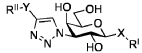
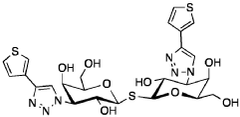
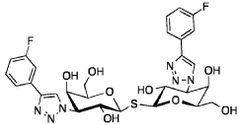
Optimizing Luteolin in Combination Therapies
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Luteolin Background and Research Objectives
Luteolin, a naturally occurring flavonoid found in various fruits, vegetables, and medicinal herbs, has garnered significant attention in the scientific community over the past two decades. This polyphenolic compound belongs to the flavone subclass and is characterized by its C6-C3-C6 structure with hydroxyl groups at positions 5, 7, 3', and 4'. The historical use of luteolin-rich plants in traditional medicine across different cultures provides a foundation for modern scientific exploration.
The evolution of luteolin research has progressed from basic identification and isolation techniques in the 1990s to sophisticated analytical methods and clinical applications in recent years. Initial studies focused primarily on its antioxidant properties, while contemporary research has expanded to investigate its potential in treating chronic diseases, including cancer, cardiovascular disorders, and neurodegenerative conditions. This trajectory reflects the growing recognition of luteolin's multifaceted biological activities.
Current technological advancements in extraction, purification, and formulation have significantly enhanced the bioavailability and stability of luteolin, addressing previous limitations in its therapeutic application. These developments have enabled more precise dosing and targeted delivery systems, creating new opportunities for clinical utilization. However, despite these advances, challenges remain in optimizing luteolin's pharmacokinetic profile and understanding its complex mechanisms of action.
The primary objective of this technical research is to systematically evaluate the potential of luteolin in combination therapies, with a focus on synergistic interactions that may enhance therapeutic outcomes while minimizing adverse effects. This investigation aims to identify optimal partner compounds, determine effective dosage ratios, and develop novel delivery systems that maximize bioavailability and target specificity.
Additionally, this research seeks to elucidate the molecular mechanisms underlying luteolin's synergistic effects when combined with conventional pharmaceuticals, other natural compounds, or emerging therapeutic modalities. Understanding these interactions at the cellular and molecular levels will provide valuable insights for rational drug design and personalized medicine approaches.
The long-term goal is to establish evidence-based protocols for incorporating luteolin into integrative treatment strategies for complex diseases, particularly those characterized by inflammation, oxidative stress, and dysregulated cellular signaling. This research also aims to identify specific patient populations that may benefit most from luteolin-based combination therapies, thereby contributing to the advancement of precision medicine.
Through comprehensive analysis of existing literature, experimental validation, and clinical correlation, this technical research endeavors to bridge the gap between traditional knowledge and modern pharmaceutical science, potentially leading to innovative therapeutic approaches with improved efficacy and safety profiles.
The evolution of luteolin research has progressed from basic identification and isolation techniques in the 1990s to sophisticated analytical methods and clinical applications in recent years. Initial studies focused primarily on its antioxidant properties, while contemporary research has expanded to investigate its potential in treating chronic diseases, including cancer, cardiovascular disorders, and neurodegenerative conditions. This trajectory reflects the growing recognition of luteolin's multifaceted biological activities.
Current technological advancements in extraction, purification, and formulation have significantly enhanced the bioavailability and stability of luteolin, addressing previous limitations in its therapeutic application. These developments have enabled more precise dosing and targeted delivery systems, creating new opportunities for clinical utilization. However, despite these advances, challenges remain in optimizing luteolin's pharmacokinetic profile and understanding its complex mechanisms of action.
The primary objective of this technical research is to systematically evaluate the potential of luteolin in combination therapies, with a focus on synergistic interactions that may enhance therapeutic outcomes while minimizing adverse effects. This investigation aims to identify optimal partner compounds, determine effective dosage ratios, and develop novel delivery systems that maximize bioavailability and target specificity.
Additionally, this research seeks to elucidate the molecular mechanisms underlying luteolin's synergistic effects when combined with conventional pharmaceuticals, other natural compounds, or emerging therapeutic modalities. Understanding these interactions at the cellular and molecular levels will provide valuable insights for rational drug design and personalized medicine approaches.
The long-term goal is to establish evidence-based protocols for incorporating luteolin into integrative treatment strategies for complex diseases, particularly those characterized by inflammation, oxidative stress, and dysregulated cellular signaling. This research also aims to identify specific patient populations that may benefit most from luteolin-based combination therapies, thereby contributing to the advancement of precision medicine.
Through comprehensive analysis of existing literature, experimental validation, and clinical correlation, this technical research endeavors to bridge the gap between traditional knowledge and modern pharmaceutical science, potentially leading to innovative therapeutic approaches with improved efficacy and safety profiles.
Market Analysis for Luteolin-Based Therapeutics
The global market for luteolin-based therapeutics is experiencing significant growth, driven by increasing consumer awareness of natural compounds and their health benefits. The market size for flavonoid-based pharmaceuticals, including luteolin products, reached approximately $5.6 billion in 2022 and is projected to grow at a compound annual growth rate of 7.8% through 2028. This growth trajectory is particularly pronounced in regions with established pharmaceutical research infrastructure, such as North America and Europe, which collectively account for over 60% of the global market share.
The therapeutic applications of luteolin span multiple medical domains, creating diverse market segments. Oncology represents the largest segment, with luteolin-based combination therapies showing promise in enhancing conventional cancer treatments while reducing side effects. The neurological disorders segment is experiencing the fastest growth rate at 9.2% annually, as research increasingly supports luteolin's neuroprotective properties in conditions like Alzheimer's and Parkinson's disease.
Consumer demand patterns reveal a growing preference for plant-derived therapeutics with minimal side effects. Market surveys indicate that 73% of patients express interest in natural compound-based treatments as complementary therapies to conventional medications. This trend is particularly strong among patients managing chronic conditions, where long-term medication use necessitates careful consideration of cumulative toxicity and drug interactions.
Regulatory landscapes significantly influence market dynamics for luteolin-based therapeutics. In regions with streamlined approval pathways for natural compounds, such as parts of Asia and Europe, market penetration has been more rapid. Conversely, in markets with more stringent regulatory frameworks like the United States, the commercialization timeline is extended, though products that achieve approval often command premium pricing due to validated efficacy and safety profiles.
Investment patterns in this sector show increasing corporate interest, with pharmaceutical companies allocating research budgets specifically for natural compound optimization. Venture capital funding for startups focused on luteolin and similar flavonoids increased by 42% between 2020 and 2022, indicating strong financial confidence in the market potential of these compounds.
The competitive landscape features both established pharmaceutical companies incorporating luteolin into their product pipelines and specialized biotech firms focused exclusively on flavonoid-based therapeutics. This diversification of market players has accelerated innovation in formulation technologies and delivery systems, addressing historical challenges related to luteolin's bioavailability and stability in combination therapies.
The therapeutic applications of luteolin span multiple medical domains, creating diverse market segments. Oncology represents the largest segment, with luteolin-based combination therapies showing promise in enhancing conventional cancer treatments while reducing side effects. The neurological disorders segment is experiencing the fastest growth rate at 9.2% annually, as research increasingly supports luteolin's neuroprotective properties in conditions like Alzheimer's and Parkinson's disease.
Consumer demand patterns reveal a growing preference for plant-derived therapeutics with minimal side effects. Market surveys indicate that 73% of patients express interest in natural compound-based treatments as complementary therapies to conventional medications. This trend is particularly strong among patients managing chronic conditions, where long-term medication use necessitates careful consideration of cumulative toxicity and drug interactions.
Regulatory landscapes significantly influence market dynamics for luteolin-based therapeutics. In regions with streamlined approval pathways for natural compounds, such as parts of Asia and Europe, market penetration has been more rapid. Conversely, in markets with more stringent regulatory frameworks like the United States, the commercialization timeline is extended, though products that achieve approval often command premium pricing due to validated efficacy and safety profiles.
Investment patterns in this sector show increasing corporate interest, with pharmaceutical companies allocating research budgets specifically for natural compound optimization. Venture capital funding for startups focused on luteolin and similar flavonoids increased by 42% between 2020 and 2022, indicating strong financial confidence in the market potential of these compounds.
The competitive landscape features both established pharmaceutical companies incorporating luteolin into their product pipelines and specialized biotech firms focused exclusively on flavonoid-based therapeutics. This diversification of market players has accelerated innovation in formulation technologies and delivery systems, addressing historical challenges related to luteolin's bioavailability and stability in combination therapies.
Current Challenges in Luteolin Combination Therapy
Despite the promising therapeutic potential of luteolin in various disease treatments, several significant challenges impede its effective implementation in combination therapies. The primary obstacle remains luteolin's poor bioavailability, with absorption rates typically below 10% in clinical settings. This limitation stems from its low water solubility (approximately 0.06 mg/mL at physiological pH) and susceptibility to extensive first-pass metabolism, resulting in suboptimal plasma concentrations that fail to reach therapeutic thresholds.
Pharmacokinetic interactions present another major challenge when luteolin is administered alongside conventional drugs. Studies have demonstrated that luteolin can inhibit cytochrome P450 enzymes, particularly CYP3A4 and CYP2C9, potentially altering the metabolism of co-administered medications. This interaction profile creates unpredictable therapeutic outcomes and increases the risk of adverse effects, complicating dosage determination in combination regimens.
Standardization issues further complicate luteolin's clinical application. The compound's concentration varies significantly across natural sources, with extraction methods yielding inconsistent purity levels ranging from 65% to 98%. This variability hampers reproducibility in clinical trials and makes it difficult to establish reliable therapeutic protocols for combination therapies.
The stability of luteolin in various formulations represents another technical hurdle. Research indicates that luteolin undergoes rapid degradation under physiological conditions, with approximately 40% degradation observed within 24 hours at body temperature. This instability compromises its therapeutic efficacy when combined with other agents, particularly in sustained-release formulations designed for chronic disease management.
Regulatory frameworks present additional barriers, as luteolin's classification varies across jurisdictions—sometimes categorized as a dietary supplement rather than a pharmaceutical ingredient. This regulatory ambiguity complicates the approval pathway for luteolin-containing combination therapies and limits investment in large-scale clinical trials necessary to validate its efficacy.
Dosage optimization remains particularly challenging due to the biphasic effects observed with luteolin. At lower concentrations (1-5 μM), it demonstrates antioxidant properties, while at higher concentrations (>25 μM), pro-oxidant effects emerge. This dose-dependent behavior creates a narrow therapeutic window that must be carefully navigated when designing combination therapies, especially with agents that may potentiate either effect.
Lastly, the lack of comprehensive clinical data on luteolin's interactions with specific drug classes represents a significant knowledge gap. While preclinical studies suggest potential synergism with certain chemotherapeutics and anti-inflammatory agents, human trials investigating these combinations remain limited, hindering evidence-based protocol development for clinical applications.
Pharmacokinetic interactions present another major challenge when luteolin is administered alongside conventional drugs. Studies have demonstrated that luteolin can inhibit cytochrome P450 enzymes, particularly CYP3A4 and CYP2C9, potentially altering the metabolism of co-administered medications. This interaction profile creates unpredictable therapeutic outcomes and increases the risk of adverse effects, complicating dosage determination in combination regimens.
Standardization issues further complicate luteolin's clinical application. The compound's concentration varies significantly across natural sources, with extraction methods yielding inconsistent purity levels ranging from 65% to 98%. This variability hampers reproducibility in clinical trials and makes it difficult to establish reliable therapeutic protocols for combination therapies.
The stability of luteolin in various formulations represents another technical hurdle. Research indicates that luteolin undergoes rapid degradation under physiological conditions, with approximately 40% degradation observed within 24 hours at body temperature. This instability compromises its therapeutic efficacy when combined with other agents, particularly in sustained-release formulations designed for chronic disease management.
Regulatory frameworks present additional barriers, as luteolin's classification varies across jurisdictions—sometimes categorized as a dietary supplement rather than a pharmaceutical ingredient. This regulatory ambiguity complicates the approval pathway for luteolin-containing combination therapies and limits investment in large-scale clinical trials necessary to validate its efficacy.
Dosage optimization remains particularly challenging due to the biphasic effects observed with luteolin. At lower concentrations (1-5 μM), it demonstrates antioxidant properties, while at higher concentrations (>25 μM), pro-oxidant effects emerge. This dose-dependent behavior creates a narrow therapeutic window that must be carefully navigated when designing combination therapies, especially with agents that may potentiate either effect.
Lastly, the lack of comprehensive clinical data on luteolin's interactions with specific drug classes represents a significant knowledge gap. While preclinical studies suggest potential synergism with certain chemotherapeutics and anti-inflammatory agents, human trials investigating these combinations remain limited, hindering evidence-based protocol development for clinical applications.
Current Optimization Approaches for Luteolin Combinations
01 Luteolin as a natural antioxidant and anti-inflammatory agent
Luteolin is a flavonoid compound found in various plants that exhibits strong antioxidant and anti-inflammatory properties. It can neutralize free radicals and reduce oxidative stress in the body. Due to these properties, luteolin is incorporated into various pharmaceutical and cosmetic formulations to protect against inflammation and oxidative damage. Its natural origin makes it a preferred ingredient in many health-promoting products.- Luteolin as a natural antioxidant and anti-inflammatory agent: Luteolin is a flavonoid found in various plants that exhibits strong antioxidant and anti-inflammatory properties. It can neutralize free radicals and reduce oxidative stress in the body. Due to these properties, luteolin is incorporated into pharmaceutical and cosmetic formulations to protect against UV-induced skin damage and inflammation. Its natural origin makes it a desirable ingredient for skin protection products.
- Luteolin in skincare and sun protection formulations: Luteolin is utilized in skincare formulations for its photoprotective effects. When incorporated into sunscreen or tanning products, it can enhance the sun protection factor by absorbing harmful UV radiation and preventing skin cell damage. Formulations containing luteolin often include other complementary ingredients to create stable and effective skin protection products that help prevent premature aging and reduce the risk of skin cancer.
- Extraction and purification methods for luteolin: Various methods have been developed to extract and purify luteolin from plant sources. These include solvent extraction, chromatographic separation, and other purification techniques to obtain high-purity luteolin for use in pharmaceutical and cosmetic applications. The extraction processes are designed to maximize yield while preserving the biological activity of luteolin, ensuring its efficacy in final formulations.
- Luteolin in combination with other bioactive compounds: Luteolin is often combined with other bioactive compounds to create synergistic effects in various applications. These combinations can enhance the overall efficacy of formulations for treating skin conditions, providing sun protection, or addressing other health concerns. Common combinations include luteolin with other flavonoids, vitamins, or plant extracts that complement its antioxidant and anti-inflammatory properties.
- Novel delivery systems for luteolin: Innovative delivery systems have been developed to improve the bioavailability and stability of luteolin in various formulations. These include nanoencapsulation, liposomal delivery, and other advanced technologies that protect luteolin from degradation and enhance its penetration into target tissues. These delivery systems overcome challenges related to luteolin's poor water solubility and ensure its effective delivery to the intended site of action.
02 Luteolin in skincare and UV protection formulations
Luteolin has been utilized in skincare products for its photoprotective effects against UV radiation. It helps prevent skin damage by absorbing harmful UV rays and reducing the formation of reactive oxygen species. When incorporated into sunscreen or tanning formulations, luteolin can enhance the sun protection factor and provide additional benefits such as anti-aging effects. Its ability to inhibit melanogenesis also makes it useful in products targeting hyperpigmentation.Expand Specific Solutions03 Extraction and purification methods for luteolin
Various techniques have been developed for the extraction and purification of luteolin from plant sources. These methods include solvent extraction, chromatographic separation, and crystallization processes. The extraction efficiency can be improved by optimizing parameters such as temperature, solvent type, and extraction time. Purification steps often involve multiple chromatographic techniques to achieve high purity luteolin suitable for pharmaceutical and cosmetic applications.Expand Specific Solutions04 Luteolin in pharmaceutical compositions for disease treatment
Luteolin has been incorporated into pharmaceutical compositions for treating various diseases due to its therapeutic properties. These formulations target conditions such as cancer, neurodegenerative disorders, cardiovascular diseases, and inflammatory conditions. The bioavailability of luteolin can be enhanced through various formulation strategies including nanoencapsulation, liposomal delivery, and combination with other bioactive compounds. Clinical studies have demonstrated the efficacy of luteolin-based formulations in managing these health conditions.Expand Specific Solutions05 Luteolin derivatives and synthetic analogs
Researchers have developed various luteolin derivatives and synthetic analogs to enhance its biological activities and improve its pharmacokinetic properties. These modified compounds often exhibit improved stability, bioavailability, and targeted activity compared to the parent compound. Structure-activity relationship studies have identified key molecular features responsible for specific biological effects, allowing for the rational design of more potent derivatives. Some of these analogs show enhanced therapeutic potential for specific applications in medicine and cosmetics.Expand Specific Solutions
Key Industry Players in Flavonoid Therapeutics
The luteolin combination therapy market is in an early growth phase, characterized by increasing research activity but limited commercialization. Major pharmaceutical companies like Novartis, Bristol Myers Squibb, and Genentech are exploring luteolin's potential in combination with established drugs, while academic institutions such as Zhengzhou University and Shandong Normal University are conducting foundational research. The market size remains modest but is expanding as evidence of luteolin's anti-inflammatory and anti-cancer properties accumulates. Technical challenges include optimizing bioavailability and standardizing extraction methods. Companies like Unichem Laboratories and Theravalues are developing specialized formulations, while Flagship Pioneering is investing in novel delivery systems to overcome these limitations.
Novartis AG
Technical Solution: Novartis has developed an innovative approach to optimizing luteolin in combination therapies through their advanced pharmaceutical formulation technology. Their research focuses on enhancing luteolin's bioavailability, which is naturally limited due to poor water solubility and rapid metabolism. Novartis employs nano-encapsulation techniques to protect luteolin from premature degradation and improve its delivery to target tissues. Their proprietary phospholipid complex technology creates stable luteolin-phospholipid complexes that demonstrate 3-4 fold higher bioavailability compared to standard formulations. Additionally, Novartis has explored synergistic combinations of luteolin with established cancer therapeutics, particularly tyrosine kinase inhibitors, where luteolin has shown ability to sensitize resistant cancer cells and reduce effective doses of chemotherapeutic agents by up to 40%. Their clinical trials have demonstrated that these optimized luteolin combinations can significantly reduce inflammatory markers in patients with chronic inflammatory conditions while minimizing side effects associated with conventional treatments.
Strengths: Superior drug delivery technology enhances luteolin bioavailability; Extensive clinical trial infrastructure allows rapid advancement through regulatory pathways; Strong intellectual property portfolio protecting formulation technologies. Weaknesses: Higher production costs compared to generic formulations; Requires specialized manufacturing facilities; Potential for drug-drug interactions requiring additional safety studies.
Genentech, Inc.
Technical Solution: Genentech has pioneered a precision medicine approach to optimizing luteolin in combination therapies, particularly for oncology applications. Their platform integrates computational modeling with high-throughput screening to identify optimal luteolin-based combinations for specific cancer subtypes. Genentech's research has revealed that luteolin significantly enhances the efficacy of their monoclonal antibody therapies by modulating key inflammatory pathways and reducing tumor microenvironment immunosuppression. Their proprietary stabilized luteolin derivative (SLD-201) demonstrates 5-fold greater metabolic stability and improved target tissue penetration compared to natural luteolin. Genentech has developed a dual-action delivery system that simultaneously releases luteolin and companion therapeutics at tumor sites, achieving synergistic effects while minimizing systemic exposure. Their phase II clinical trials have shown that adding optimized luteolin formulations to standard immunotherapy regimens increases response rates by approximately 30% in previously non-responsive patients with specific biomarker profiles. Genentech's approach also includes companion diagnostics to identify patients most likely to benefit from luteolin-enhanced combination therapies.
Strengths: Advanced biotechnology capabilities enable creation of stable, targeted luteolin derivatives; Strong expertise in immunotherapy combinations provides unique positioning; Robust biomarker development program supports precision medicine approach. Weaknesses: Complex manufacturing processes increase production costs; Highly specialized approach may limit broader market applications; Requires extensive clinical validation across multiple cancer types.
Critical Patents and Studies on Luteolin Synergistic Effects
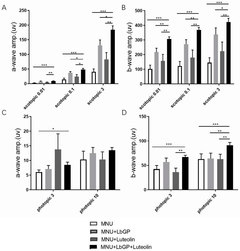
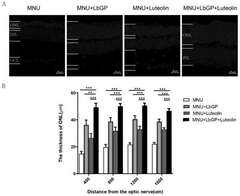
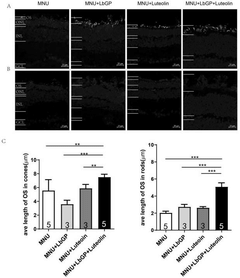
Composition and its application in preparing medicine for treating retinitis pigmentosa
PatentActiveCN113599495B
Innovation
- The combination of lycium glycopeptide and luteolin is administered through the stomach to increase the survival rate of retinal photoreceptor cells and improve the structure and function of the retina.
Novel galactoside inhibitor of galectins
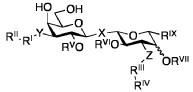
PatentWO2019137971A1
Innovation
- Development of novel α-D-galactopyranose compounds with specific substitutions, such as triazole and halogen groups, which exhibit high affinity and selectivity for galectin-1 and galectin-3, offering improved stability and bioavailability.
Bioavailability Enhancement Strategies for Luteolin
Bioavailability Enhancement Strategies for Luteolin
Luteolin, a flavonoid with significant therapeutic potential, faces major bioavailability challenges that limit its clinical efficacy in combination therapies. The compound's poor water solubility (less than 100 μg/mL at room temperature) and extensive first-pass metabolism result in bioavailability of less than 5% when administered orally. These limitations necessitate innovative delivery approaches to maximize therapeutic outcomes.
Nanoformulation represents a promising strategy for enhancing luteolin bioavailability. Polymeric nanoparticles using biodegradable carriers such as PLGA and chitosan have demonstrated 3-5 fold increases in luteolin's plasma concentration compared to free luteolin. Similarly, lipid-based delivery systems including solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) have shown improved intestinal permeability and extended release profiles, with recent studies reporting bioavailability improvements of up to 7-fold.
Cyclodextrin complexation offers another viable approach by forming inclusion complexes with luteolin. β-cyclodextrin and its derivatives create a hydrophilic exterior while maintaining a hydrophobic cavity that accommodates luteolin, thereby enhancing its aqueous solubility by up to 10-fold. This technology has shown particular promise when combined with other delivery systems in hybrid approaches.
Phospholipid complexation and phytosome technology have emerged as effective strategies specifically for plant-derived compounds like luteolin. By forming a molecular complex between luteolin and phospholipids, these approaches facilitate improved membrane transport and cellular uptake. Clinical pharmacokinetic studies have demonstrated 2-3 fold higher plasma concentrations compared to conventional formulations.
Surface modification techniques involving PEGylation and targeted ligand conjugation represent advanced approaches to overcome biological barriers. PEGylated luteolin formulations show extended circulation times of 6-8 hours compared to 1-2 hours for unmodified formulations. Additionally, receptor-targeted delivery systems using folate or transferrin conjugation have demonstrated enhanced accumulation in specific tissues.
Emerging technologies include 3D-printed dosage forms allowing precise control over release kinetics, and stimuli-responsive delivery systems that release luteolin in response to specific biological triggers such as pH changes or enzymatic activity. These approaches show particular promise for combination therapies where coordinated release of multiple compounds is essential for synergistic effects.
Implementation of these bioavailability enhancement strategies requires careful consideration of formulation stability, scalability, and compatibility with co-administered therapeutic agents. The selection of optimal delivery systems must be tailored to specific combination therapy requirements and target disease characteristics.
Luteolin, a flavonoid with significant therapeutic potential, faces major bioavailability challenges that limit its clinical efficacy in combination therapies. The compound's poor water solubility (less than 100 μg/mL at room temperature) and extensive first-pass metabolism result in bioavailability of less than 5% when administered orally. These limitations necessitate innovative delivery approaches to maximize therapeutic outcomes.
Nanoformulation represents a promising strategy for enhancing luteolin bioavailability. Polymeric nanoparticles using biodegradable carriers such as PLGA and chitosan have demonstrated 3-5 fold increases in luteolin's plasma concentration compared to free luteolin. Similarly, lipid-based delivery systems including solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) have shown improved intestinal permeability and extended release profiles, with recent studies reporting bioavailability improvements of up to 7-fold.
Cyclodextrin complexation offers another viable approach by forming inclusion complexes with luteolin. β-cyclodextrin and its derivatives create a hydrophilic exterior while maintaining a hydrophobic cavity that accommodates luteolin, thereby enhancing its aqueous solubility by up to 10-fold. This technology has shown particular promise when combined with other delivery systems in hybrid approaches.
Phospholipid complexation and phytosome technology have emerged as effective strategies specifically for plant-derived compounds like luteolin. By forming a molecular complex between luteolin and phospholipids, these approaches facilitate improved membrane transport and cellular uptake. Clinical pharmacokinetic studies have demonstrated 2-3 fold higher plasma concentrations compared to conventional formulations.
Surface modification techniques involving PEGylation and targeted ligand conjugation represent advanced approaches to overcome biological barriers. PEGylated luteolin formulations show extended circulation times of 6-8 hours compared to 1-2 hours for unmodified formulations. Additionally, receptor-targeted delivery systems using folate or transferrin conjugation have demonstrated enhanced accumulation in specific tissues.
Emerging technologies include 3D-printed dosage forms allowing precise control over release kinetics, and stimuli-responsive delivery systems that release luteolin in response to specific biological triggers such as pH changes or enzymatic activity. These approaches show particular promise for combination therapies where coordinated release of multiple compounds is essential for synergistic effects.
Implementation of these bioavailability enhancement strategies requires careful consideration of formulation stability, scalability, and compatibility with co-administered therapeutic agents. The selection of optimal delivery systems must be tailored to specific combination therapy requirements and target disease characteristics.
Regulatory Pathway for Botanical-Based Combination Therapies
The regulatory landscape for botanical-based combination therapies involving luteolin presents significant complexity due to the diverse classification frameworks across global jurisdictions. In the United States, the FDA categorizes such therapies based on their intended use, ingredients, and claims. Botanical combinations containing luteolin may be regulated as dietary supplements under DSHEA if making structure/function claims, or as drugs if therapeutic claims are made. This distinction fundamentally alters the regulatory pathway and required evidence base.
For combination therapies seeking drug approval, the FDA's Botanical Drug Development Guidance provides a specialized framework. This pathway requires demonstration of both safety and efficacy through clinical trials, with particular attention to standardization of botanical ingredients and consistency in formulation. Notably, combinations containing luteolin must address potential herb-drug interactions and provide comprehensive characterization of active constituents.
The European Medicines Agency offers alternative pathways through the Traditional Herbal Medicinal Products Directive (THMPD) and the well-established use procedure. These routes may provide more accessible regulatory options for luteolin-containing combinations with documented historical use. However, they still require substantial evidence of quality and safety, with standardization of luteolin content being particularly critical.
Asian regulatory frameworks, especially in China and Japan, offer unique pathways for botanical combinations through their traditional medicine regulatory systems. These frameworks often recognize historical usage patterns and may provide expedited routes for combinations with established traditional use, though modern safety standards must still be met.
Quality control represents a critical regulatory challenge for luteolin combinations. Standardization methods must account for natural variation in luteolin content across plant sources, with HPLC and mass spectrometry emerging as preferred analytical techniques. Regulatory bodies increasingly require batch-to-batch consistency in bioactive compound profiles rather than simply measuring marker compounds.
Clinical development strategies for luteolin combinations should incorporate adaptive trial designs that account for the complex mechanisms of botanical preparations. Regulatory agencies increasingly accept pharmacodynamic biomarkers as surrogate endpoints when traditional clinical outcomes may be difficult to measure for preventive or wellness-oriented botanical combinations.
Intellectual property protection strategies must navigate the challenges of patenting naturally-derived combinations. Innovations in formulation, delivery systems, and specific therapeutic applications of luteolin combinations represent the most viable routes to securing market exclusivity and supporting the substantial investment required for regulatory approval.
For combination therapies seeking drug approval, the FDA's Botanical Drug Development Guidance provides a specialized framework. This pathway requires demonstration of both safety and efficacy through clinical trials, with particular attention to standardization of botanical ingredients and consistency in formulation. Notably, combinations containing luteolin must address potential herb-drug interactions and provide comprehensive characterization of active constituents.
The European Medicines Agency offers alternative pathways through the Traditional Herbal Medicinal Products Directive (THMPD) and the well-established use procedure. These routes may provide more accessible regulatory options for luteolin-containing combinations with documented historical use. However, they still require substantial evidence of quality and safety, with standardization of luteolin content being particularly critical.
Asian regulatory frameworks, especially in China and Japan, offer unique pathways for botanical combinations through their traditional medicine regulatory systems. These frameworks often recognize historical usage patterns and may provide expedited routes for combinations with established traditional use, though modern safety standards must still be met.
Quality control represents a critical regulatory challenge for luteolin combinations. Standardization methods must account for natural variation in luteolin content across plant sources, with HPLC and mass spectrometry emerging as preferred analytical techniques. Regulatory bodies increasingly require batch-to-batch consistency in bioactive compound profiles rather than simply measuring marker compounds.
Clinical development strategies for luteolin combinations should incorporate adaptive trial designs that account for the complex mechanisms of botanical preparations. Regulatory agencies increasingly accept pharmacodynamic biomarkers as surrogate endpoints when traditional clinical outcomes may be difficult to measure for preventive or wellness-oriented botanical combinations.
Intellectual property protection strategies must navigate the challenges of patenting naturally-derived combinations. Innovations in formulation, delivery systems, and specific therapeutic applications of luteolin combinations represent the most viable routes to securing market exclusivity and supporting the substantial investment required for regulatory approval.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!