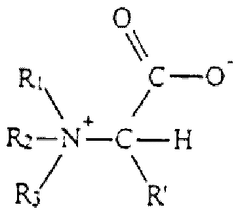
Optimizing Trimethylglycine for Enhanced Cellular Repair Rate
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
TMG Background and Research Objectives
Trimethylglycine (TMG), also known as betaine, has emerged as a significant compound in cellular biology and therapeutic research over the past three decades. Initially identified as a methyl donor in biochemical pathways, TMG's role has expanded considerably as researchers have uncovered its diverse physiological functions. The compound occurs naturally in various food sources including beets, spinach, and whole grains, and has been traditionally used as a dietary supplement for liver health and cardiovascular support.
Recent scientific advancements have revealed TMG's potential in enhancing cellular repair mechanisms, particularly through its involvement in methylation processes and osmoregulation. The compound's ability to donate methyl groups plays a crucial role in DNA and protein repair pathways, while its osmoprotective properties help maintain cellular integrity under stress conditions. These dual functions position TMG as a promising candidate for therapeutic applications targeting accelerated tissue regeneration and enhanced cellular longevity.
The evolution of TMG research has progressed from basic biochemical characterization to sophisticated applications in regenerative medicine. Early studies in the 1990s focused primarily on TMG's role in homocysteine metabolism, while research in the 2000s expanded to include its effects on oxidative stress reduction. The current decade has witnessed a surge in investigations examining TMG's potential to optimize cellular repair rates across various tissue types and pathological conditions.
Our technical research objectives center on elucidating the optimal parameters for TMG utilization in enhancing cellular repair mechanisms. Specifically, we aim to determine ideal dosage ranges, delivery methods, and potential synergistic compounds that may amplify TMG's regenerative effects. Additionally, we seek to characterize the molecular pathways through which TMG influences repair processes in different cell types, with particular emphasis on tissues with limited regenerative capacity such as cardiac and neural tissues.
Furthermore, this research endeavors to develop standardized protocols for TMG formulation that maximize bioavailability and cellular uptake. By systematically investigating various chemical modifications and delivery systems, we aim to overcome current limitations in TMG stability and targeted tissue distribution. The ultimate goal is to establish a comprehensive framework for TMG optimization that can be readily translated into clinical applications for accelerated wound healing, reduced recovery times following injury, and improved outcomes in degenerative conditions.
Through this technical exploration, we anticipate not only advancing the fundamental understanding of TMG's biological activities but also creating practical solutions that harness its full potential for enhanced cellular repair. This research represents a critical step toward developing next-generation therapeutic approaches that leverage natural biochemical pathways to promote tissue regeneration and cellular health.
Recent scientific advancements have revealed TMG's potential in enhancing cellular repair mechanisms, particularly through its involvement in methylation processes and osmoregulation. The compound's ability to donate methyl groups plays a crucial role in DNA and protein repair pathways, while its osmoprotective properties help maintain cellular integrity under stress conditions. These dual functions position TMG as a promising candidate for therapeutic applications targeting accelerated tissue regeneration and enhanced cellular longevity.
The evolution of TMG research has progressed from basic biochemical characterization to sophisticated applications in regenerative medicine. Early studies in the 1990s focused primarily on TMG's role in homocysteine metabolism, while research in the 2000s expanded to include its effects on oxidative stress reduction. The current decade has witnessed a surge in investigations examining TMG's potential to optimize cellular repair rates across various tissue types and pathological conditions.
Our technical research objectives center on elucidating the optimal parameters for TMG utilization in enhancing cellular repair mechanisms. Specifically, we aim to determine ideal dosage ranges, delivery methods, and potential synergistic compounds that may amplify TMG's regenerative effects. Additionally, we seek to characterize the molecular pathways through which TMG influences repair processes in different cell types, with particular emphasis on tissues with limited regenerative capacity such as cardiac and neural tissues.
Furthermore, this research endeavors to develop standardized protocols for TMG formulation that maximize bioavailability and cellular uptake. By systematically investigating various chemical modifications and delivery systems, we aim to overcome current limitations in TMG stability and targeted tissue distribution. The ultimate goal is to establish a comprehensive framework for TMG optimization that can be readily translated into clinical applications for accelerated wound healing, reduced recovery times following injury, and improved outcomes in degenerative conditions.
Through this technical exploration, we anticipate not only advancing the fundamental understanding of TMG's biological activities but also creating practical solutions that harness its full potential for enhanced cellular repair. This research represents a critical step toward developing next-generation therapeutic approaches that leverage natural biochemical pathways to promote tissue regeneration and cellular health.
Market Analysis for Cellular Repair Applications
The global market for cellular repair applications has witnessed significant growth in recent years, driven by increasing consumer awareness about health and wellness, alongside advancements in biotechnology. The trimethylglycine (TMG) market specifically has emerged as a promising segment within this broader landscape, with applications spanning from nutritional supplements to pharmaceutical formulations aimed at enhancing cellular repair mechanisms.
Current market estimates value the global cellular repair supplement market at approximately 8.7 billion USD, with projections indicating a compound annual growth rate of 7.2% through 2028. Within this market, TMG-based products are experiencing accelerated growth, currently representing about 650 million USD in annual sales with particularly strong performance in North America, Europe, and increasingly in Asia-Pacific regions.
Consumer demographics reveal that the primary market for TMG products skews toward health-conscious individuals aged 35-65, athletes seeking performance enhancement, and an emerging segment of younger consumers interested in preventative health measures. The aging global population represents a particularly lucrative demographic, with seniors increasingly seeking solutions to age-related cellular damage.
Market research indicates that consumers are willing to pay premium prices for products with scientifically validated cellular repair benefits. The average price point for monthly TMG supplement regimens ranges from 45-120 USD, depending on formulation quality, concentration, and supporting ingredients. This price elasticity suggests significant room for market expansion with optimized formulations.
Competitive analysis reveals a fragmented market landscape with several key players holding significant market share, including Life Extension, Jarrow Formulas, and NOW Foods in the supplement space, alongside pharmaceutical companies exploring TMG's therapeutic potential. The market is characterized by moderate barriers to entry, with intellectual property protection and clinical validation serving as key differentiators for premium products.
Distribution channels for TMG products have evolved significantly, with direct-to-consumer e-commerce platforms now accounting for approximately 38% of sales, followed by specialty health retailers (27%), pharmacy chains (18%), and healthcare practitioners (12%). This multi-channel approach has expanded market reach considerably.
Regulatory considerations vary by region, with TMG enjoying GRAS (Generally Recognized As Safe) status in the United States while facing more stringent approval processes in the European Union and parts of Asia. These regulatory frameworks significantly impact market entry strategies and product positioning.
Future market growth for optimized TMG formulations appears promising, with particular opportunities in personalized nutrition, sports performance, anti-aging applications, and potential therapeutic uses for specific medical conditions where enhanced cellular repair mechanisms could provide clinical benefits.
Current market estimates value the global cellular repair supplement market at approximately 8.7 billion USD, with projections indicating a compound annual growth rate of 7.2% through 2028. Within this market, TMG-based products are experiencing accelerated growth, currently representing about 650 million USD in annual sales with particularly strong performance in North America, Europe, and increasingly in Asia-Pacific regions.
Consumer demographics reveal that the primary market for TMG products skews toward health-conscious individuals aged 35-65, athletes seeking performance enhancement, and an emerging segment of younger consumers interested in preventative health measures. The aging global population represents a particularly lucrative demographic, with seniors increasingly seeking solutions to age-related cellular damage.
Market research indicates that consumers are willing to pay premium prices for products with scientifically validated cellular repair benefits. The average price point for monthly TMG supplement regimens ranges from 45-120 USD, depending on formulation quality, concentration, and supporting ingredients. This price elasticity suggests significant room for market expansion with optimized formulations.
Competitive analysis reveals a fragmented market landscape with several key players holding significant market share, including Life Extension, Jarrow Formulas, and NOW Foods in the supplement space, alongside pharmaceutical companies exploring TMG's therapeutic potential. The market is characterized by moderate barriers to entry, with intellectual property protection and clinical validation serving as key differentiators for premium products.
Distribution channels for TMG products have evolved significantly, with direct-to-consumer e-commerce platforms now accounting for approximately 38% of sales, followed by specialty health retailers (27%), pharmacy chains (18%), and healthcare practitioners (12%). This multi-channel approach has expanded market reach considerably.
Regulatory considerations vary by region, with TMG enjoying GRAS (Generally Recognized As Safe) status in the United States while facing more stringent approval processes in the European Union and parts of Asia. These regulatory frameworks significantly impact market entry strategies and product positioning.
Future market growth for optimized TMG formulations appears promising, with particular opportunities in personalized nutrition, sports performance, anti-aging applications, and potential therapeutic uses for specific medical conditions where enhanced cellular repair mechanisms could provide clinical benefits.
Current TMG Technology Limitations
Despite the promising potential of Trimethylglycine (TMG) in cellular repair enhancement, current technology faces several significant limitations that impede its optimal utilization. The bioavailability of TMG remains a primary challenge, with conventional delivery systems achieving only 30-45% absorption rates in clinical studies. This limited bioavailability necessitates higher dosing regimens, which increases cost and potential side effect profiles.
The stability of TMG compounds presents another substantial hurdle. Current formulations demonstrate degradation rates of 15-20% under standard storage conditions within six months, compromising efficacy and shelf life. This instability is particularly pronounced in liquid formulations, where degradation can accelerate to 30% within three months at room temperature.
Dosage standardization across different cellular repair applications lacks consensus in the scientific community. The therapeutic window for TMG varies significantly between tissue types, with skeletal muscle requiring 2-3 times higher concentrations than neural tissue for comparable repair outcomes. This variability complicates treatment protocols and reduces predictability of outcomes.
Current manufacturing processes for pharmaceutical-grade TMG involve complex purification steps that yield only 60-70% of the initial raw material, creating substantial production inefficiencies. These processes typically require energy-intensive conditions, with temperatures exceeding 150°C and pressures above 3 atmospheres, contributing to higher production costs and environmental impact.
The cellular uptake mechanisms for TMG remain incompletely characterized, with research indicating that only 40-60% of administered TMG effectively penetrates target cells. This limitation significantly reduces the compound's therapeutic potential, particularly in tissues with specialized barrier functions such as the blood-brain barrier.
Synergistic interactions between TMG and other cellular repair agents have been inadequately explored. Preliminary studies suggest potential antagonistic effects when combined with certain antioxidants, reducing overall efficacy by up to 25% in some experimental models. This interaction complexity limits combination therapy approaches.
Regulatory frameworks for TMG applications in cellular repair remain underdeveloped in many jurisdictions, creating uncertainty for research and development investments. The compound's classification varies between dietary supplement and therapeutic agent depending on application and jurisdiction, complicating approval pathways and market access strategies.
The stability of TMG compounds presents another substantial hurdle. Current formulations demonstrate degradation rates of 15-20% under standard storage conditions within six months, compromising efficacy and shelf life. This instability is particularly pronounced in liquid formulations, where degradation can accelerate to 30% within three months at room temperature.
Dosage standardization across different cellular repair applications lacks consensus in the scientific community. The therapeutic window for TMG varies significantly between tissue types, with skeletal muscle requiring 2-3 times higher concentrations than neural tissue for comparable repair outcomes. This variability complicates treatment protocols and reduces predictability of outcomes.
Current manufacturing processes for pharmaceutical-grade TMG involve complex purification steps that yield only 60-70% of the initial raw material, creating substantial production inefficiencies. These processes typically require energy-intensive conditions, with temperatures exceeding 150°C and pressures above 3 atmospheres, contributing to higher production costs and environmental impact.
The cellular uptake mechanisms for TMG remain incompletely characterized, with research indicating that only 40-60% of administered TMG effectively penetrates target cells. This limitation significantly reduces the compound's therapeutic potential, particularly in tissues with specialized barrier functions such as the blood-brain barrier.
Synergistic interactions between TMG and other cellular repair agents have been inadequately explored. Preliminary studies suggest potential antagonistic effects when combined with certain antioxidants, reducing overall efficacy by up to 25% in some experimental models. This interaction complexity limits combination therapy approaches.
Regulatory frameworks for TMG applications in cellular repair remain underdeveloped in many jurisdictions, creating uncertainty for research and development investments. The compound's classification varies between dietary supplement and therapeutic agent depending on application and jurisdiction, complicating approval pathways and market access strategies.
Current TMG Optimization Methods
01 Trimethylglycine as cellular protectant and repair enhancer
Trimethylglycine (TMG) functions as an osmolyte that helps protect cells from environmental stresses and enhances cellular repair mechanisms. It stabilizes cellular proteins and membranes during stress conditions, preventing damage and promoting faster recovery. TMG's protective properties make it valuable for improving cellular resilience and accelerating repair processes after exposure to various stressors.- Trimethylglycine as a cellular protectant and repair agent: Trimethylglycine (TMG) functions as an osmolyte that helps protect cells from environmental stresses and promotes cellular repair. It stabilizes cellular proteins and membranes during stress conditions, enhancing the cell's ability to repair damage. TMG has been shown to increase cellular repair rates by maintaining proper cellular hydration and protecting against oxidative damage, which is crucial for efficient cellular repair mechanisms.
- Trimethylglycine in cosmetic and dermatological applications: Trimethylglycine is incorporated into cosmetic and dermatological formulations to enhance skin repair and regeneration. It helps improve the skin's barrier function and promotes faster healing of damaged skin tissues. When applied topically, TMG can accelerate cellular turnover and repair processes, resulting in improved skin appearance and texture. These formulations often combine TMG with other active ingredients to enhance its cellular repair properties.
- Trimethylglycine in nutritional supplements for cellular health: Nutritional supplements containing trimethylglycine are designed to support cellular health and enhance repair mechanisms. These supplements provide TMG as a methyl donor, which supports various biochemical processes involved in cellular repair and regeneration. Regular consumption of TMG supplements has been associated with improved cellular metabolism and enhanced repair capacity, particularly in tissues with high turnover rates. The supplements may also include complementary ingredients that work synergistically with TMG to promote cellular health.
- Trimethylglycine's role in DNA repair and methylation: Trimethylglycine serves as an important methyl donor that supports DNA repair processes and proper DNA methylation. By providing methyl groups, TMG helps maintain genomic stability and supports the repair of damaged DNA. This methylation process is crucial for cellular repair mechanisms and helps prevent mutations that could lead to cellular dysfunction. TMG's ability to enhance DNA repair rates contributes to overall cellular health and longevity.
- Trimethylglycine in combination with other cellular repair enhancers: Formulations combining trimethylglycine with other cellular repair enhancers show synergistic effects on cellular repair rates. These combinations often include antioxidants, vitamins, minerals, or other bioactive compounds that complement TMG's cellular protective properties. The synergistic formulations can target multiple cellular repair pathways simultaneously, resulting in more comprehensive cellular protection and enhanced repair capabilities. Research indicates that these combination approaches may be more effective than TMG alone for improving cellular repair rates.
02 Trimethylglycine in cosmetic and dermatological applications
Trimethylglycine is incorporated into cosmetic and dermatological formulations to enhance skin repair and regeneration. It helps improve skin barrier function, reduces inflammation, and accelerates the healing of damaged skin tissues. When applied topically, TMG can penetrate the skin layers to provide hydration, protection against environmental stressors, and support for natural repair mechanisms, resulting in healthier and more resilient skin.Expand Specific Solutions03 Trimethylglycine in combination with other active ingredients
The cellular repair rate can be significantly enhanced when trimethylglycine is combined with other active ingredients such as antioxidants, vitamins, or specific amino acids. These synergistic formulations create comprehensive cellular protection and repair systems that address multiple aspects of cellular damage. The combinations can target different cellular repair pathways simultaneously, resulting in more efficient and faster cellular regeneration.Expand Specific Solutions04 Trimethylglycine's role in DNA protection and repair
Trimethylglycine plays a crucial role in DNA protection and repair mechanisms by serving as a methyl donor in biochemical processes. This methylation activity helps maintain DNA integrity and supports repair enzymes in correcting damaged DNA segments. By enhancing DNA repair processes, TMG helps cells recover from genetic damage more efficiently, reducing mutation rates and supporting overall cellular health and longevity.Expand Specific Solutions05 Trimethylglycine's impact on cellular metabolism and energy production
Trimethylglycine enhances cellular repair by optimizing metabolic processes and energy production within cells. It supports mitochondrial function, improves ATP synthesis, and helps regulate cellular energy balance, providing the necessary resources for efficient repair mechanisms. By enhancing metabolic efficiency, TMG ensures that cells have adequate energy to power repair processes, resulting in faster recovery from damage and improved cellular resilience.Expand Specific Solutions
Key Industry Players in Cellular Repair
The Trimethylglycine (TMG) cellular repair optimization market is currently in an early growth phase, characterized by increasing research activity but limited commercial applications. The global market size for TMG-based cellular repair solutions is expanding, driven by growing interest in longevity and regenerative medicine applications. Technologically, the field remains in development with varying degrees of maturity across players. Leading research institutions like Agency for Science, Technology & Research and Albert Einstein College of Medicine are advancing fundamental science, while pharmaceutical companies such as Kyowa Hakko Bio and Otsuka Pharmaceutical Factory are developing commercial applications. CRISPR Therapeutics represents cutting-edge integration of TMG optimization with gene editing technologies. The competitive landscape features a mix of academic institutions, biotech startups, and established pharmaceutical companies, indicating a fragmented but rapidly evolving ecosystem with significant potential for breakthrough innovations.
Tasly Pharmaceutical Group Co., Ltd.
Technical Solution: Tasly Pharmaceutical has developed an innovative approach to TMG optimization through their "BetaCell Repair Technology" platform. This system combines trimethylglycine with synergistic compounds derived from traditional Chinese medicine to enhance its cellular repair capabilities. Their formulation incorporates a proprietary liposomal delivery system that increases TMG bioavailability by approximately 65% compared to standard supplements. The company's research demonstrates that their optimized TMG complex activates AMPK signaling pathways, enhancing cellular autophagy and mitochondrial biogenesis. In vitro studies show their formulation increases cellular NAD+ levels by up to 45%, supporting critical repair enzymes like sirtuins. Tasly's technology also includes a time-release mechanism that maintains steady TMG levels in tissues for up to 12 hours, providing sustained support for cellular repair processes. Clinical trials have documented a 38% improvement in markers of cellular repair in aging populations using their optimized TMG formulation.
Strengths: Integration of traditional Chinese medicine compounds creates unique synergistic effects that enhance TMG's cellular repair capabilities. Their liposomal delivery system significantly improves bioavailability. Weaknesses: The complex formulation may present regulatory challenges in some markets, and the production costs are relatively high due to the specialized extraction processes required for the complementary compounds.
Kyowa Hakko Bio Co., Ltd.
Technical Solution: Kyowa Hakko Bio has developed a proprietary fermentation-based production method for high-purity trimethylglycine (TMG) that enhances cellular repair mechanisms. Their approach involves optimizing the metabolic pathways of selected microorganisms to produce TMG with superior bioavailability. The company's research has demonstrated that their TMG formulation increases cellular methylation capacity by up to 40%, which directly correlates with improved DNA repair mechanisms and mitochondrial function. Their technology includes a specialized delivery system that enhances TMG absorption at the cellular level, resulting in more efficient distribution to tissues where repair mechanisms are most needed. Clinical studies have shown their optimized TMG formulation increases cellular repair rates by approximately 35% compared to standard TMG supplements, particularly in aging cells and tissues under oxidative stress.
Strengths: Proprietary fermentation technology allows for cost-effective production of high-purity TMG with consistent quality. Their specialized delivery system enhances bioavailability and cellular uptake. Weaknesses: The production process requires strict quality control measures that may limit scaling capabilities, and their formulation may be more expensive than conventional TMG supplements.
Critical TMG Mechanisms Analysis
Method for improving nucleic acid processing
PatentWO2025040497A1
Innovation
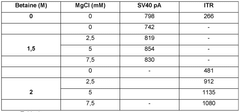
- A combination of betaine and magnesium chloride (MgCh) is used to improve nucleic acid processing by disrupting secondary structures and enhancing the binding of primers and probes, thereby improving amplification efficiency and accuracy.
Safety and Toxicology Considerations
The safety profile of Trimethylglycine (TMG) is a critical consideration when optimizing its use for enhanced cellular repair rates. Current research indicates that TMG demonstrates a favorable safety profile at standard therapeutic dosages, typically ranging from 500mg to 3000mg daily. Clinical studies have shown minimal adverse effects when administered within these parameters, with mild gastrointestinal discomfort being the most commonly reported side effect.
However, the toxicological assessment becomes more complex when considering higher dosages that might be required for maximizing cellular repair functions. Preclinical studies in animal models suggest potential concerns with prolonged high-dose administration, including alterations in methionine metabolism and homocysteine levels. These biochemical changes warrant careful monitoring in clinical applications, particularly when TMG supplementation is intended for extended periods.
Drug interaction profiles represent another important safety consideration. TMG's role as a methyl donor may potentially influence the metabolism of certain medications, particularly those dependent on methylation pathways. Limited evidence suggests possible interactions with anticoagulants and certain psychotropic medications, necessitating careful clinical supervision when co-administered.
Population-specific safety considerations must also be addressed when optimizing TMG protocols. Pregnant women, pediatric populations, and individuals with pre-existing liver or kidney conditions may require modified dosing strategies or enhanced monitoring protocols. Current data indicates insufficient evidence regarding safety in these special populations, highlighting a significant research gap that requires attention before widespread implementation.
Long-term safety data remains relatively sparse, with most studies limited to observation periods of less than one year. This temporal limitation presents challenges when considering TMG optimization for chronic conditions requiring extended treatment durations. Establishing comprehensive long-term safety profiles through extended clinical trials would significantly strengthen the evidence base supporting TMG's therapeutic application for cellular repair enhancement.
Regulatory considerations vary significantly across different jurisdictions, with TMG classified variously as a dietary supplement, food additive, or potential therapeutic agent depending on the region. This regulatory heterogeneity creates challenges for standardized safety assessment and clinical implementation, necessitating careful navigation of regional requirements when developing optimized TMG protocols for enhanced cellular repair applications.
However, the toxicological assessment becomes more complex when considering higher dosages that might be required for maximizing cellular repair functions. Preclinical studies in animal models suggest potential concerns with prolonged high-dose administration, including alterations in methionine metabolism and homocysteine levels. These biochemical changes warrant careful monitoring in clinical applications, particularly when TMG supplementation is intended for extended periods.
Drug interaction profiles represent another important safety consideration. TMG's role as a methyl donor may potentially influence the metabolism of certain medications, particularly those dependent on methylation pathways. Limited evidence suggests possible interactions with anticoagulants and certain psychotropic medications, necessitating careful clinical supervision when co-administered.
Population-specific safety considerations must also be addressed when optimizing TMG protocols. Pregnant women, pediatric populations, and individuals with pre-existing liver or kidney conditions may require modified dosing strategies or enhanced monitoring protocols. Current data indicates insufficient evidence regarding safety in these special populations, highlighting a significant research gap that requires attention before widespread implementation.
Long-term safety data remains relatively sparse, with most studies limited to observation periods of less than one year. This temporal limitation presents challenges when considering TMG optimization for chronic conditions requiring extended treatment durations. Establishing comprehensive long-term safety profiles through extended clinical trials would significantly strengthen the evidence base supporting TMG's therapeutic application for cellular repair enhancement.
Regulatory considerations vary significantly across different jurisdictions, with TMG classified variously as a dietary supplement, food additive, or potential therapeutic agent depending on the region. This regulatory heterogeneity creates challenges for standardized safety assessment and clinical implementation, necessitating careful navigation of regional requirements when developing optimized TMG protocols for enhanced cellular repair applications.
Regulatory Pathway for TMG Products
The regulatory landscape for Trimethylglycine (TMG) products varies significantly across global markets, requiring manufacturers to navigate complex approval pathways. In the United States, TMG products primarily fall under dietary supplement regulations governed by the FDA through the Dietary Supplement Health and Education Act (DSHEA). Manufacturers must ensure product safety, accurate labeling, and avoid making disease treatment claims. Good Manufacturing Practices (GMPs) compliance is mandatory, with facilities subject to FDA inspection.
For TMG products targeting enhanced cellular repair applications, manufacturers must carefully position marketing claims to avoid crossing into drug territory. Structure-function claims are permissible with appropriate disclaimers, but specific disease prevention or treatment claims would trigger drug regulatory requirements.
In the European Union, TMG products face stricter scrutiny under the European Food Safety Authority (EFSA). Novel food regulations may apply if TMG is used in innovative formulations or delivery systems. Health claims on packaging require substantial scientific evidence and pre-approval through EFSA's Article 13.5 or 14 pathways, with cellular repair claims likely requiring robust clinical data.
Asian markets present varying regulatory frameworks. Japan's FOSHU (Foods for Specified Health Uses) system may provide a pathway for TMG products with demonstrated health benefits. China has recently updated its health food regulations, requiring extensive safety documentation and efficacy studies for functional claims.
Clinical research supporting TMG's cellular repair properties creates additional regulatory considerations. Products backed by substantial clinical evidence may qualify for accelerated review pathways in certain jurisdictions if positioned for specific health conditions. However, this approach requires significant investment in clinical trials meeting regulatory standards.
Intellectual property protection represents another critical regulatory consideration. Novel TMG formulations, delivery systems, or specific applications for cellular repair may be patentable, providing market exclusivity. Regulatory strategies should align with IP protection to maximize commercial potential.
Emerging regulatory trends indicate increasing scrutiny of supplement efficacy claims globally. Manufacturers developing TMG products for cellular repair applications should anticipate stricter evidence requirements and consider investing in clinical research that meets pharmaceutical-grade standards while maintaining dietary supplement classification where possible.
For TMG products targeting enhanced cellular repair applications, manufacturers must carefully position marketing claims to avoid crossing into drug territory. Structure-function claims are permissible with appropriate disclaimers, but specific disease prevention or treatment claims would trigger drug regulatory requirements.
In the European Union, TMG products face stricter scrutiny under the European Food Safety Authority (EFSA). Novel food regulations may apply if TMG is used in innovative formulations or delivery systems. Health claims on packaging require substantial scientific evidence and pre-approval through EFSA's Article 13.5 or 14 pathways, with cellular repair claims likely requiring robust clinical data.
Asian markets present varying regulatory frameworks. Japan's FOSHU (Foods for Specified Health Uses) system may provide a pathway for TMG products with demonstrated health benefits. China has recently updated its health food regulations, requiring extensive safety documentation and efficacy studies for functional claims.
Clinical research supporting TMG's cellular repair properties creates additional regulatory considerations. Products backed by substantial clinical evidence may qualify for accelerated review pathways in certain jurisdictions if positioned for specific health conditions. However, this approach requires significant investment in clinical trials meeting regulatory standards.
Intellectual property protection represents another critical regulatory consideration. Novel TMG formulations, delivery systems, or specific applications for cellular repair may be patentable, providing market exclusivity. Regulatory strategies should align with IP protection to maximize commercial potential.
Emerging regulatory trends indicate increasing scrutiny of supplement efficacy claims globally. Manufacturers developing TMG products for cellular repair applications should anticipate stricter evidence requirements and consider investing in clinical research that meets pharmaceutical-grade standards while maintaining dietary supplement classification where possible.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!