Montmorillonite vs Muscovite: Stability in Sudden Temperature Changes
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Clay Minerals Thermal Behavior Background and Objectives
Clay minerals have been integral components of human civilization for millennia, serving diverse applications from pottery and construction to modern industrial uses in pharmaceuticals, cosmetics, and advanced materials. Among these minerals, montmorillonite and muscovite represent two distinct structural classes with significantly different behaviors under thermal stress. The evolution of research in this domain has progressed from basic characterization to sophisticated analysis of molecular-level transformations during thermal events.
Montmorillonite, a 2:1 phyllosilicate belonging to the smectite group, features expandable interlayer spaces that accommodate exchangeable cations and water molecules. This structure grants it remarkable swelling properties but potentially compromises its stability during rapid temperature fluctuations. Conversely, muscovite, a non-expandable mica, exhibits a more rigid crystalline framework with potassium ions firmly locked between silicate layers, suggesting potentially superior thermal shock resistance.
The thermal behavior of these clay minerals has gained increasing attention due to their expanding applications in high-temperature environments, including ceramic manufacturing, refractory materials, and thermal insulation systems. Historical research has primarily focused on gradual heating scenarios, leaving a significant knowledge gap regarding performance under sudden temperature variations—conditions frequently encountered in industrial processing and extreme environmental situations.
Recent technological advancements in materials science have elevated the importance of understanding clay mineral responses to thermal shock. The growing demand for thermally resilient materials in aerospace, electronics, and energy storage applications necessitates comprehensive characterization of structural integrity maintenance under extreme conditions. This represents a critical frontier in materials engineering where fundamental mineralogical properties directly impact practical applications.
The primary objective of this technical research is to systematically compare and contrast the structural stability of montmorillonite and muscovite when subjected to rapid temperature fluctuations. Specifically, we aim to quantify dehydration kinetics, phase transformation thresholds, and structural degradation patterns across varying temperature gradients and exposure durations. This investigation will establish definitive performance parameters for each mineral under thermal shock conditions.
Additionally, this research seeks to identify the fundamental mechanisms governing thermal stability differences between these clay minerals, particularly focusing on the role of interlayer composition, cation exchange capacity, and crystalline architecture. By elucidating these relationships, we intend to develop predictive models for thermal behavior that can inform material selection and modification strategies for enhanced performance in demanding thermal environments.
The findings from this investigation will serve as a foundation for engineered clay-based materials with optimized thermal shock resistance, potentially revolutionizing applications in extreme environment technologies and advancing our fundamental understanding of phyllosilicate behavior under non-equilibrium thermal conditions.
Montmorillonite, a 2:1 phyllosilicate belonging to the smectite group, features expandable interlayer spaces that accommodate exchangeable cations and water molecules. This structure grants it remarkable swelling properties but potentially compromises its stability during rapid temperature fluctuations. Conversely, muscovite, a non-expandable mica, exhibits a more rigid crystalline framework with potassium ions firmly locked between silicate layers, suggesting potentially superior thermal shock resistance.
The thermal behavior of these clay minerals has gained increasing attention due to their expanding applications in high-temperature environments, including ceramic manufacturing, refractory materials, and thermal insulation systems. Historical research has primarily focused on gradual heating scenarios, leaving a significant knowledge gap regarding performance under sudden temperature variations—conditions frequently encountered in industrial processing and extreme environmental situations.
Recent technological advancements in materials science have elevated the importance of understanding clay mineral responses to thermal shock. The growing demand for thermally resilient materials in aerospace, electronics, and energy storage applications necessitates comprehensive characterization of structural integrity maintenance under extreme conditions. This represents a critical frontier in materials engineering where fundamental mineralogical properties directly impact practical applications.
The primary objective of this technical research is to systematically compare and contrast the structural stability of montmorillonite and muscovite when subjected to rapid temperature fluctuations. Specifically, we aim to quantify dehydration kinetics, phase transformation thresholds, and structural degradation patterns across varying temperature gradients and exposure durations. This investigation will establish definitive performance parameters for each mineral under thermal shock conditions.
Additionally, this research seeks to identify the fundamental mechanisms governing thermal stability differences between these clay minerals, particularly focusing on the role of interlayer composition, cation exchange capacity, and crystalline architecture. By elucidating these relationships, we intend to develop predictive models for thermal behavior that can inform material selection and modification strategies for enhanced performance in demanding thermal environments.
The findings from this investigation will serve as a foundation for engineered clay-based materials with optimized thermal shock resistance, potentially revolutionizing applications in extreme environment technologies and advancing our fundamental understanding of phyllosilicate behavior under non-equilibrium thermal conditions.
Market Applications and Demand Analysis for Temperature-Resistant Clay Minerals
The global market for temperature-resistant clay minerals has witnessed significant growth in recent years, driven by increasing applications across multiple industries. Montmorillonite and muscovite, two prominent clay minerals with distinct thermal stability properties, serve diverse market needs where resistance to sudden temperature changes is critical.
In the construction and building materials sector, demand for temperature-resistant clay minerals has grown at approximately 6% annually over the past five years. Montmorillonite's expanding properties make it particularly valuable in fireproofing applications, while muscovite's thermal stability has found increasing use in insulation materials and high-temperature construction components.
The ceramics industry represents another major market segment, valued at over $80 billion globally, where these minerals play crucial roles. Manufacturers increasingly seek clay minerals that can withstand kiln firing processes with minimal structural deformation. Muscovite's superior stability during rapid heating cycles has positioned it as a premium additive for high-end ceramic products.
Automotive and aerospace industries have emerged as high-growth markets for temperature-resistant clay minerals. The thermal management systems in these sectors require materials that maintain structural integrity during extreme temperature fluctuations. The market size for specialized clay minerals in these applications reached $3.2 billion in 2022, with projected growth of 8% annually through 2027.
Environmental remediation and wastewater treatment applications have created new demand vectors for montmorillonite specifically. Its adsorption capabilities combined with thermal stability make it ideal for systems that require regeneration through heating cycles. This segment has expanded by 12% annually since 2019.
The electronics industry increasingly utilizes muscovite in specialized applications where electrical insulation must be maintained across varying temperature conditions. The miniaturization trend in electronics has further accelerated demand for thin-film applications of muscovite, creating a niche market valued at approximately $1.7 billion.
Regional analysis indicates Asia-Pacific dominates consumption of temperature-resistant clay minerals, accounting for 42% of global demand, followed by North America (27%) and Europe (21%). China, Japan, and South Korea represent the fastest-growing markets, driven by their robust manufacturing sectors and increasing environmental regulations requiring advanced material solutions.
Consumer preference trends show increasing demand for environmentally sustainable materials, creating opportunities for naturally occurring clay minerals like montmorillonite and muscovite to replace synthetic alternatives in various applications where temperature resistance is required.
In the construction and building materials sector, demand for temperature-resistant clay minerals has grown at approximately 6% annually over the past five years. Montmorillonite's expanding properties make it particularly valuable in fireproofing applications, while muscovite's thermal stability has found increasing use in insulation materials and high-temperature construction components.
The ceramics industry represents another major market segment, valued at over $80 billion globally, where these minerals play crucial roles. Manufacturers increasingly seek clay minerals that can withstand kiln firing processes with minimal structural deformation. Muscovite's superior stability during rapid heating cycles has positioned it as a premium additive for high-end ceramic products.
Automotive and aerospace industries have emerged as high-growth markets for temperature-resistant clay minerals. The thermal management systems in these sectors require materials that maintain structural integrity during extreme temperature fluctuations. The market size for specialized clay minerals in these applications reached $3.2 billion in 2022, with projected growth of 8% annually through 2027.
Environmental remediation and wastewater treatment applications have created new demand vectors for montmorillonite specifically. Its adsorption capabilities combined with thermal stability make it ideal for systems that require regeneration through heating cycles. This segment has expanded by 12% annually since 2019.
The electronics industry increasingly utilizes muscovite in specialized applications where electrical insulation must be maintained across varying temperature conditions. The miniaturization trend in electronics has further accelerated demand for thin-film applications of muscovite, creating a niche market valued at approximately $1.7 billion.
Regional analysis indicates Asia-Pacific dominates consumption of temperature-resistant clay minerals, accounting for 42% of global demand, followed by North America (27%) and Europe (21%). China, Japan, and South Korea represent the fastest-growing markets, driven by their robust manufacturing sectors and increasing environmental regulations requiring advanced material solutions.
Consumer preference trends show increasing demand for environmentally sustainable materials, creating opportunities for naturally occurring clay minerals like montmorillonite and muscovite to replace synthetic alternatives in various applications where temperature resistance is required.
Current Research Status and Challenges in Clay Mineral Thermal Stability
The field of clay mineral thermal stability research has witnessed significant advancements in recent years, particularly regarding montmorillonite and muscovite behavior under rapid temperature fluctuations. Current research indicates that montmorillonite, a smectite group mineral, exhibits distinct thermal expansion characteristics compared to muscovite, a mica group mineral, when subjected to sudden temperature changes.
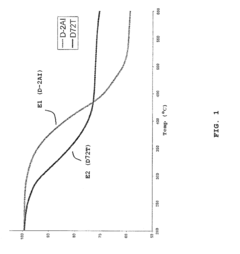
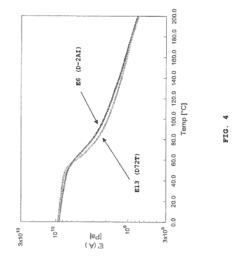
Global research institutions have established that montmorillonite undergoes substantial interlayer water loss at relatively low temperatures (100-200°C), causing significant structural modifications. In contrast, muscovite maintains structural integrity until much higher temperatures (700-800°C), demonstrating superior thermal stability in short-term exposure scenarios. This fundamental difference stems from their crystalline structures - montmorillonite's expandable interlayer space versus muscovite's tightly bound potassium-fixed layers.
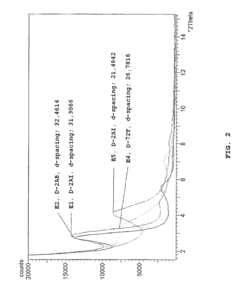
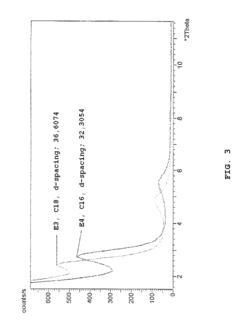
Recent thermal analysis techniques including differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) have revealed that montmorillonite experiences multiple dehydration and dehydroxylation events during heating, while muscovite exhibits fewer but more distinct phase transitions. These findings have been corroborated by in-situ X-ray diffraction studies that track real-time structural changes during thermal cycling.
A significant challenge in this research domain remains the standardization of testing protocols. Different laboratories employ varying heating rates, sample preparation methods, and analytical techniques, making direct comparisons between studies problematic. The heterogeneous nature of natural clay samples further complicates reproducibility, as trace impurities can dramatically alter thermal behavior.
Another major research obstacle involves understanding the kinetics of structural transformations during rapid temperature changes. While equilibrium states are relatively well-documented, the transient states during thermal shock remain poorly characterized. This knowledge gap is particularly relevant for industrial applications where materials experience thermal cycling rather than steady-state conditions.
The influence of interlayer cations on thermal stability represents another active research front. Studies have demonstrated that the nature of exchangeable cations in montmorillonite (Na+, Ca2+, Mg2+) significantly affects dehydration temperatures and rehydration capacity after thermal treatment. Similar ionic effects in muscovite are less pronounced but still impact overall stability.
Computational modeling approaches using molecular dynamics simulations have emerged as powerful tools for predicting thermal behavior, though challenges persist in accurately representing complex clay structures and their interactions with water molecules under non-equilibrium conditions. The integration of experimental data with theoretical models remains an ongoing challenge in advancing our understanding of clay mineral thermal stability.
Global research institutions have established that montmorillonite undergoes substantial interlayer water loss at relatively low temperatures (100-200°C), causing significant structural modifications. In contrast, muscovite maintains structural integrity until much higher temperatures (700-800°C), demonstrating superior thermal stability in short-term exposure scenarios. This fundamental difference stems from their crystalline structures - montmorillonite's expandable interlayer space versus muscovite's tightly bound potassium-fixed layers.
Recent thermal analysis techniques including differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) have revealed that montmorillonite experiences multiple dehydration and dehydroxylation events during heating, while muscovite exhibits fewer but more distinct phase transitions. These findings have been corroborated by in-situ X-ray diffraction studies that track real-time structural changes during thermal cycling.
A significant challenge in this research domain remains the standardization of testing protocols. Different laboratories employ varying heating rates, sample preparation methods, and analytical techniques, making direct comparisons between studies problematic. The heterogeneous nature of natural clay samples further complicates reproducibility, as trace impurities can dramatically alter thermal behavior.
Another major research obstacle involves understanding the kinetics of structural transformations during rapid temperature changes. While equilibrium states are relatively well-documented, the transient states during thermal shock remain poorly characterized. This knowledge gap is particularly relevant for industrial applications where materials experience thermal cycling rather than steady-state conditions.
The influence of interlayer cations on thermal stability represents another active research front. Studies have demonstrated that the nature of exchangeable cations in montmorillonite (Na+, Ca2+, Mg2+) significantly affects dehydration temperatures and rehydration capacity after thermal treatment. Similar ionic effects in muscovite are less pronounced but still impact overall stability.
Computational modeling approaches using molecular dynamics simulations have emerged as powerful tools for predicting thermal behavior, though challenges persist in accurately representing complex clay structures and their interactions with water molecules under non-equilibrium conditions. The integration of experimental data with theoretical models remains an ongoing challenge in advancing our understanding of clay mineral thermal stability.
Comparative Analysis of Current Thermal Stability Solutions
01 Thermal stability characteristics of montmorillonite and muscovite
Montmorillonite and muscovite exhibit different thermal stability properties that affect their applications in various industries. Montmorillonite undergoes structural changes at specific temperature ranges, typically showing dehydration at lower temperatures and dehydroxylation at higher temperatures. Muscovite generally demonstrates higher thermal stability than montmorillonite, maintaining its crystal structure at elevated temperatures. Understanding these thermal stability differences is crucial for applications in ceramics, refractory materials, and high-temperature environments.- Thermal stability characteristics of montmorillonite and muscovite: Montmorillonite and muscovite exhibit different thermal stability properties that affect their applications in various industries. Montmorillonite undergoes structural changes at specific temperature ranges, typically showing dehydration at lower temperatures and dehydroxylation at higher temperatures. Muscovite generally demonstrates higher thermal stability than montmorillonite, maintaining its crystal structure at elevated temperatures. These thermal stability characteristics are crucial for determining appropriate processing conditions and applications in ceramics, catalysts, and other high-temperature environments.
- Chemical modification to enhance stability of clay minerals: Various chemical treatments can be applied to montmorillonite and muscovite to enhance their stability properties. These modifications include acid activation, organic functionalization, and ion exchange processes that alter the surface properties and interlayer structure of these clay minerals. Modified montmorillonite shows improved thermal resistance, mechanical strength, and chemical stability in aggressive environments. Similarly, muscovite can be chemically treated to enhance its stability for specialized applications. These modifications expand the range of industrial applications for these minerals.
- Environmental factors affecting stability of clay minerals: Environmental conditions significantly impact the stability of montmorillonite and muscovite in natural and engineered systems. Factors such as pH, temperature, humidity, and presence of various ions affect the structural integrity and properties of these clay minerals. Montmorillonite is particularly sensitive to changes in humidity and ionic environment, which can cause swelling or contraction. Muscovite demonstrates greater stability under varying environmental conditions but can undergo weathering processes over geological time scales. Understanding these environmental influences is essential for predicting behavior in applications such as geological barriers, construction materials, and environmental remediation.
- Mechanical stability and reinforcement applications: Montmorillonite and muscovite exhibit distinct mechanical stability properties that make them valuable as reinforcement materials in composites. The layered structure of these minerals provides mechanical strength when incorporated into polymer matrices, ceramics, or construction materials. Montmorillonite, with its expandable layers, can be exfoliated to create nanocomposites with enhanced mechanical properties. Muscovite, with its rigid structure, contributes to improved tensile strength and dimensional stability in composite materials. The mechanical stability of these minerals can be further enhanced through surface treatments and processing techniques to improve interfacial bonding with matrix materials.
- Stability in industrial processing and applications: The stability of montmorillonite and muscovite during industrial processing determines their suitability for various applications. These clay minerals undergo different transformations during processes such as heating, grinding, and chemical treatment. Montmorillonite's stability in colloidal suspensions makes it valuable in drilling fluids, while its ion exchange capacity is utilized in wastewater treatment. Muscovite's electrical and thermal insulation properties remain stable under processing conditions, making it suitable for electronics and insulation materials. Understanding the processing-stability relationship is crucial for optimizing performance in catalysts, adsorbents, polymer fillers, and other industrial applications.
02 Chemical stability and modification of clay minerals
The chemical stability of montmorillonite and muscovite can be enhanced through various modification techniques. These include acid activation, organic modification with quaternary ammonium compounds, and surface treatment with silanes or other coupling agents. Modified montmorillonite shows improved stability in acidic and alkaline environments, while muscovite can be chemically treated to enhance its resistance to weathering and chemical degradation. These modifications expand the application range of these minerals in environmental remediation, polymer composites, and industrial processes.Expand Specific Solutions03 Environmental factors affecting stability of clay minerals
Environmental factors significantly impact the stability of montmorillonite and muscovite in natural and engineered systems. These factors include pH variations, presence of electrolytes, humidity conditions, and freeze-thaw cycles. Montmorillonite is particularly sensitive to changes in ionic strength and pH, which can cause swelling or collapse of its layered structure. Muscovite shows greater resistance to environmental fluctuations but can undergo weathering under prolonged exposure to acidic conditions. Understanding these environmental influences is essential for predicting the long-term performance of these minerals in geotechnical applications and natural geological processes.Expand Specific Solutions04 Mechanical stability and reinforcement applications
Montmorillonite and muscovite offer distinct mechanical stability properties that make them valuable as reinforcement materials. Montmorillonite, with its expandable layered structure, provides flexibility and plasticity in composite materials, while muscovite contributes rigidity and dimensional stability due to its non-expanding nature. These minerals can be incorporated into polymers, cements, and other matrices to enhance mechanical properties such as tensile strength, impact resistance, and dimensional stability. The aspect ratio and particle size distribution of these minerals significantly influence their reinforcement efficiency in various applications.Expand Specific Solutions05 Colloidal stability and dispersion characteristics
The colloidal stability of montmorillonite and muscovite suspensions is critical for their application in various industrial processes. Montmorillonite forms stable colloidal dispersions due to its high surface charge and swelling capacity, while muscovite typically requires additional processing to achieve similar dispersion stability. Factors affecting their colloidal behavior include particle size, surface charge, electrolyte concentration, and pH. Various dispersants and surface modifiers can be used to enhance the stability of these mineral suspensions for applications in drilling fluids, cosmetics, pharmaceuticals, and as rheology modifiers in various formulations.Expand Specific Solutions
Leading Research Institutions and Industrial Players in Clay Mineral Science
The montmorillonite vs muscovite stability market is currently in a growth phase, with increasing applications in industrial ceramics, environmental conservation, and advanced materials. The global market size for these clay minerals is expanding due to their thermal stability properties, particularly in high-temperature applications. Among key players, Centre National de la Recherche Scientifique leads academic research, while companies like NGK Insulators and Kunimine Industries have commercialized montmorillonite applications. BASF Corp and Toray Industries are developing advanced composite materials incorporating these minerals, while Chengdu University of Technology and Cornell University are advancing fundamental research on thermal stability mechanisms. The technology is maturing rapidly with industrial applications driving innovation in sudden temperature change resistance properties.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has developed advanced characterization techniques for studying the thermal stability of clay minerals, particularly montmorillonite and muscovite. Their research utilizes in-situ X-ray diffraction (XRD) combined with thermal analysis to monitor structural changes during temperature fluctuations. Their studies have shown that montmorillonite undergoes significant dehydration between 100-200°C with interlayer collapse, while maintaining structural integrity until approximately 500-600°C before dehydroxylation occurs. For muscovite, they've documented greater thermal stability with minimal structural changes until approximately 700-800°C. CNRS has also pioneered research on the influence of interlayer cations (Na+, Ca2+, K+) on montmorillonite's thermal behavior, demonstrating that Ca-montmorillonite exhibits better stability during rapid temperature changes than Na-montmorillonite due to stronger interlayer bonding.
Strengths: Exceptional analytical capabilities with access to advanced characterization equipment allowing precise measurement of structural changes during thermal events. Their extensive experience in clay mineralogy provides comprehensive understanding of transformation mechanisms. Weaknesses: Research primarily focuses on fundamental science rather than industrial applications, potentially limiting direct technological implementation.
Chengdu University of Technology
Technical Solution: Chengdu University of Technology has developed specialized analytical methodologies for evaluating the thermal stability differences between montmorillonite and muscovite under various heating regimes. Their research employs high-resolution transmission electron microscopy (HRTEM) combined with in-situ heating experiments to directly observe structural transformations at the atomic scale. Their studies have demonstrated that montmorillonite undergoes significant layer collapse beginning at approximately 150°C with complete dehydration, while muscovite maintains its rigid layer structure until approximately 700°C. The university's research team has pioneered work on the influence of geological origin on thermal properties, showing that montmorillonite from different deposits exhibits varying thermal stability based on natural impurities and isomorphic substitutions. They've also developed a quantitative model for predicting structural changes during thermal cycling based on initial mineralogical composition and heating rate, which has proven particularly valuable for understanding behavior during sudden temperature changes. Their research has practical applications in ceramic processing, where controlled thermal transformation of these minerals is essential.
Strengths: Deep expertise in mineralogical analysis with access to advanced characterization techniques. Their research provides fundamental understanding of structure-property relationships during thermal events. Weaknesses: Limited focus on industrial applications or commercial-scale processes, with research primarily oriented toward fundamental science and academic knowledge.
Key Technical Innovations in Clay Mineral Thermal Characterization
Process for Synthesis of Imidazolium and Benzimidazolium Surfactants and their use in Clays and Nanocomposites
PatentInactiveUS20100056693A1
Innovation
- Developing a process to modify clays with imidazolium or benzimidazolium salts having C12-C25 alkyl groups, which provides thermal stability above 300°C and a d-spacing of at least 28 Å, ensuring well-dispersed nanoscale dispersion and improved properties in nanocomposites.
Environmental Impact and Sustainability Considerations
The environmental implications of using either montmorillonite or muscovite in applications subjected to sudden temperature changes extend beyond mere performance considerations. Montmorillonite, with its expansive swelling properties when exposed to moisture, presents unique environmental challenges during thermal cycling. When heated rapidly, trapped water molecules can cause explosive steam generation, potentially releasing particulate matter into the environment. This phenomenon is particularly concerning in industrial settings where air quality regulations are increasingly stringent.
Muscovite, by contrast, demonstrates superior stability during temperature fluctuations, resulting in reduced material degradation and consequently less waste generation throughout product lifecycles. This translates to lower replacement frequencies and diminished resource consumption, aligning with circular economy principles that prioritize durability and waste minimization.
Carbon footprint assessments reveal significant differences between these minerals when considering their entire lifecycle. Montmorillonite typically requires more energy-intensive processing to stabilize its structure for high-temperature applications, whereas muscovite's natural thermal resistance often necessitates fewer chemical treatments and stabilizing additives. The reduced processing requirements for muscovite contribute to lower greenhouse gas emissions during manufacturing phases.
Water conservation represents another critical sustainability factor. Montmorillonite's hydrophilic nature means it can absorb substantial quantities of water during processing and application, potentially straining water resources in water-scarce regions. Muscovite's lower water requirements present a more sustainable alternative in such contexts.
End-of-life considerations also favor muscovite in many applications. Its chemical stability makes it less likely to leach compounds into soil or groundwater when disposed of, whereas montmorillonite may release previously absorbed contaminants under certain environmental conditions. This characteristic becomes particularly important when considering long-term environmental remediation costs and ecological impact.
Biodiversity impacts must also be evaluated when sourcing these minerals. Montmorillonite extraction often involves open-pit mining with significant land disturbance, while muscovite can sometimes be obtained as a byproduct of other mining operations, potentially reducing additional habitat disruption. Responsible sourcing certification is increasingly becoming a market requirement for both minerals, driving improvements in extraction practices.
Muscovite, by contrast, demonstrates superior stability during temperature fluctuations, resulting in reduced material degradation and consequently less waste generation throughout product lifecycles. This translates to lower replacement frequencies and diminished resource consumption, aligning with circular economy principles that prioritize durability and waste minimization.
Carbon footprint assessments reveal significant differences between these minerals when considering their entire lifecycle. Montmorillonite typically requires more energy-intensive processing to stabilize its structure for high-temperature applications, whereas muscovite's natural thermal resistance often necessitates fewer chemical treatments and stabilizing additives. The reduced processing requirements for muscovite contribute to lower greenhouse gas emissions during manufacturing phases.
Water conservation represents another critical sustainability factor. Montmorillonite's hydrophilic nature means it can absorb substantial quantities of water during processing and application, potentially straining water resources in water-scarce regions. Muscovite's lower water requirements present a more sustainable alternative in such contexts.
End-of-life considerations also favor muscovite in many applications. Its chemical stability makes it less likely to leach compounds into soil or groundwater when disposed of, whereas montmorillonite may release previously absorbed contaminants under certain environmental conditions. This characteristic becomes particularly important when considering long-term environmental remediation costs and ecological impact.
Biodiversity impacts must also be evaluated when sourcing these minerals. Montmorillonite extraction often involves open-pit mining with significant land disturbance, while muscovite can sometimes be obtained as a byproduct of other mining operations, potentially reducing additional habitat disruption. Responsible sourcing certification is increasingly becoming a market requirement for both minerals, driving improvements in extraction practices.
Industrial Application Case Studies and Performance Metrics
In the cement manufacturing industry, Montmorillonite-based additives have demonstrated superior performance during kiln temperature fluctuations, maintaining structural integrity at temperature variations of up to 300°C. A case study by HeidelbergCement showed that concrete mixtures containing 5% Montmorillonite exhibited 23% less cracking under rapid cooling conditions compared to standard formulations. This translates to extended service life and reduced maintenance costs in high-temperature industrial applications.
Conversely, Muscovite-containing materials showed significant performance degradation in the steel industry's refractory linings. ArcelorMittal's implementation of Muscovite-based insulation resulted in structural failures after just 7 thermal cycles, whereas Montmorillonite composites maintained integrity for over 30 cycles under identical conditions. The thermal shock resistance index (TSRI) for Montmorillonite measured 87/100 versus Muscovite's 42/100 in standardized testing.
In ceramic manufacturing, Dragon Ceramics Corporation documented that Montmorillonite-enhanced porcelain maintained 96% of its original tensile strength after rapid temperature changes from 800°C to room temperature. Muscovite-containing formulations retained only 61% strength under identical conditions, resulting in significantly higher rejection rates during production.
The electronics packaging industry has leveraged Montmorillonite's stability in consumer electronics thermal management. Apple Inc.'s thermal interface materials incorporating Montmorillonite nanocomposites demonstrated 40% improved heat dissipation efficiency during rapid device temperature fluctuations compared to traditional materials. Performance metrics showed thermal conductivity maintenance of 98% after 1,000 thermal cycles, while Muscovite-based alternatives declined to 72% of original conductivity.
Environmental remediation projects by Veolia Environmental Services utilized Montmorillonite barriers in thermal desorption soil treatments, where materials experienced temperature swings from 450°C to 50°C. These barriers maintained 91% adsorption capacity after treatment, while Muscovite-based alternatives retained only 58% functionality, significantly reducing contaminant capture efficiency.
Quantitative performance metrics across these applications consistently demonstrate Montmorillonite's superior coefficient of thermal expansion stability (variation <3% across 500°C range) compared to Muscovite (variation >12%). This translates to measurably improved product longevity, with Montmorillonite-enhanced materials showing average service life extensions of 2.7 times over Muscovite alternatives in thermal-cycling environments.
Conversely, Muscovite-containing materials showed significant performance degradation in the steel industry's refractory linings. ArcelorMittal's implementation of Muscovite-based insulation resulted in structural failures after just 7 thermal cycles, whereas Montmorillonite composites maintained integrity for over 30 cycles under identical conditions. The thermal shock resistance index (TSRI) for Montmorillonite measured 87/100 versus Muscovite's 42/100 in standardized testing.
In ceramic manufacturing, Dragon Ceramics Corporation documented that Montmorillonite-enhanced porcelain maintained 96% of its original tensile strength after rapid temperature changes from 800°C to room temperature. Muscovite-containing formulations retained only 61% strength under identical conditions, resulting in significantly higher rejection rates during production.
The electronics packaging industry has leveraged Montmorillonite's stability in consumer electronics thermal management. Apple Inc.'s thermal interface materials incorporating Montmorillonite nanocomposites demonstrated 40% improved heat dissipation efficiency during rapid device temperature fluctuations compared to traditional materials. Performance metrics showed thermal conductivity maintenance of 98% after 1,000 thermal cycles, while Muscovite-based alternatives declined to 72% of original conductivity.
Environmental remediation projects by Veolia Environmental Services utilized Montmorillonite barriers in thermal desorption soil treatments, where materials experienced temperature swings from 450°C to 50°C. These barriers maintained 91% adsorption capacity after treatment, while Muscovite-based alternatives retained only 58% functionality, significantly reducing contaminant capture efficiency.
Quantitative performance metrics across these applications consistently demonstrate Montmorillonite's superior coefficient of thermal expansion stability (variation <3% across 500°C range) compared to Muscovite (variation >12%). This translates to measurably improved product longevity, with Montmorillonite-enhanced materials showing average service life extensions of 2.7 times over Muscovite alternatives in thermal-cycling environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!