increasing surgical procedures, and the expanding geriatric population worldwide.
The pharmaceutical packaging industry has also been a major driver of LDPE demand. LDPE is extensively used in the production of bottles, containers, and flexible packaging for medications and medical supplies. The pharmaceutical packaging market has been expanding due to the increasing production of generic drugs, the growth of the biopharmaceutical sector, and the rising demand for innovative drug delivery systems.
Another factor contributing to the market demand for LDPE-based medical solutions is the growing emphasis on infection control and prevention in healthcare settings. LDPE's resistance to chemicals and its ability to maintain sterility make it an excellent choice for packaging medical instruments and supplies. This has become particularly important in light of recent global health crises, which have heightened awareness of the need for effective sterilization and packaging solutions.
The COVID-19 pandemic has further accelerated the demand for LDPE in medical applications. The sudden surge in demand for personal protective equipment (PPE), testing kits, and other medical supplies has led to increased production of LDPE-based products. This trend is expected to continue even post-pandemic, as healthcare systems worldwide focus on improving their preparedness for future health emergencies.
Emerging markets, particularly in Asia-Pacific and Latin America, are presenting significant growth opportunities for LDPE-based medical solutions. The rapid expansion of healthcare infrastructure, increasing healthcare expenditure, and growing awareness about hygiene and infection control in these regions are driving the demand for high-quality medical devices and packaging solutions.
However, the market for LDPE-based medical solutions also faces challenges. Environmental concerns related to plastic waste have led to increased scrutiny of single-use plastics, including those used in medical applications. This has spurred research into biodegradable alternatives and recycling technologies for medical plastics. Despite these challenges, the unique properties of LDPE and its established role in ensuring patient safety continue to sustain its demand in the medical sector.
Current LDPE Applications and Challenges in Healthcare
Low-density polyethylene (LDPE) has become a cornerstone material in the healthcare industry, particularly in medical devices, due to its unique properties and versatility. Its flexibility, durability, and ability to maintain sterility have made it an ideal choice for a wide range of medical applications.
In the realm of medical packaging, LDPE is extensively used for creating sterile barriers and protective coverings. It is commonly employed in the production of medical bags, pouches, and wraps that safeguard instruments, implants, and other medical supplies from contamination. The material's excellent moisture barrier properties and resistance to punctures ensure the integrity of sterile products during storage and transportation.
LDPE also plays a crucial role in the manufacturing of medical tubing and catheters. Its flexibility allows for the creation of soft, pliable tubes that can navigate through complex anatomical structures with minimal discomfort to patients. This characteristic is particularly valuable in applications such as intravenous lines, drainage tubes, and feeding tubes, where patient comfort and ease of insertion are paramount.
In the field of prosthetics and orthotics, LDPE is utilized in the fabrication of custom-fit devices. Its malleability at relatively low temperatures enables the creation of personalized orthopedic supports, braces, and prosthetic components that can be easily molded to individual patient needs.
Despite its widespread use, LDPE faces several challenges in healthcare applications. One significant concern is its potential for leaching chemicals, particularly when exposed to certain medications or bodily fluids. This has led to increased scrutiny and the need for rigorous testing to ensure the safety of LDPE-based medical devices.
Another challenge lies in the material's limited resistance to high temperatures, which can restrict its use in applications requiring frequent sterilization through autoclaving. This limitation has prompted research into developing LDPE formulations with enhanced thermal stability or exploring alternative sterilization methods compatible with the material.
The growing emphasis on sustainability in healthcare has also presented challenges for LDPE usage. While the material is recyclable, the complex nature of medical waste often makes recycling difficult or impractical. This has spurred efforts to develop more environmentally friendly alternatives or improve the recyclability of LDPE-based medical products.
Lastly, the increasing demand for antimicrobial properties in medical devices has led to research into incorporating antimicrobial agents into LDPE. While promising, this approach faces challenges in maintaining the material's original properties and ensuring long-term effectiveness of the antimicrobial additives.
In the realm of medical packaging, LDPE is extensively used for creating sterile barriers and protective coverings. It is commonly employed in the production of medical bags, pouches, and wraps that safeguard instruments, implants, and other medical supplies from contamination. The material's excellent moisture barrier properties and resistance to punctures ensure the integrity of sterile products during storage and transportation.
LDPE also plays a crucial role in the manufacturing of medical tubing and catheters. Its flexibility allows for the creation of soft, pliable tubes that can navigate through complex anatomical structures with minimal discomfort to patients. This characteristic is particularly valuable in applications such as intravenous lines, drainage tubes, and feeding tubes, where patient comfort and ease of insertion are paramount.
In the field of prosthetics and orthotics, LDPE is utilized in the fabrication of custom-fit devices. Its malleability at relatively low temperatures enables the creation of personalized orthopedic supports, braces, and prosthetic components that can be easily molded to individual patient needs.
Despite its widespread use, LDPE faces several challenges in healthcare applications. One significant concern is its potential for leaching chemicals, particularly when exposed to certain medications or bodily fluids. This has led to increased scrutiny and the need for rigorous testing to ensure the safety of LDPE-based medical devices.
Another challenge lies in the material's limited resistance to high temperatures, which can restrict its use in applications requiring frequent sterilization through autoclaving. This limitation has prompted research into developing LDPE formulations with enhanced thermal stability or exploring alternative sterilization methods compatible with the material.
The growing emphasis on sustainability in healthcare has also presented challenges for LDPE usage. While the material is recyclable, the complex nature of medical waste often makes recycling difficult or impractical. This has spurred efforts to develop more environmentally friendly alternatives or improve the recyclability of LDPE-based medical products.
Lastly, the increasing demand for antimicrobial properties in medical devices has led to research into incorporating antimicrobial agents into LDPE. While promising, this approach faces challenges in maintaining the material's original properties and ensuring long-term effectiveness of the antimicrobial additives.
Existing LDPE Solutions for Medical Applications
01 Enhancing LDPE flexibility through blending
LDPE flexibility can be improved by blending it with other polymers or additives. This method allows for the customization of the material's properties, including increased flexibility, while maintaining other desirable characteristics of LDPE. The blending process can involve various compatible materials, resulting in a composite that offers enhanced flexibility for specific applications.- Enhancing LDPE flexibility through blending: LDPE flexibility can be improved by blending it with other polymers or additives. This method allows for the customization of the material's properties, including increased flexibility, while maintaining other desirable characteristics of LDPE. The blending process can involve various compatible materials, resulting in a composite that offers enhanced flexibility for specific applications.
- Modification of LDPE molecular structure: The flexibility of LDPE can be enhanced by modifying its molecular structure. This can be achieved through various methods such as controlled branching, adjusting molecular weight distribution, or introducing specific functional groups. These modifications can lead to improved chain mobility and reduced crystallinity, resulting in a more flexible LDPE material.
- Incorporation of plasticizers in LDPE: Plasticizers can be incorporated into LDPE to increase its flexibility. These additives work by reducing the intermolecular forces between polymer chains, allowing for greater movement and flexibility. The selection of appropriate plasticizers and their concentration can be tailored to achieve the desired level of flexibility while maintaining other important properties of the LDPE.
- Processing techniques for flexible LDPE: Various processing techniques can be employed to enhance the flexibility of LDPE. These may include specific extrusion methods, controlled cooling rates, or post-processing treatments. By optimizing these processes, the molecular orientation and crystalline structure of LDPE can be manipulated to improve its flexibility without compromising other essential properties.
- Nanocomposite approach for LDPE flexibility: Incorporating nanoparticles or nanofillers into LDPE can significantly enhance its flexibility. These nanocomposites can alter the polymer matrix structure, leading to improved chain mobility and reduced crystallinity. The selection of appropriate nanofillers and their dispersion within the LDPE matrix are crucial factors in achieving the desired flexibility enhancement.
02 Modification of LDPE molecular structure
The flexibility of LDPE can be enhanced by modifying its molecular structure. This can be achieved through various methods such as controlled branching, adjusting molecular weight distribution, or introducing specific functional groups. These modifications can lead to improved chain mobility and reduced crystallinity, resulting in increased flexibility of the polymer.Expand Specific Solutions03 Incorporation of plasticizers in LDPE
Plasticizers can be incorporated into LDPE to increase its flexibility. These additives work by reducing the intermolecular forces between polymer chains, allowing for greater mobility and flexibility. The selection of appropriate plasticizers and their concentration can be tailored to achieve the desired level of flexibility while maintaining other important properties of the material.Expand Specific Solutions04 Processing techniques for flexible LDPE
Various processing techniques can be employed to enhance the flexibility of LDPE. These may include specific extrusion methods, controlled cooling rates, or post-processing treatments. By optimizing these processes, the molecular orientation and crystalline structure of LDPE can be manipulated to achieve improved flexibility without compromising other essential properties.Expand Specific Solutions05 Development of flexible LDPE composites
Flexible LDPE composites can be developed by incorporating specific fillers or reinforcing materials. These composites combine the inherent properties of LDPE with the characteristics of the added components, resulting in materials with enhanced flexibility and potentially improved mechanical properties. The selection of appropriate fillers and their distribution within the LDPE matrix are crucial for achieving the desired flexibility.Expand Specific Solutions
Key Players in LDPE Medical Device Manufacturing
The LDPE medical device market is in a mature growth stage, characterized by established players and steady demand. The global market size for LDPE in medical devices is estimated to be in the billions of dollars, driven by increasing healthcare expenditure and aging populations. Technologically, LDPE production and application in medical devices are well-developed, with companies like Basell Polyolefine GmbH, Shandong Weigao Group, and China Petroleum & Chemical Corp leading in manufacturing. Innovation focuses on enhancing LDPE properties for specific medical applications. Major medical device manufacturers such as Roche Diagnostics, Baxter International, and Becton, Dickinson & Co are key end-users, integrating LDPE into various products. The competitive landscape is characterized by a mix of large petrochemical companies and specialized medical material suppliers.
Basell Polyolefine GmbH
Technical Solution: Basell Polyolefine GmbH has developed advanced LDPE formulations specifically tailored for medical device applications. Their LDPE solutions offer enhanced flexibility and sterilization resistance, crucial for medical products. The company utilizes a proprietary catalyst system that allows for precise control of molecular weight distribution, resulting in LDPE with improved mechanical properties and processability[1]. Their medical-grade LDPE undergoes rigorous testing to ensure compliance with ISO 10993 biocompatibility standards[2]. Basell's LDPE formulations also incorporate additives that enhance resistance to gamma and e-beam sterilization methods commonly used in the medical industry[3].
Strengths: Superior flexibility and sterilization resistance, precise control over material properties. Weaknesses: Potentially higher cost compared to standard LDPE grades, limited to specific medical applications.
Shandong Weigao Group Medical Polymer Co. Ltd.
Technical Solution: Shandong Weigao Group specializes in medical-grade LDPE for various healthcare applications. Their LDPE formulations are engineered to meet the stringent requirements of medical devices, offering excellent chemical resistance and low extractables[4]. The company employs a multi-stage polymerization process that results in LDPE with a balanced combination of flexibility and strength. Weigao's LDPE products undergo extensive quality control measures, including advanced chromatography techniques to ensure purity and consistency[5]. They have also developed LDPE blends that incorporate antimicrobial agents, enhancing the material's suitability for infection-sensitive medical applications[6].
Strengths: Specialized in medical-grade polymers, advanced quality control processes. Weaknesses: Limited global presence compared to larger multinational corporations.
Core Innovations in LDPE for Medical Use
High pressure LDPE for medical applications
PatentActiveUS20120220738A1
Innovation
- Development of a new LDPE with higher density and crystallinity, achieved through radical polymerization, allowing for higher melting and softening temperatures while maintaining a high melt flow rate, enabling faster and more effective sterilization processes.
Disposable sterilised medical bedpan protection
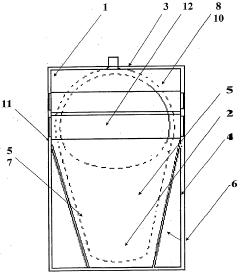
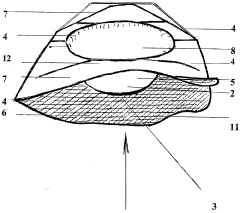
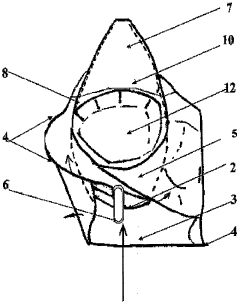
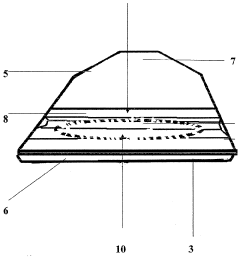
PatentWO2006035120A1
Innovation
- A disposable sterilized basin protector with a soluble or incinerable envelope that isolates the patient's skin from the medical basin, using soluble PVA film and incinerable materials, respectively, to create a waterproof barrier and absorbent sachet for urine and liquids, ensuring easy cleaning and disposal.
Regulatory Framework for LDPE in Medical Devices
The regulatory framework for LDPE in medical devices is a complex and evolving landscape that manufacturers must navigate to ensure compliance and patient safety. In the United States, the Food and Drug Administration (FDA) oversees the regulation of medical devices, including those containing LDPE. The FDA classifies medical devices into three categories based on their risk level, with Class I being the lowest risk and Class III being the highest.
For LDPE-containing medical devices, the classification depends on the specific application and intended use. Many LDPE devices fall under Class I or Class II, requiring manufacturers to follow general controls and, in some cases, special controls. General controls include registration of the manufacturing facility, device listing, and adherence to Good Manufacturing Practices (GMP).
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern the use of LDPE in medical devices. These regulations emphasize a risk-based approach to classification and require manufacturers to demonstrate the safety and performance of their devices through clinical evaluation and post-market surveillance.
The International Organization for Standardization (ISO) provides several standards relevant to LDPE in medical devices, such as ISO 10993 for biocompatibility testing and ISO 13485 for quality management systems. Adherence to these standards is often necessary to meet regulatory requirements in various markets.
Sterilization is a critical aspect of LDPE medical devices, and regulatory bodies require validation of sterilization processes. Common methods for LDPE devices include ethylene oxide (EtO) sterilization and gamma irradiation, both of which must be validated according to standards like ISO 11135 and ISO 11137, respectively.
Manufacturers must also consider regulations related to packaging and labeling of LDPE medical devices. This includes ensuring that packaging maintains sterility and providing clear instructions for use and disposal. In many jurisdictions, unique device identification (UDI) systems are becoming mandatory, requiring manufacturers to implement traceability measures throughout the product lifecycle.
As environmental concerns grow, regulations surrounding the disposal and recycling of LDPE medical devices are becoming more stringent. Manufacturers are increasingly required to consider the entire lifecycle of their products, including end-of-life management, in their regulatory strategies.
For LDPE-containing medical devices, the classification depends on the specific application and intended use. Many LDPE devices fall under Class I or Class II, requiring manufacturers to follow general controls and, in some cases, special controls. General controls include registration of the manufacturing facility, device listing, and adherence to Good Manufacturing Practices (GMP).
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern the use of LDPE in medical devices. These regulations emphasize a risk-based approach to classification and require manufacturers to demonstrate the safety and performance of their devices through clinical evaluation and post-market surveillance.
The International Organization for Standardization (ISO) provides several standards relevant to LDPE in medical devices, such as ISO 10993 for biocompatibility testing and ISO 13485 for quality management systems. Adherence to these standards is often necessary to meet regulatory requirements in various markets.
Sterilization is a critical aspect of LDPE medical devices, and regulatory bodies require validation of sterilization processes. Common methods for LDPE devices include ethylene oxide (EtO) sterilization and gamma irradiation, both of which must be validated according to standards like ISO 11135 and ISO 11137, respectively.
Manufacturers must also consider regulations related to packaging and labeling of LDPE medical devices. This includes ensuring that packaging maintains sterility and providing clear instructions for use and disposal. In many jurisdictions, unique device identification (UDI) systems are becoming mandatory, requiring manufacturers to implement traceability measures throughout the product lifecycle.
As environmental concerns grow, regulations surrounding the disposal and recycling of LDPE medical devices are becoming more stringent. Manufacturers are increasingly required to consider the entire lifecycle of their products, including end-of-life management, in their regulatory strategies.
Sustainability Aspects of LDPE in Healthcare
The sustainability aspects of LDPE in healthcare are becoming increasingly important as the medical industry seeks to balance patient care with environmental responsibility. LDPE, or Low-Density Polyethylene, has been a staple material in medical devices due to its flexibility, durability, and sterility. However, its environmental impact has come under scrutiny in recent years.
One of the primary sustainability concerns with LDPE in healthcare is its end-of-life management. Medical devices made from LDPE are often single-use items, contributing to the growing problem of medical waste. The disposal of these items in landfills or through incineration can lead to environmental pollution and greenhouse gas emissions. To address this issue, some healthcare facilities are implementing recycling programs specifically for LDPE medical waste, although challenges remain in terms of contamination and sorting.
The production of LDPE also raises sustainability questions. The material is derived from fossil fuels, a non-renewable resource. The extraction and processing of these raw materials contribute to carbon emissions and environmental degradation. However, advancements in manufacturing technologies are gradually reducing the carbon footprint of LDPE production. Some manufacturers are exploring the use of bio-based feedstocks to create more sustainable versions of LDPE, though these alternatives are not yet widely adopted in the medical field.
Energy consumption during the lifecycle of LDPE medical devices is another aspect of sustainability to consider. While the material itself is lightweight, which can reduce transportation-related emissions, the energy required for sterilization processes can be significant. Healthcare facilities are increasingly looking at more energy-efficient sterilization methods to mitigate this impact.
The durability of LDPE can be viewed as both a sustainability advantage and challenge. On one hand, its resistance to wear and tear means that LDPE devices can have a longer useful life, potentially reducing the need for frequent replacements. On the other hand, this durability makes LDPE persist in the environment for hundreds of years when not properly disposed of or recycled.
Efforts to improve the sustainability of LDPE in healthcare are ongoing. Research into biodegradable alternatives that maintain the necessary properties for medical use is progressing, although finding materials that match LDPE's performance while offering improved environmental profiles remains challenging. Additionally, some healthcare providers are reevaluating their use of disposable LDPE items, opting for reusable alternatives where possible without compromising patient safety or care quality.
The regulatory landscape is also evolving to address sustainability concerns. Some regions are implementing stricter regulations on plastic use in healthcare, pushing manufacturers to innovate and develop more environmentally friendly solutions. This regulatory pressure, combined with growing consumer awareness, is driving the industry towards more sustainable practices in the use and disposal of LDPE medical devices.
One of the primary sustainability concerns with LDPE in healthcare is its end-of-life management. Medical devices made from LDPE are often single-use items, contributing to the growing problem of medical waste. The disposal of these items in landfills or through incineration can lead to environmental pollution and greenhouse gas emissions. To address this issue, some healthcare facilities are implementing recycling programs specifically for LDPE medical waste, although challenges remain in terms of contamination and sorting.
The production of LDPE also raises sustainability questions. The material is derived from fossil fuels, a non-renewable resource. The extraction and processing of these raw materials contribute to carbon emissions and environmental degradation. However, advancements in manufacturing technologies are gradually reducing the carbon footprint of LDPE production. Some manufacturers are exploring the use of bio-based feedstocks to create more sustainable versions of LDPE, though these alternatives are not yet widely adopted in the medical field.
Energy consumption during the lifecycle of LDPE medical devices is another aspect of sustainability to consider. While the material itself is lightweight, which can reduce transportation-related emissions, the energy required for sterilization processes can be significant. Healthcare facilities are increasingly looking at more energy-efficient sterilization methods to mitigate this impact.
The durability of LDPE can be viewed as both a sustainability advantage and challenge. On one hand, its resistance to wear and tear means that LDPE devices can have a longer useful life, potentially reducing the need for frequent replacements. On the other hand, this durability makes LDPE persist in the environment for hundreds of years when not properly disposed of or recycled.
Efforts to improve the sustainability of LDPE in healthcare are ongoing. Research into biodegradable alternatives that maintain the necessary properties for medical use is progressing, although finding materials that match LDPE's performance while offering improved environmental profiles remains challenging. Additionally, some healthcare providers are reevaluating their use of disposable LDPE items, opting for reusable alternatives where possible without compromising patient safety or care quality.
The regulatory landscape is also evolving to address sustainability concerns. Some regions are implementing stricter regulations on plastic use in healthcare, pushing manufacturers to innovate and develop more environmentally friendly solutions. This regulatory pressure, combined with growing consumer awareness, is driving the industry towards more sustainable practices in the use and disposal of LDPE medical devices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!