Luminol Applications in Chemical Reaction Analysis
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Luminol Chemistry Background
Luminol, a versatile chemiluminescent compound, has been a cornerstone in forensic science and analytical chemistry for decades. Its unique property of emitting light when oxidized has made it an invaluable tool in various chemical reaction analyses. The chemistry behind luminol's luminescence is rooted in its molecular structure, which consists of a cyclic diacylhydrazide.
The luminol reaction is fundamentally an oxidation process. When luminol encounters an oxidizing agent in the presence of a catalyst, it undergoes a series of chemical transformations. This process results in the formation of an excited state intermediate, 3-aminophthalate. As this intermediate returns to its ground state, it releases energy in the form of light, producing the characteristic blue glow associated with luminol.
The oxidation of luminol can be triggered by various substances, including hydrogen peroxide, hypochlorite, and certain metal ions. In forensic applications, the iron in hemoglobin acts as a catalyst, making luminol particularly effective in detecting trace amounts of blood at crime scenes. This sensitivity to blood has made luminol an indispensable tool in criminal investigations for over half a century.
In the realm of chemical reaction analysis, luminol's applications extend far beyond forensics. Its chemiluminescent properties have been harnessed for a wide array of analytical techniques. For instance, in environmental monitoring, luminol-based systems are used to detect trace amounts of metal ions in water samples. The intensity of the light emitted is proportional to the concentration of the analyte, allowing for quantitative analysis.
The versatility of luminol chemistry has led to its integration into various analytical instruments. Flow injection analysis systems, for example, utilize luminol reactions for rapid and sensitive detection of analytes in continuous flow setups. These systems have found applications in pharmaceutical quality control, food safety testing, and clinical diagnostics.
Recent advancements in luminol chemistry have focused on enhancing its sensitivity and specificity. Researchers have developed modified luminol derivatives with improved luminescence properties and tailored reactivity. These innovations have expanded the range of detectable analytes and lowered detection limits, pushing the boundaries of chemical analysis.
The ongoing exploration of luminol's potential has also led to its application in emerging fields such as nanotechnology and biosensing. Luminol-functionalized nanoparticles have been developed for ultra-sensitive detection of biomolecules, opening new avenues in medical diagnostics and environmental monitoring.
As we continue to unravel the intricacies of luminol chemistry, its role in chemical reaction analysis is likely to grow. The combination of its sensitivity, versatility, and the non-destructive nature of chemiluminescence makes luminol an enduring and evolving tool in the analytical chemist's arsenal.
The luminol reaction is fundamentally an oxidation process. When luminol encounters an oxidizing agent in the presence of a catalyst, it undergoes a series of chemical transformations. This process results in the formation of an excited state intermediate, 3-aminophthalate. As this intermediate returns to its ground state, it releases energy in the form of light, producing the characteristic blue glow associated with luminol.
The oxidation of luminol can be triggered by various substances, including hydrogen peroxide, hypochlorite, and certain metal ions. In forensic applications, the iron in hemoglobin acts as a catalyst, making luminol particularly effective in detecting trace amounts of blood at crime scenes. This sensitivity to blood has made luminol an indispensable tool in criminal investigations for over half a century.
In the realm of chemical reaction analysis, luminol's applications extend far beyond forensics. Its chemiluminescent properties have been harnessed for a wide array of analytical techniques. For instance, in environmental monitoring, luminol-based systems are used to detect trace amounts of metal ions in water samples. The intensity of the light emitted is proportional to the concentration of the analyte, allowing for quantitative analysis.
The versatility of luminol chemistry has led to its integration into various analytical instruments. Flow injection analysis systems, for example, utilize luminol reactions for rapid and sensitive detection of analytes in continuous flow setups. These systems have found applications in pharmaceutical quality control, food safety testing, and clinical diagnostics.
Recent advancements in luminol chemistry have focused on enhancing its sensitivity and specificity. Researchers have developed modified luminol derivatives with improved luminescence properties and tailored reactivity. These innovations have expanded the range of detectable analytes and lowered detection limits, pushing the boundaries of chemical analysis.
The ongoing exploration of luminol's potential has also led to its application in emerging fields such as nanotechnology and biosensing. Luminol-functionalized nanoparticles have been developed for ultra-sensitive detection of biomolecules, opening new avenues in medical diagnostics and environmental monitoring.
As we continue to unravel the intricacies of luminol chemistry, its role in chemical reaction analysis is likely to grow. The combination of its sensitivity, versatility, and the non-destructive nature of chemiluminescence makes luminol an enduring and evolving tool in the analytical chemist's arsenal.
Market Demand Analysis
The market demand for luminol applications in chemical reaction analysis has been steadily growing, driven by advancements in forensic science, environmental monitoring, and biomedical research. Luminol, a chemiluminescent compound, has gained significant traction due to its unique ability to detect trace amounts of blood and other biological materials, making it invaluable in crime scene investigations and medical diagnostics.
In the forensic science sector, the global market for luminol-based products is expanding rapidly. Law enforcement agencies and forensic laboratories worldwide are increasingly adopting luminol-based technologies for crime scene analysis, particularly in cases involving blood evidence detection. This trend is expected to continue as the need for more accurate and sensitive detection methods grows in response to evolving criminal techniques.
The environmental monitoring industry has also recognized the potential of luminol in detecting pollutants and contaminants in water bodies. As global concerns about water quality and environmental safety intensify, the demand for luminol-based sensors and testing kits is projected to rise. These applications are particularly relevant in industrial areas where chemical spills and contamination are significant risks.
In the biomedical research field, luminol's chemiluminescent properties have found applications in various analytical techniques, including Western blotting and immunoassays. The increasing focus on personalized medicine and the need for more sensitive diagnostic tools are driving the demand for luminol-based reagents in medical laboratories and research institutions.
The pharmaceutical industry has shown growing interest in luminol applications for drug discovery and development processes. Luminol-based assays are being used to study enzyme kinetics, cellular processes, and drug interactions, contributing to more efficient and cost-effective drug development pipelines.
Market analysts predict that the global market for luminol and related chemiluminescent compounds in chemical reaction analysis will experience substantial growth over the next five years. This growth is attributed to the compound's versatility, sensitivity, and cost-effectiveness compared to alternative detection methods.
However, the market faces challenges such as the need for specialized training in luminol application techniques and the development of more stable formulations to extend shelf life and improve reliability in field conditions. These challenges present opportunities for innovation and product development in the luminol market.
As research continues to uncover new applications for luminol in chemical reaction analysis, the market is expected to diversify further. Emerging areas of interest include its use in food safety testing, environmental remediation, and even in the development of novel imaging techniques for medical diagnostics.
In the forensic science sector, the global market for luminol-based products is expanding rapidly. Law enforcement agencies and forensic laboratories worldwide are increasingly adopting luminol-based technologies for crime scene analysis, particularly in cases involving blood evidence detection. This trend is expected to continue as the need for more accurate and sensitive detection methods grows in response to evolving criminal techniques.
The environmental monitoring industry has also recognized the potential of luminol in detecting pollutants and contaminants in water bodies. As global concerns about water quality and environmental safety intensify, the demand for luminol-based sensors and testing kits is projected to rise. These applications are particularly relevant in industrial areas where chemical spills and contamination are significant risks.
In the biomedical research field, luminol's chemiluminescent properties have found applications in various analytical techniques, including Western blotting and immunoassays. The increasing focus on personalized medicine and the need for more sensitive diagnostic tools are driving the demand for luminol-based reagents in medical laboratories and research institutions.
The pharmaceutical industry has shown growing interest in luminol applications for drug discovery and development processes. Luminol-based assays are being used to study enzyme kinetics, cellular processes, and drug interactions, contributing to more efficient and cost-effective drug development pipelines.
Market analysts predict that the global market for luminol and related chemiluminescent compounds in chemical reaction analysis will experience substantial growth over the next five years. This growth is attributed to the compound's versatility, sensitivity, and cost-effectiveness compared to alternative detection methods.
However, the market faces challenges such as the need for specialized training in luminol application techniques and the development of more stable formulations to extend shelf life and improve reliability in field conditions. These challenges present opportunities for innovation and product development in the luminol market.
As research continues to uncover new applications for luminol in chemical reaction analysis, the market is expected to diversify further. Emerging areas of interest include its use in food safety testing, environmental remediation, and even in the development of novel imaging techniques for medical diagnostics.
Current Challenges
Despite the widespread use of luminol in chemical reaction analysis, several challenges persist in its application. One of the primary issues is the sensitivity of luminol to environmental factors, particularly pH levels. The luminescent reaction is highly dependent on the pH of the solution, with optimal results typically achieved in alkaline conditions. This sensitivity can lead to inconsistent results when analyzing samples with varying pH levels, requiring careful calibration and standardization of test conditions.
Another significant challenge is the potential for interference from other substances present in the sample. Luminol can react with various compounds, including metal ions, peroxides, and certain organic molecules, which may produce false-positive results or mask the presence of the target analyte. This interference can be particularly problematic in complex matrices such as biological samples or environmental specimens, where multiple chemical species coexist.
The stability of luminol solutions over time also presents a challenge for long-term storage and consistent analysis. Luminol solutions can degrade when exposed to light or heat, leading to a reduction in chemiluminescent intensity and potentially affecting the accuracy of quantitative measurements. This necessitates careful handling and storage protocols, as well as frequent preparation of fresh solutions to maintain reliability.
Quantification of analytes using luminol-based methods can be challenging due to the transient nature of the chemiluminescent signal. The light emission occurs rapidly and decays over time, making it difficult to achieve precise and reproducible measurements, especially for low-concentration analytes. This issue is compounded by the fact that the intensity of the luminescent signal is not always directly proportional to the concentration of the target compound, particularly at high concentrations where quenching effects may occur.
The limited selectivity of luminol reactions poses another challenge in chemical analysis. While luminol is sensitive to a range of oxidizing agents and catalysts, it lacks specificity for individual compounds. This can make it difficult to distinguish between different analytes that may trigger a similar chemiluminescent response, necessitating additional separation or identification steps in complex analytical procedures.
Lastly, the optimization of reaction conditions for specific analytical applications remains a significant challenge. Factors such as reagent concentrations, reaction time, and the presence of enhancers or catalysts can all affect the intensity and duration of the chemiluminescent signal. Developing robust and reproducible methods for different types of samples and analytes requires extensive experimentation and validation, which can be time-consuming and resource-intensive.
Another significant challenge is the potential for interference from other substances present in the sample. Luminol can react with various compounds, including metal ions, peroxides, and certain organic molecules, which may produce false-positive results or mask the presence of the target analyte. This interference can be particularly problematic in complex matrices such as biological samples or environmental specimens, where multiple chemical species coexist.
The stability of luminol solutions over time also presents a challenge for long-term storage and consistent analysis. Luminol solutions can degrade when exposed to light or heat, leading to a reduction in chemiluminescent intensity and potentially affecting the accuracy of quantitative measurements. This necessitates careful handling and storage protocols, as well as frequent preparation of fresh solutions to maintain reliability.
Quantification of analytes using luminol-based methods can be challenging due to the transient nature of the chemiluminescent signal. The light emission occurs rapidly and decays over time, making it difficult to achieve precise and reproducible measurements, especially for low-concentration analytes. This issue is compounded by the fact that the intensity of the luminescent signal is not always directly proportional to the concentration of the target compound, particularly at high concentrations where quenching effects may occur.
The limited selectivity of luminol reactions poses another challenge in chemical analysis. While luminol is sensitive to a range of oxidizing agents and catalysts, it lacks specificity for individual compounds. This can make it difficult to distinguish between different analytes that may trigger a similar chemiluminescent response, necessitating additional separation or identification steps in complex analytical procedures.
Lastly, the optimization of reaction conditions for specific analytical applications remains a significant challenge. Factors such as reagent concentrations, reaction time, and the presence of enhancers or catalysts can all affect the intensity and duration of the chemiluminescent signal. Developing robust and reproducible methods for different types of samples and analytes requires extensive experimentation and validation, which can be time-consuming and resource-intensive.
Existing Luminol Solutions
01 Luminol in forensic applications
Luminol is widely used in forensic science for detecting trace amounts of blood at crime scenes. When luminol comes into contact with the iron in hemoglobin, it produces a bright blue chemiluminescence. This reaction is highly sensitive and can detect blood even after cleaning attempts. Forensic investigators use luminol-based solutions to spray suspected areas and photograph the resulting luminescence in darkened conditions.- Luminol in chemiluminescence detection: Luminol is widely used in chemiluminescence detection methods for various applications. It produces a bright blue light when oxidized, making it useful for detecting blood traces in forensic investigations, analyzing biological samples, and environmental monitoring. The chemiluminescent reaction of luminol can be enhanced or modified to improve sensitivity and specificity in different detection systems.
- Luminol-based biosensors and immunoassays: Luminol is incorporated into biosensors and immunoassay systems for detecting specific biomolecules or pathogens. These systems often combine luminol with enzymes, antibodies, or other biological recognition elements to create highly sensitive and selective detection methods. The chemiluminescent signal produced by luminol can be used to quantify target analytes in medical diagnostics, food safety testing, and environmental analysis.
- Luminol derivatives and modifications: Research focuses on developing luminol derivatives and modifications to enhance its properties for specific applications. These modifications can improve solubility, stability, or reactivity of luminol, leading to more efficient chemiluminescent reactions. Some derivatives are designed to emit light at different wavelengths or to be more sensitive to specific catalysts or oxidizing agents.
- Luminol in imaging and visualization techniques: Luminol is utilized in various imaging and visualization techniques, particularly in biological and medical research. It can be used to visualize enzyme activity, detect reactive oxygen species in cells, or image blood flow in tissues. Advanced imaging systems combine luminol-based chemiluminescence with other detection methods to provide detailed information about biological processes or material properties.
- Industrial and environmental applications of luminol: Luminol finds applications in industrial processes and environmental monitoring. It can be used to detect metal ions, assess water quality, or monitor industrial effluents. In some cases, luminol-based systems are integrated into automated monitoring equipment or portable devices for on-site testing. The chemiluminescent properties of luminol make it valuable for detecting trace contaminants or process inefficiencies in various industrial settings.
02 Luminol in medical diagnostics
Luminol has applications in medical diagnostics, particularly in detecting and quantifying various biological molecules. It can be used in immunoassays, where the luminol reaction is coupled with specific antibodies to detect target proteins or other biomolecules. This technique is valuable in clinical laboratories for diagnosing diseases and monitoring treatment progress.Expand Specific Solutions03 Enhanced luminol formulations
Researchers have developed enhanced luminol formulations to improve its sensitivity and specificity. These formulations may include additives that intensify the chemiluminescence, stabilize the reaction, or reduce interference from other substances. Some formulations also incorporate nanoparticles or other advanced materials to enhance the luminol's performance in various applications.Expand Specific Solutions04 Luminol in environmental monitoring
Luminol-based systems are used in environmental monitoring to detect pollutants and contaminants. These applications include testing for heavy metals in water samples, monitoring industrial effluents, and assessing soil contamination. The high sensitivity of luminol reactions allows for the detection of trace amounts of certain pollutants, making it a valuable tool in environmental protection efforts.Expand Specific Solutions05 Luminol in analytical chemistry
In analytical chemistry, luminol is used in various techniques for quantitative and qualitative analysis. It serves as a chemiluminescent indicator in flow injection analysis, high-performance liquid chromatography, and other analytical methods. These applications allow for the detection and measurement of a wide range of chemical species, including metal ions, organic compounds, and enzymes.Expand Specific Solutions
Key Industry Players
The luminol applications in chemical reaction analysis market is in a growth phase, driven by increasing demand for sensitive detection methods in forensics, biochemistry, and environmental monitoring. The market size is expanding, with a projected CAGR of 5-7% over the next five years. Technologically, luminol-based techniques are mature but continue to evolve, with companies like Promega Corp. and Cyanagen Srl leading in reagent development. Academic institutions such as Washington University in St. Louis and the University of South Florida are advancing research in novel applications. While established players dominate, there's room for innovation, particularly in enhancing sensitivity and specificity of luminol-based assays.
Promega Corp.
Technical Solution: Promega Corp. has developed advanced luminol-based chemiluminescence systems for chemical reaction analysis. Their technology utilizes enhanced luminol derivatives and optimized reaction conditions to achieve higher sensitivity and longer-lasting light emission[1]. The company's luminol reagents are designed for various applications, including forensic blood detection, environmental contaminant analysis, and biomedical research. Promega's luminol kits incorporate stabilizers and enhancers that improve the overall performance and reliability of the chemiluminescent reaction[2]. They have also introduced automated systems for high-throughput luminol-based assays, enabling rapid and accurate chemical analysis in research and industrial settings[3].
Strengths: High sensitivity, long-lasting emission, versatile applications. Weaknesses: May require specialized equipment, potential for false positives in some applications.
Cyanagen Srl
Technical Solution: Cyanagen Srl specializes in chemiluminescence reagents and has developed proprietary luminol-based formulations for chemical reaction analysis. Their technology focuses on enhancing the stability and intensity of luminol reactions through the use of novel catalysts and reaction accelerators[4]. Cyanagen's luminol systems are designed for ultra-sensitive detection of various analytes in complex matrices, with applications in environmental monitoring, food safety, and clinical diagnostics. The company has also introduced luminol derivatives with improved solubility and reduced background noise, allowing for more precise quantification of chemical reactions[5]. Cyanagen's luminol reagents are compatible with a wide range of detection instruments, making them suitable for both research and industrial applications[6].
Strengths: Ultra-sensitive detection, reduced background noise, broad instrument compatibility. Weaknesses: May be more expensive than traditional luminol reagents, requires optimization for specific applications.
Core Luminol Innovations
Photodynamic therapy using chemiluminescence and a ligand-photosensitiser conjugate
PatentInactiveUS20100297762A1
Innovation
- A method involving a ligand-toxin conjugate (LTC) comprising a photosensitizer like hematoporphyrin conjugated with transferrin, combined with a chemiluminescent agent such as luminol, which activates the photosensitizer intracellularly to produce reactive oxygen species, thereby enhancing target cell destruction without requiring external light.
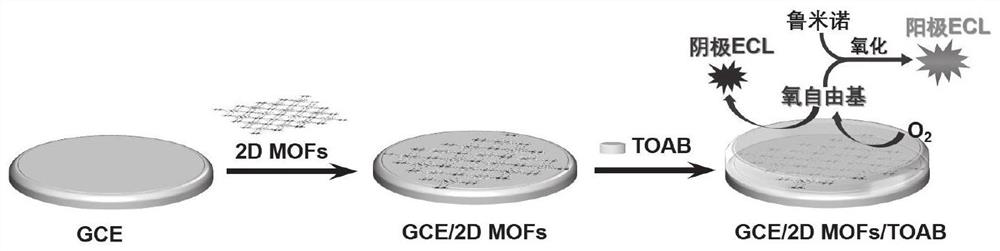
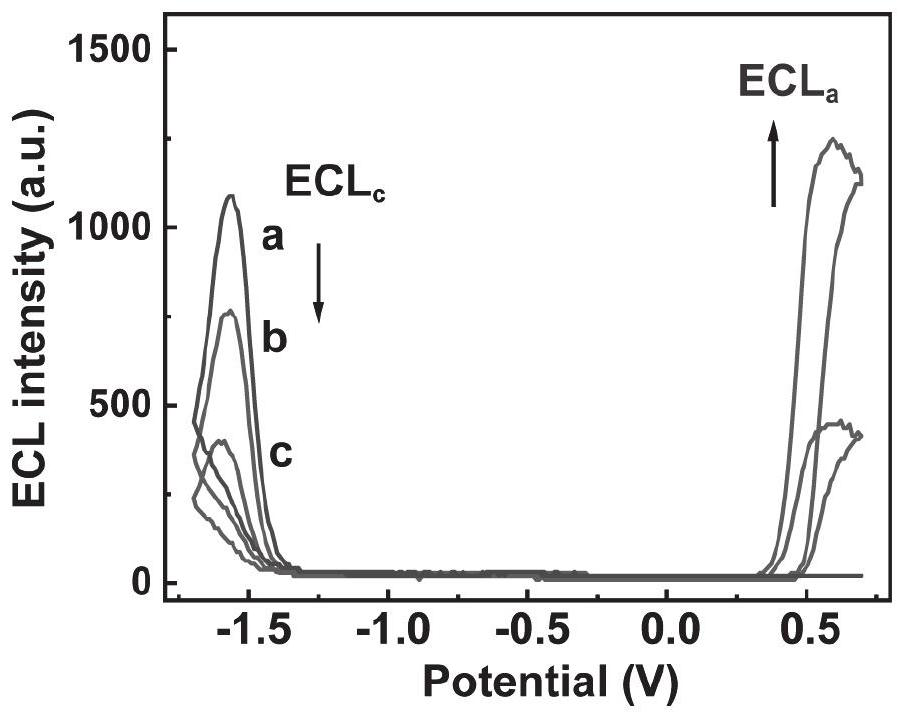
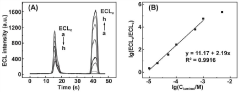
Two-dimensional porphyrin MOFs/TOAB modified electrode and preparation method and application thereof
PatentActiveCN113916953A
Innovation
- The two-dimensional porphyrin MOFs/TOAB modified electrode is used to produce a competitive catalytic reaction with luminol in an aqueous electrolyte containing dissolved oxygen. Through the two-dimensional porphyrin metal organic framework material and tetraoctylammonium bromide (TOAB) Modified glassy carbon electrode to achieve highly sensitive detection of luminol.
Safety and Handling
Luminol, a versatile chemiluminescent compound, requires careful handling and safety considerations in chemical reaction analysis applications. Proper safety protocols are essential to protect researchers and ensure accurate results. Personal protective equipment (PPE) is mandatory when working with luminol, including safety goggles, lab coats, and gloves to prevent skin contact or accidental ingestion.
Luminol solutions should be prepared in a well-ventilated area or fume hood to minimize inhalation risks. The compound is sensitive to light and temperature, necessitating storage in dark, cool conditions to maintain its efficacy. Researchers must be aware of luminol's potential to cause skin and eye irritation, implementing immediate washing procedures in case of exposure.
When conducting experiments, it is crucial to use appropriate containment measures to prevent spills or unintended reactions. Luminol should not be mixed with incompatible substances, as this may lead to hazardous reactions or compromise analytical results. Proper disposal of luminol-containing waste is essential, adhering to local regulations and environmental guidelines.
Training and education on luminol's properties and handling procedures are vital for all personnel involved in its use. This includes understanding the compound's reactivity, potential hazards, and emergency response protocols. Regular safety audits and equipment checks should be conducted to ensure compliance with established safety standards.
In the context of chemical reaction analysis, researchers must consider the potential interference of luminol with other reagents or analytes. Careful experimental design and control measures are necessary to isolate luminol's effects and maintain the integrity of analytical results. Additionally, the use of luminol in forensic applications requires strict chain-of-custody procedures to preserve evidence integrity.
Environmental considerations are also important when handling luminol. While generally considered low-toxicity, proper containment and disposal practices prevent environmental contamination. Researchers should be aware of any regulatory requirements or reporting obligations related to luminol use in their specific applications.
By implementing comprehensive safety and handling protocols, researchers can harness the full potential of luminol in chemical reaction analysis while minimizing risks and ensuring reliable, reproducible results. These measures not only protect personnel but also contribute to the overall quality and credibility of scientific investigations utilizing luminol-based techniques.
Luminol solutions should be prepared in a well-ventilated area or fume hood to minimize inhalation risks. The compound is sensitive to light and temperature, necessitating storage in dark, cool conditions to maintain its efficacy. Researchers must be aware of luminol's potential to cause skin and eye irritation, implementing immediate washing procedures in case of exposure.
When conducting experiments, it is crucial to use appropriate containment measures to prevent spills or unintended reactions. Luminol should not be mixed with incompatible substances, as this may lead to hazardous reactions or compromise analytical results. Proper disposal of luminol-containing waste is essential, adhering to local regulations and environmental guidelines.
Training and education on luminol's properties and handling procedures are vital for all personnel involved in its use. This includes understanding the compound's reactivity, potential hazards, and emergency response protocols. Regular safety audits and equipment checks should be conducted to ensure compliance with established safety standards.
In the context of chemical reaction analysis, researchers must consider the potential interference of luminol with other reagents or analytes. Careful experimental design and control measures are necessary to isolate luminol's effects and maintain the integrity of analytical results. Additionally, the use of luminol in forensic applications requires strict chain-of-custody procedures to preserve evidence integrity.
Environmental considerations are also important when handling luminol. While generally considered low-toxicity, proper containment and disposal practices prevent environmental contamination. Researchers should be aware of any regulatory requirements or reporting obligations related to luminol use in their specific applications.
By implementing comprehensive safety and handling protocols, researchers can harness the full potential of luminol in chemical reaction analysis while minimizing risks and ensuring reliable, reproducible results. These measures not only protect personnel but also contribute to the overall quality and credibility of scientific investigations utilizing luminol-based techniques.
Environmental Impact
The environmental impact of luminol applications in chemical reaction analysis is a crucial aspect to consider, given the increasing focus on sustainable practices in scientific research. Luminol, a chemiluminescent compound widely used in forensic science and analytical chemistry, has both positive and negative implications for the environment.
One of the primary environmental benefits of luminol-based analysis is its high sensitivity, which allows for the detection of minute traces of substances. This sensitivity reduces the need for large sample sizes, thereby minimizing waste generation and resource consumption in laboratory settings. Additionally, the non-destructive nature of luminol reactions preserves evidence integrity, potentially reducing the need for repeated sampling and analysis.
However, the production and disposal of luminol and its associated reagents pose potential environmental risks. The synthesis of luminol involves several chemical processes that may generate hazardous by-products. Proper waste management protocols are essential to prevent these chemicals from entering ecosystems through improper disposal or accidental release.
The alkaline conditions required for luminol reactions can also be a concern. If not properly neutralized, the high pH solutions used in luminol-based analyses could potentially harm aquatic environments if released into water systems. This underscores the importance of implementing robust waste treatment procedures in laboratories utilizing luminol techniques.
Furthermore, the use of hydrogen peroxide as an oxidizing agent in luminol reactions contributes to the overall environmental footprint. While hydrogen peroxide eventually breaks down into water and oxygen, its production and transportation have associated carbon emissions and energy costs that should be considered in a comprehensive environmental assessment.
On a positive note, the development of more environmentally friendly luminol formulations and reaction conditions is an active area of research. Scientists are exploring ways to reduce the environmental impact of luminol applications, such as using alternative oxidizing agents or developing recyclable luminol-based systems.
In conclusion, while luminol applications in chemical reaction analysis offer significant benefits in terms of sensitivity and efficiency, their environmental impact requires careful consideration. Balancing the analytical advantages with responsible environmental practices is crucial for the sustainable use of luminol in scientific research and forensic applications.
One of the primary environmental benefits of luminol-based analysis is its high sensitivity, which allows for the detection of minute traces of substances. This sensitivity reduces the need for large sample sizes, thereby minimizing waste generation and resource consumption in laboratory settings. Additionally, the non-destructive nature of luminol reactions preserves evidence integrity, potentially reducing the need for repeated sampling and analysis.
However, the production and disposal of luminol and its associated reagents pose potential environmental risks. The synthesis of luminol involves several chemical processes that may generate hazardous by-products. Proper waste management protocols are essential to prevent these chemicals from entering ecosystems through improper disposal or accidental release.
The alkaline conditions required for luminol reactions can also be a concern. If not properly neutralized, the high pH solutions used in luminol-based analyses could potentially harm aquatic environments if released into water systems. This underscores the importance of implementing robust waste treatment procedures in laboratories utilizing luminol techniques.
Furthermore, the use of hydrogen peroxide as an oxidizing agent in luminol reactions contributes to the overall environmental footprint. While hydrogen peroxide eventually breaks down into water and oxygen, its production and transportation have associated carbon emissions and energy costs that should be considered in a comprehensive environmental assessment.
On a positive note, the development of more environmentally friendly luminol formulations and reaction conditions is an active area of research. Scientists are exploring ways to reduce the environmental impact of luminol applications, such as using alternative oxidizing agents or developing recyclable luminol-based systems.
In conclusion, while luminol applications in chemical reaction analysis offer significant benefits in terms of sensitivity and efficiency, their environmental impact requires careful consideration. Balancing the analytical advantages with responsible environmental practices is crucial for the sustainable use of luminol in scientific research and forensic applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!