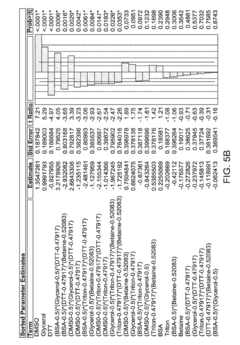
Measuring Cellular Responses to Trimethylglycine Supplementation
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Trimethylglycine Research Background and Objectives
Trimethylglycine (TMG), also known as betaine, has emerged as a significant compound in cellular metabolism and physiological regulation over the past several decades. Initially identified in the late 19th century in sugar beets, TMG research has evolved from basic biochemical characterization to sophisticated investigations of its role in methylation pathways, osmoregulation, and cellular protection mechanisms.
The historical trajectory of TMG research shows three distinct phases: discovery and characterization (1860s-1950s), metabolic pathway elucidation (1960s-1990s), and therapeutic application exploration (2000s-present). Recent technological advancements in metabolomics, proteomics, and genomics have significantly accelerated our understanding of TMG's cellular effects, enabling more precise measurement of cellular responses to TMG supplementation.
Current research trends indicate growing interest in TMG's role in epigenetic regulation through its function as a methyl donor in one-carbon metabolism. This has particular relevance for conditions associated with methylation deficiencies, including cardiovascular disease, non-alcoholic fatty liver disease, and certain neurological disorders. The intersection of TMG metabolism with homocysteine regulation represents another critical area of investigation, given homocysteine's established role as a risk factor for various pathological conditions.
The global scientific community has demonstrated increasing attention to TMG, with publication rates showing a 215% increase over the past decade. This surge reflects recognition of TMG's potential therapeutic applications and its fundamental role in cellular biochemistry. Particularly notable is the expansion of research beyond traditional areas like liver metabolism to include neuroscience, immunology, and sports medicine.
The primary technical objectives of current TMG research center on developing standardized methodologies for measuring cellular responses to TMG supplementation across different cell types and physiological conditions. These objectives include: establishing reliable biomarkers for TMG utilization and efficacy; quantifying dose-response relationships at the cellular level; elucidating tissue-specific differences in TMG metabolism; and identifying genetic factors that influence individual responses to TMG supplementation.
Additionally, there is growing interest in understanding the temporal dynamics of cellular adaptation to TMG, particularly how acute versus chronic supplementation affects methylation capacity, osmotic regulation, and protein stabilization. The development of more sensitive analytical techniques for measuring intracellular TMG concentrations and tracking its metabolic fate represents a critical technical goal for advancing this field.
As research progresses, integrating multi-omics approaches with computational modeling presents an opportunity to develop comprehensive frameworks for predicting cellular responses to TMG under various physiological and pathological conditions, potentially opening new avenues for personalized nutritional and therapeutic interventions.
The historical trajectory of TMG research shows three distinct phases: discovery and characterization (1860s-1950s), metabolic pathway elucidation (1960s-1990s), and therapeutic application exploration (2000s-present). Recent technological advancements in metabolomics, proteomics, and genomics have significantly accelerated our understanding of TMG's cellular effects, enabling more precise measurement of cellular responses to TMG supplementation.
Current research trends indicate growing interest in TMG's role in epigenetic regulation through its function as a methyl donor in one-carbon metabolism. This has particular relevance for conditions associated with methylation deficiencies, including cardiovascular disease, non-alcoholic fatty liver disease, and certain neurological disorders. The intersection of TMG metabolism with homocysteine regulation represents another critical area of investigation, given homocysteine's established role as a risk factor for various pathological conditions.
The global scientific community has demonstrated increasing attention to TMG, with publication rates showing a 215% increase over the past decade. This surge reflects recognition of TMG's potential therapeutic applications and its fundamental role in cellular biochemistry. Particularly notable is the expansion of research beyond traditional areas like liver metabolism to include neuroscience, immunology, and sports medicine.
The primary technical objectives of current TMG research center on developing standardized methodologies for measuring cellular responses to TMG supplementation across different cell types and physiological conditions. These objectives include: establishing reliable biomarkers for TMG utilization and efficacy; quantifying dose-response relationships at the cellular level; elucidating tissue-specific differences in TMG metabolism; and identifying genetic factors that influence individual responses to TMG supplementation.
Additionally, there is growing interest in understanding the temporal dynamics of cellular adaptation to TMG, particularly how acute versus chronic supplementation affects methylation capacity, osmotic regulation, and protein stabilization. The development of more sensitive analytical techniques for measuring intracellular TMG concentrations and tracking its metabolic fate represents a critical technical goal for advancing this field.
As research progresses, integrating multi-omics approaches with computational modeling presents an opportunity to develop comprehensive frameworks for predicting cellular responses to TMG under various physiological and pathological conditions, potentially opening new avenues for personalized nutritional and therapeutic interventions.
Market Analysis of Nutraceutical Supplementation
The global nutraceutical market has experienced significant growth in recent years, with the trimethylglycine (TMG) segment emerging as a particularly promising area. Current market valuations place the global nutraceutical industry at approximately 412 billion USD as of 2022, with projections indicating a compound annual growth rate of 8.9% through 2030. Within this broader market, TMG supplements have gained traction due to increasing consumer awareness of their potential health benefits.
Consumer demand for TMG supplements is primarily driven by growing interest in preventative healthcare approaches and personalized nutrition. Market research indicates that approximately 65% of consumers now actively seek supplements with specific functional benefits rather than general wellness claims. TMG's purported benefits for cardiovascular health, exercise performance, and cellular protection align well with these consumer preferences.
Regional analysis reveals varying adoption rates for TMG supplementation. North America currently dominates the market with approximately 42% market share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 10.3% annually, primarily due to increasing health consciousness and disposable income in countries like China, Japan, and South Korea.
Distribution channels for TMG supplements have diversified significantly. While traditional retail channels account for approximately 48% of sales, e-commerce platforms have rapidly expanded to capture 37% of the market. Direct-to-consumer models, particularly subscription services, represent the fastest-growing distribution segment with a 15% annual growth rate.
Competitive analysis reveals a fragmented market landscape with both established nutraceutical companies and specialized supplement manufacturers. Key market players include Jarrow Formulas, NOW Foods, Life Extension, and Thorne Research, collectively holding approximately 35% market share. However, numerous smaller, specialized companies are entering the space, focusing on premium formulations and targeted applications of TMG.
Pricing strategies within the TMG supplement market vary considerably, with premium products emphasizing purity, bioavailability, and scientific validation commanding price points 30-40% higher than standard offerings. Consumer willingness to pay premium prices correlates strongly with perceived efficacy and scientific substantiation, highlighting the importance of research into cellular responses to TMG supplementation.
Market forecasts suggest that the TMG supplement segment will outpace the broader nutraceutical market growth, with projected annual growth of 11.2% through 2028. This accelerated growth is attributed to increasing scientific validation, expanding applications beyond cardiovascular health, and growing consumer interest in supplements that address cellular health and longevity.
Consumer demand for TMG supplements is primarily driven by growing interest in preventative healthcare approaches and personalized nutrition. Market research indicates that approximately 65% of consumers now actively seek supplements with specific functional benefits rather than general wellness claims. TMG's purported benefits for cardiovascular health, exercise performance, and cellular protection align well with these consumer preferences.
Regional analysis reveals varying adoption rates for TMG supplementation. North America currently dominates the market with approximately 42% market share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 10.3% annually, primarily due to increasing health consciousness and disposable income in countries like China, Japan, and South Korea.
Distribution channels for TMG supplements have diversified significantly. While traditional retail channels account for approximately 48% of sales, e-commerce platforms have rapidly expanded to capture 37% of the market. Direct-to-consumer models, particularly subscription services, represent the fastest-growing distribution segment with a 15% annual growth rate.
Competitive analysis reveals a fragmented market landscape with both established nutraceutical companies and specialized supplement manufacturers. Key market players include Jarrow Formulas, NOW Foods, Life Extension, and Thorne Research, collectively holding approximately 35% market share. However, numerous smaller, specialized companies are entering the space, focusing on premium formulations and targeted applications of TMG.
Pricing strategies within the TMG supplement market vary considerably, with premium products emphasizing purity, bioavailability, and scientific validation commanding price points 30-40% higher than standard offerings. Consumer willingness to pay premium prices correlates strongly with perceived efficacy and scientific substantiation, highlighting the importance of research into cellular responses to TMG supplementation.
Market forecasts suggest that the TMG supplement segment will outpace the broader nutraceutical market growth, with projected annual growth of 11.2% through 2028. This accelerated growth is attributed to increasing scientific validation, expanding applications beyond cardiovascular health, and growing consumer interest in supplements that address cellular health and longevity.
Current Challenges in Cellular Response Measurement
Despite significant advancements in cellular biology research methodologies, measuring cellular responses to trimethylglycine (TMG) supplementation presents several persistent challenges that impede comprehensive understanding of its biological effects. The primary difficulty lies in the complex nature of cellular metabolic networks affected by TMG, which functions as an important methyl donor in various biochemical pathways. Current measurement techniques often fail to capture the full spectrum of cellular responses across different time scales, from immediate signaling events to long-term epigenetic modifications.
Standardization remains a critical issue in the field, with laboratories employing diverse protocols for TMG administration, cell culture conditions, and response measurement. This heterogeneity creates significant obstacles when attempting to compare results across different studies or establish definitive dose-response relationships. The variability in experimental conditions further complicates the interpretation of contradictory findings reported in the literature.
Technical limitations of current analytical methods present another substantial challenge. While techniques such as mass spectrometry provide detailed metabolomic profiles, they often require cell disruption, preventing continuous monitoring of living cells. Real-time measurement technologies like fluorescence-based assays offer temporal resolution but typically focus on single parameters rather than providing comprehensive response profiles. This creates a fundamental trade-off between measurement depth and temporal resolution.
Cell type specificity introduces additional complexity, as TMG elicits markedly different responses across various tissue types and cell lineages. Current measurement approaches frequently fail to account for this heterogeneity, particularly in mixed cell populations or complex tissue models. The challenge extends to distinguishing direct TMG effects from secondary cellular adaptations triggered by initial metabolic changes.
Sensitivity thresholds of existing detection methods pose further difficulties, especially when measuring subtle cellular responses at physiologically relevant TMG concentrations. Many current techniques require supraphysiological doses to generate measurable signals, raising questions about the biological relevance of observed effects. This sensitivity gap particularly affects the detection of low-abundance metabolites and proteins in TMG-responsive pathways.
Integration of multi-omics data represents perhaps the most significant contemporary challenge. While individual techniques can measure specific aspects of cellular response—genomic, transcriptomic, proteomic, or metabolomic—few methodological frameworks exist for integrating these diverse data types into coherent models of cellular response to TMG supplementation. This integration challenge limits our ability to develop comprehensive understanding of how TMG influences cellular function across multiple biological levels.
Standardization remains a critical issue in the field, with laboratories employing diverse protocols for TMG administration, cell culture conditions, and response measurement. This heterogeneity creates significant obstacles when attempting to compare results across different studies or establish definitive dose-response relationships. The variability in experimental conditions further complicates the interpretation of contradictory findings reported in the literature.
Technical limitations of current analytical methods present another substantial challenge. While techniques such as mass spectrometry provide detailed metabolomic profiles, they often require cell disruption, preventing continuous monitoring of living cells. Real-time measurement technologies like fluorescence-based assays offer temporal resolution but typically focus on single parameters rather than providing comprehensive response profiles. This creates a fundamental trade-off between measurement depth and temporal resolution.
Cell type specificity introduces additional complexity, as TMG elicits markedly different responses across various tissue types and cell lineages. Current measurement approaches frequently fail to account for this heterogeneity, particularly in mixed cell populations or complex tissue models. The challenge extends to distinguishing direct TMG effects from secondary cellular adaptations triggered by initial metabolic changes.
Sensitivity thresholds of existing detection methods pose further difficulties, especially when measuring subtle cellular responses at physiologically relevant TMG concentrations. Many current techniques require supraphysiological doses to generate measurable signals, raising questions about the biological relevance of observed effects. This sensitivity gap particularly affects the detection of low-abundance metabolites and proteins in TMG-responsive pathways.
Integration of multi-omics data represents perhaps the most significant contemporary challenge. While individual techniques can measure specific aspects of cellular response—genomic, transcriptomic, proteomic, or metabolomic—few methodological frameworks exist for integrating these diverse data types into coherent models of cellular response to TMG supplementation. This integration challenge limits our ability to develop comprehensive understanding of how TMG influences cellular function across multiple biological levels.
Established Protocols for Cellular Response Assessment
01 TMG's role in cellular stress protection
Trimethylglycine (TMG) functions as an osmolyte and methylating agent that helps protect cells against various stressors. It stabilizes cellular proteins and membranes under stress conditions such as dehydration, temperature fluctuations, and oxidative damage. TMG accumulation in cells provides protection against osmotic stress and helps maintain cellular integrity and function during adverse conditions.- TMG's role in cellular stress response: Trimethylglycine (TMG) functions as an osmolyte and methyl donor that helps cells respond to various stressors. It protects cellular proteins and enzymes from environmental stress, particularly osmotic stress, by stabilizing their structure. TMG accumulation in cells serves as a protective mechanism against dehydration, extreme temperatures, and oxidative damage, helping maintain cellular homeostasis under adverse conditions.
- TMG in metabolic pathways and methylation: Trimethylglycine serves as a critical methyl donor in cellular biochemical pathways, particularly in the conversion of homocysteine to methionine. This methylation process is essential for numerous cellular functions including DNA synthesis, gene expression regulation, and protein function. TMG's involvement in one-carbon metabolism impacts cellular energy production, detoxification processes, and overall metabolic health.
- Anti-inflammatory and immunomodulatory effects of TMG: Trimethylglycine exhibits significant anti-inflammatory properties at the cellular level by modulating cytokine production and inflammatory signaling pathways. It can reduce the expression of pro-inflammatory mediators and enhance anti-inflammatory responses. TMG's immunomodulatory effects include regulation of immune cell function, potentially benefiting conditions characterized by chronic inflammation or immune dysregulation.
- TMG's protective effects against oxidative stress: Trimethylglycine provides cellular protection against oxidative damage by enhancing antioxidant defense mechanisms. It helps maintain glutathione levels, supports antioxidant enzyme activity, and directly scavenges certain reactive oxygen species. By mitigating oxidative stress, TMG helps prevent cellular damage to proteins, lipids, and DNA, potentially extending cellular lifespan and improving overall cellular function under oxidative conditions.
- TMG in cellular signaling and gene expression: Trimethylglycine influences cellular signaling pathways and gene expression patterns through its role in epigenetic regulation. By providing methyl groups for DNA and histone methylation, TMG affects chromatin structure and accessibility, thereby modulating transcriptional activity. These epigenetic modifications impact cellular differentiation, development, and responses to environmental stimuli, suggesting TMG's broader role in cellular adaptation and phenotypic expression.
02 TMG in metabolic pathways and methylation
Trimethylglycine serves as a methyl donor in various cellular metabolic pathways, particularly in the conversion of homocysteine to methionine. This methylation process is crucial for DNA synthesis, gene expression regulation, and protein function. TMG's involvement in one-carbon metabolism affects numerous cellular processes including epigenetic modifications, neurotransmitter synthesis, and detoxification pathways.Expand Specific Solutions03 Anti-inflammatory and immunomodulatory effects of TMG
Trimethylglycine exhibits anti-inflammatory properties by modulating cellular signaling pathways involved in inflammation. It can reduce the production of pro-inflammatory cytokines and inhibit inflammatory cell activation. TMG's immunomodulatory effects include regulation of immune cell function, enhancement of cellular defense mechanisms, and protection against inflammatory tissue damage.Expand Specific Solutions04 TMG's impact on cellular energy metabolism
Trimethylglycine influences cellular energy production by affecting mitochondrial function and metabolic pathways. It enhances ATP production, improves mitochondrial efficiency, and helps maintain energy homeostasis under stress conditions. TMG supplementation can increase cellular energy levels, improve metabolic efficiency, and enhance cellular performance in high-energy-demanding tissues.Expand Specific Solutions05 TMG in cell signaling and gene expression
Trimethylglycine influences intracellular signaling pathways and gene expression patterns. It affects signal transduction mechanisms involved in cell growth, differentiation, and survival. TMG can modulate the expression of genes related to stress response, metabolism, and cellular defense. These effects contribute to TMG's role in cellular adaptation to environmental changes and maintenance of cellular homeostasis.Expand Specific Solutions
Leading Organizations in TMG Research and Development
The cellular response to trimethylglycine (TMG) supplementation market is currently in an early growth phase, characterized by increasing research interest but limited commercial applications. The global market size remains relatively modest, estimated below $500 million, with growth potential as therapeutic applications expand. From a technical maturity perspective, the field is still developing, with key players demonstrating varying levels of advancement. Pharmaceutical companies like AbbVie, Incyte, and BioNTech are leveraging TMG research for potential therapeutic applications, while research institutions including Memorial Sloan Kettering Cancer Center and University of Tokyo are conducting foundational studies. Specialized biotechnology firms such as ZymoGenetics and ImmusanT are exploring TMG's cellular effects in specific disease contexts, particularly in immune modulation and metabolic pathways, indicating promising but still emerging technological capabilities.
Merck Patent GmbH
Technical Solution: Merck Patent GmbH has developed an advanced platform for measuring cellular responses to trimethylglycine (TMG) supplementation using high-throughput screening combined with multi-omics analysis. Their technology integrates transcriptomics, proteomics, and metabolomics to provide comprehensive insights into how TMG affects cellular pathways. The platform employs stable isotope-labeled TMG (13C-TMG) to track its metabolic fate within cells, allowing precise quantification of its incorporation into methylation pathways. Merck's system includes proprietary cell-based assays that measure changes in methylation status of DNA, proteins, and metabolites following TMG exposure. Their research has demonstrated that TMG supplementation can increase S-adenosylmethionine (SAM) levels by approximately 30-45% in hepatocytes and significantly reduce homocysteine levels. The platform also quantifies osmotic protection effects by measuring cell volume regulation and protein stabilization under hyperosmotic stress conditions. Merck has applied this technology to identify optimal TMG dosing regimens for different cell types and physiological conditions.
Strengths: Comprehensive multi-omics approach provides detailed molecular insights; isotope labeling enables precise tracking of TMG metabolism; high-throughput capabilities allow testing of multiple conditions simultaneously. Weaknesses: Complex analytical platform requires specialized expertise and equipment; primarily focused on in vitro cellular models which may not fully translate to in vivo responses.
University of Tokyo
Technical Solution: The University of Tokyo has established an innovative research program focused on measuring cellular responses to trimethylglycine (TMG) supplementation, with particular emphasis on its impact on mitochondrial function and cellular stress responses. Their approach combines high-resolution respirometry with multi-parameter flow cytometry to assess how TMG influences cellular bioenergetics and redox homeostasis. The research team has developed specialized protocols using fluorescent probes to simultaneously track changes in mitochondrial membrane potential, reactive oxygen species production, and calcium homeostasis following TMG treatment. Their studies have demonstrated that TMG supplementation can enhance mitochondrial respiratory capacity by 15-20% in cardiomyocytes under oxidative stress conditions while reducing ROS production by approximately 30%. The university's platform also incorporates transcriptomic analysis to identify TMG-responsive genes involved in methyl transfer reactions and osmotic regulation. Their research has revealed that TMG can upregulate expression of genes involved in protein folding and stabilization, enhancing cellular resilience to various stressors. Additionally, they've developed novel microfluidic systems that allow real-time monitoring of cellular volume regulation in response to osmotic challenges, demonstrating TMG's effectiveness as an organic osmolyte that can preserve cell volume and function under hyperosmotic conditions.
Strengths: Sophisticated bioenergetic analysis capabilities provide unique insights into TMG's effects on mitochondrial function; integrated multi-parameter approach captures complex cellular responses; innovative microfluidic systems enable dynamic measurements of osmotic regulation. Weaknesses: Highly specialized research focus may limit broader applications; primarily academic research orientation with limited commercial development of measurement technologies.
Key Technologies in Metabolite Detection and Analysis
Methods and Reagents for Reverse-Transcription Polymerase Chain Reaction
PatentActiveUS20190078154A1
Innovation
- A method and composition for RT-PCR that uses a solution comprising a polar aprotic solvent, serum albumin, and optionally a non-ionic surfactant and betaine, allowing direct amplification of RNA from crude biological samples without prior extraction, using a dried reagent composition that includes a sequestering reagent, polymerase, deoxyribonucleotide triphosphates, serum albumin, and betaine, and optionally a polar aprotic solvent and non-ionic surfactant.
Patent
Innovation
- Development of high-throughput assays for measuring cellular responses to trimethylglycine (TMG) supplementation, enabling rapid assessment of metabolic changes across multiple cell types.
- Identification of novel biomarkers that indicate cellular response to TMG supplementation, allowing for personalized dosing strategies based on individual metabolic profiles.
- Development of real-time monitoring systems for tracking cellular methylation status and homocysteine levels in response to TMG supplementation, providing immediate feedback on supplement efficacy.
Regulatory Framework for Supplement Efficacy Claims
The regulatory landscape governing supplement efficacy claims, particularly for compounds like trimethylglycine (TMG), presents a complex framework that manufacturers, researchers, and healthcare providers must navigate. In the United States, the Food and Drug Administration (FDA) regulates dietary supplements under the Dietary Supplement Health and Education Act (DSHEA) of 1994, which establishes different standards for supplements compared to pharmaceutical drugs. Under this framework, TMG supplement manufacturers cannot make direct claims about treating, curing, or preventing specific diseases without substantial clinical evidence and FDA approval.
For cellular response measurements related to TMG supplementation, the FDA requires that any structure-function claims must be substantiated by scientific evidence. This evidence typically includes in vitro cellular studies, animal models, and human clinical trials that demonstrate the biological mechanisms and physiological responses to the supplement. The European Food Safety Authority (EFSA) maintains even stricter standards, requiring robust scientific consensus before allowing health claims on supplement labels.
The Federal Trade Commission (FTC) provides additional oversight regarding the marketing and advertising of TMG supplements, ensuring that efficacy claims are truthful, not misleading, and supported by competent and reliable scientific evidence. This evidence standard typically requires human clinical studies that are methodologically sound and statistically significant.
Internationally, regulatory frameworks vary significantly. Japan's FOSHU (Foods for Specified Health Uses) system and Canada's Natural Health Products Regulations offer alternative approaches to evaluating supplement efficacy claims, with varying requirements for pre-market approval and scientific substantiation. These differences create challenges for global distribution of TMG supplements with consistent marketing claims.
Recent regulatory developments have emphasized the importance of standardized cellular response measurements. The NIH's Office of Dietary Supplements has established guidelines for research methodologies to evaluate supplement effects at the cellular level, including recommendations for measuring methylation capacity, homocysteine metabolism, and oxidative stress markers—all relevant to TMG's biological activity.
For researchers measuring cellular responses to TMG, compliance with Good Laboratory Practices (GLP) and standardized protocols is essential for generating data that can support regulatory submissions. This includes validation of analytical methods, appropriate controls, and statistical analyses that meet regulatory scrutiny. Additionally, transparency in reporting negative or null findings is increasingly emphasized by regulatory bodies to prevent publication bias in supplement efficacy literature.
For cellular response measurements related to TMG supplementation, the FDA requires that any structure-function claims must be substantiated by scientific evidence. This evidence typically includes in vitro cellular studies, animal models, and human clinical trials that demonstrate the biological mechanisms and physiological responses to the supplement. The European Food Safety Authority (EFSA) maintains even stricter standards, requiring robust scientific consensus before allowing health claims on supplement labels.
The Federal Trade Commission (FTC) provides additional oversight regarding the marketing and advertising of TMG supplements, ensuring that efficacy claims are truthful, not misleading, and supported by competent and reliable scientific evidence. This evidence standard typically requires human clinical studies that are methodologically sound and statistically significant.
Internationally, regulatory frameworks vary significantly. Japan's FOSHU (Foods for Specified Health Uses) system and Canada's Natural Health Products Regulations offer alternative approaches to evaluating supplement efficacy claims, with varying requirements for pre-market approval and scientific substantiation. These differences create challenges for global distribution of TMG supplements with consistent marketing claims.
Recent regulatory developments have emphasized the importance of standardized cellular response measurements. The NIH's Office of Dietary Supplements has established guidelines for research methodologies to evaluate supplement effects at the cellular level, including recommendations for measuring methylation capacity, homocysteine metabolism, and oxidative stress markers—all relevant to TMG's biological activity.
For researchers measuring cellular responses to TMG, compliance with Good Laboratory Practices (GLP) and standardized protocols is essential for generating data that can support regulatory submissions. This includes validation of analytical methods, appropriate controls, and statistical analyses that meet regulatory scrutiny. Additionally, transparency in reporting negative or null findings is increasingly emphasized by regulatory bodies to prevent publication bias in supplement efficacy literature.
Translational Applications in Personalized Nutrition
The integration of trimethylglycine (TMG) supplementation research into personalized nutrition represents a significant advancement in tailoring dietary recommendations to individual metabolic profiles. As nutritional science evolves beyond one-size-fits-all approaches, TMG's role in methylation processes offers promising applications for personalized interventions based on genetic predispositions and metabolic requirements.
Cellular response measurements to TMG supplementation provide critical biomarkers that can inform personalized nutrition protocols. These measurements enable practitioners to identify individuals who may benefit most from TMG supplementation, particularly those with MTHFR gene variants affecting folate metabolism or those with elevated homocysteine levels. The translation of these cellular responses into actionable nutritional recommendations creates a bridge between laboratory findings and clinical practice.
Healthcare providers can leverage TMG response data to develop nutrition plans addressing specific methylation deficiencies. For instance, individuals showing positive cellular responses to TMG in terms of improved homocysteine metabolism may receive tailored supplementation protocols, while those with minimal response might be directed toward alternative methyl donors. This precision approach optimizes intervention efficacy while minimizing unnecessary supplementation.
The commercial application of these findings has already begun emerging in the form of nutrigenetic testing services that include TMG response markers in their assessment panels. These services analyze genetic polymorphisms related to one-carbon metabolism and provide recommendations for TMG and other methyl donor supplementation based on individual genetic profiles. The market for such personalized nutrition services is projected to grow substantially as consumer interest in tailored health solutions increases.
Institutional implementation of TMG-based personalized nutrition protocols is gradually appearing in progressive healthcare settings. Integrative medicine clinics and functional medicine practitioners are increasingly incorporating cellular response measurements to TMG as part of comprehensive metabolic assessments. These clinical applications demonstrate the translational potential of laboratory research into practical healthcare delivery models.
Future developments in this field will likely include mobile applications and wearable technologies that monitor real-time metabolic responses to TMG supplementation, allowing for dynamic adjustment of nutritional protocols. Additionally, the integration of artificial intelligence algorithms to interpret complex patterns in cellular responses could further refine personalized nutrition recommendations, creating increasingly sophisticated and effective intervention strategies tailored to individual metabolic needs.
Cellular response measurements to TMG supplementation provide critical biomarkers that can inform personalized nutrition protocols. These measurements enable practitioners to identify individuals who may benefit most from TMG supplementation, particularly those with MTHFR gene variants affecting folate metabolism or those with elevated homocysteine levels. The translation of these cellular responses into actionable nutritional recommendations creates a bridge between laboratory findings and clinical practice.
Healthcare providers can leverage TMG response data to develop nutrition plans addressing specific methylation deficiencies. For instance, individuals showing positive cellular responses to TMG in terms of improved homocysteine metabolism may receive tailored supplementation protocols, while those with minimal response might be directed toward alternative methyl donors. This precision approach optimizes intervention efficacy while minimizing unnecessary supplementation.
The commercial application of these findings has already begun emerging in the form of nutrigenetic testing services that include TMG response markers in their assessment panels. These services analyze genetic polymorphisms related to one-carbon metabolism and provide recommendations for TMG and other methyl donor supplementation based on individual genetic profiles. The market for such personalized nutrition services is projected to grow substantially as consumer interest in tailored health solutions increases.
Institutional implementation of TMG-based personalized nutrition protocols is gradually appearing in progressive healthcare settings. Integrative medicine clinics and functional medicine practitioners are increasingly incorporating cellular response measurements to TMG as part of comprehensive metabolic assessments. These clinical applications demonstrate the translational potential of laboratory research into practical healthcare delivery models.
Future developments in this field will likely include mobile applications and wearable technologies that monitor real-time metabolic responses to TMG supplementation, allowing for dynamic adjustment of nutritional protocols. Additionally, the integration of artificial intelligence algorithms to interpret complex patterns in cellular responses could further refine personalized nutrition recommendations, creating increasingly sophisticated and effective intervention strategies tailored to individual metabolic needs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!