Glycerol as a Carrier in Targeted Drug Delivery Systems
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycerol in Drug Delivery: Background and Objectives
Glycerol, a simple polyol compound, has emerged as a promising carrier in targeted drug delivery systems, revolutionizing the field of pharmaceutical research. The evolution of drug delivery technologies has been driven by the need for more efficient and targeted therapeutic approaches, with glycerol playing an increasingly significant role in recent years.
The primary objective of utilizing glycerol as a carrier in targeted drug delivery systems is to enhance the efficacy and safety of pharmaceutical compounds. By leveraging glycerol's unique properties, researchers aim to improve drug solubility, stability, and bioavailability while minimizing side effects and reducing dosage requirements. This approach aligns with the broader goals of personalized medicine and precision therapeutics.
Historically, the use of glycerol in pharmaceutical applications dates back several decades. Initially employed as a sweetening agent and preservative, its potential as a drug carrier has only recently been fully recognized. The growing interest in glycerol-based delivery systems stems from its biocompatibility, non-toxicity, and versatile chemical structure, which allows for various modifications and functionalization.
The technological trajectory of glycerol in drug delivery has been marked by significant milestones. Early research focused on its use as a simple solvent or stabilizer. However, advancements in nanotechnology and polymer science have led to the development of more sophisticated glycerol-based carriers, including nanoparticles, liposomes, and hydrogels.
Current research objectives in this field encompass several key areas. Scientists are exploring the synthesis of novel glycerol derivatives to enhance drug loading capacity and release kinetics. There is also a focus on developing smart delivery systems that respond to specific physiological triggers, enabling precise spatial and temporal control of drug release.
Another critical objective is to optimize the targeting capabilities of glycerol-based carriers. This involves engineering the surface properties of these carriers to interact selectively with specific cell types or tissues, thereby improving therapeutic outcomes and reducing off-target effects. Additionally, researchers are investigating the potential of glycerol-based systems in overcoming biological barriers, such as the blood-brain barrier, to address challenging therapeutic targets.
The integration of glycerol into targeted drug delivery systems also aligns with the broader trend towards sustainable and green chemistry in pharmaceutical research. As a biodegradable and renewable resource, glycerol offers an environmentally friendly alternative to many synthetic carriers, addressing growing concerns about the environmental impact of pharmaceutical products.
The primary objective of utilizing glycerol as a carrier in targeted drug delivery systems is to enhance the efficacy and safety of pharmaceutical compounds. By leveraging glycerol's unique properties, researchers aim to improve drug solubility, stability, and bioavailability while minimizing side effects and reducing dosage requirements. This approach aligns with the broader goals of personalized medicine and precision therapeutics.
Historically, the use of glycerol in pharmaceutical applications dates back several decades. Initially employed as a sweetening agent and preservative, its potential as a drug carrier has only recently been fully recognized. The growing interest in glycerol-based delivery systems stems from its biocompatibility, non-toxicity, and versatile chemical structure, which allows for various modifications and functionalization.
The technological trajectory of glycerol in drug delivery has been marked by significant milestones. Early research focused on its use as a simple solvent or stabilizer. However, advancements in nanotechnology and polymer science have led to the development of more sophisticated glycerol-based carriers, including nanoparticles, liposomes, and hydrogels.
Current research objectives in this field encompass several key areas. Scientists are exploring the synthesis of novel glycerol derivatives to enhance drug loading capacity and release kinetics. There is also a focus on developing smart delivery systems that respond to specific physiological triggers, enabling precise spatial and temporal control of drug release.
Another critical objective is to optimize the targeting capabilities of glycerol-based carriers. This involves engineering the surface properties of these carriers to interact selectively with specific cell types or tissues, thereby improving therapeutic outcomes and reducing off-target effects. Additionally, researchers are investigating the potential of glycerol-based systems in overcoming biological barriers, such as the blood-brain barrier, to address challenging therapeutic targets.
The integration of glycerol into targeted drug delivery systems also aligns with the broader trend towards sustainable and green chemistry in pharmaceutical research. As a biodegradable and renewable resource, glycerol offers an environmentally friendly alternative to many synthetic carriers, addressing growing concerns about the environmental impact of pharmaceutical products.
Market Analysis for Glycerol-based Drug Carriers
The market for glycerol-based drug carriers in targeted drug delivery systems has shown significant growth potential in recent years. This trend is driven by the increasing demand for more effective and efficient drug delivery methods, particularly in the treatment of complex diseases such as cancer and chronic conditions. Glycerol, a naturally occurring compound with excellent biocompatibility and versatility, has emerged as a promising carrier material in this field.
The global market for targeted drug delivery systems is expected to expand rapidly, with glycerol-based carriers playing a crucial role in this growth. The pharmaceutical industry's focus on personalized medicine and the need for improved drug efficacy and reduced side effects are key factors driving the adoption of these advanced delivery systems. Additionally, the growing prevalence of chronic diseases and the aging population in many developed countries contribute to the increasing demand for innovative drug delivery solutions.
In the context of glycerol-based drug carriers, several market segments show particular promise. The oncology sector represents a significant portion of the market, as targeted drug delivery is especially beneficial in cancer treatment, allowing for more precise and effective therapies with reduced systemic toxicity. Other important segments include cardiovascular diseases, neurological disorders, and inflammatory conditions, where targeted delivery can enhance therapeutic outcomes and patient compliance.
The market for glycerol-based drug carriers is also influenced by technological advancements in nanotechnology and materials science. These developments have led to the creation of more sophisticated and efficient delivery systems, such as glycerol-based nanoparticles and liposomes, which offer improved drug encapsulation, stability, and release profiles. As research in this area continues to progress, it is expected to further expand the market opportunities for glycerol-based carriers.
Geographically, North America and Europe currently dominate the market for advanced drug delivery systems, including those based on glycerol. This is primarily due to the presence of well-established pharmaceutical industries, significant R&D investments, and favorable regulatory environments in these regions. However, emerging markets in Asia-Pacific, particularly China and India, are expected to experience rapid growth in this sector, driven by increasing healthcare expenditure, growing awareness of advanced therapies, and improving research infrastructure.
The competitive landscape of the glycerol-based drug carrier market is characterized by a mix of large pharmaceutical companies, specialized drug delivery firms, and academic research institutions. Collaborations between these entities are becoming increasingly common, as they combine expertise in drug development, delivery technologies, and clinical applications to bring innovative products to market.
The global market for targeted drug delivery systems is expected to expand rapidly, with glycerol-based carriers playing a crucial role in this growth. The pharmaceutical industry's focus on personalized medicine and the need for improved drug efficacy and reduced side effects are key factors driving the adoption of these advanced delivery systems. Additionally, the growing prevalence of chronic diseases and the aging population in many developed countries contribute to the increasing demand for innovative drug delivery solutions.
In the context of glycerol-based drug carriers, several market segments show particular promise. The oncology sector represents a significant portion of the market, as targeted drug delivery is especially beneficial in cancer treatment, allowing for more precise and effective therapies with reduced systemic toxicity. Other important segments include cardiovascular diseases, neurological disorders, and inflammatory conditions, where targeted delivery can enhance therapeutic outcomes and patient compliance.
The market for glycerol-based drug carriers is also influenced by technological advancements in nanotechnology and materials science. These developments have led to the creation of more sophisticated and efficient delivery systems, such as glycerol-based nanoparticles and liposomes, which offer improved drug encapsulation, stability, and release profiles. As research in this area continues to progress, it is expected to further expand the market opportunities for glycerol-based carriers.
Geographically, North America and Europe currently dominate the market for advanced drug delivery systems, including those based on glycerol. This is primarily due to the presence of well-established pharmaceutical industries, significant R&D investments, and favorable regulatory environments in these regions. However, emerging markets in Asia-Pacific, particularly China and India, are expected to experience rapid growth in this sector, driven by increasing healthcare expenditure, growing awareness of advanced therapies, and improving research infrastructure.
The competitive landscape of the glycerol-based drug carrier market is characterized by a mix of large pharmaceutical companies, specialized drug delivery firms, and academic research institutions. Collaborations between these entities are becoming increasingly common, as they combine expertise in drug development, delivery technologies, and clinical applications to bring innovative products to market.
Current Challenges in Glycerol-based Drug Delivery
Despite the promising potential of glycerol as a carrier in targeted drug delivery systems, several challenges persist in its practical application. One of the primary obstacles is the high viscosity of glycerol, which can impede the formulation process and affect the overall drug release kinetics. This property makes it difficult to achieve consistent and controlled drug release profiles, potentially limiting the efficacy of the delivery system.
Another significant challenge lies in the hydrophilic nature of glycerol. While this characteristic enhances its biocompatibility, it can lead to rapid clearance from the body, reducing the residence time of the drug-carrier complex in the target tissues. This rapid elimination may necessitate more frequent dosing, potentially increasing side effects and patient discomfort.
The stability of glycerol-based drug delivery systems under various physiological conditions remains a concern. Changes in pH, temperature, and enzymatic activity in different body compartments can affect the integrity of the carrier system, potentially leading to premature drug release or degradation of the active pharmaceutical ingredients.
Furthermore, the interaction between glycerol and certain drug molecules poses challenges in maintaining drug stability and activity. Some drugs may undergo chemical modifications or degradation when in prolonged contact with glycerol, necessitating careful formulation strategies and extensive stability studies.
The development of targeted delivery mechanisms using glycerol-based systems is also an area of ongoing research. While glycerol itself is biocompatible, achieving specific targeting to diseased tissues or cells requires the incorporation of additional targeting moieties. The integration of these targeting elements without compromising the overall stability and efficacy of the delivery system remains a complex task.
Scalability and manufacturing considerations present additional hurdles. The production of consistent, high-quality glycerol-based drug delivery systems on an industrial scale requires sophisticated processing techniques and quality control measures. Ensuring batch-to-batch reproducibility and long-term stability during storage and transportation are critical aspects that need to be addressed.
Regulatory challenges also play a significant role in the development of glycerol-based drug delivery systems. As a novel carrier, extensive safety and efficacy data are required to gain regulatory approval. The lack of established regulatory pathways for such innovative delivery systems can lead to prolonged development timelines and increased costs.
Another significant challenge lies in the hydrophilic nature of glycerol. While this characteristic enhances its biocompatibility, it can lead to rapid clearance from the body, reducing the residence time of the drug-carrier complex in the target tissues. This rapid elimination may necessitate more frequent dosing, potentially increasing side effects and patient discomfort.
The stability of glycerol-based drug delivery systems under various physiological conditions remains a concern. Changes in pH, temperature, and enzymatic activity in different body compartments can affect the integrity of the carrier system, potentially leading to premature drug release or degradation of the active pharmaceutical ingredients.
Furthermore, the interaction between glycerol and certain drug molecules poses challenges in maintaining drug stability and activity. Some drugs may undergo chemical modifications or degradation when in prolonged contact with glycerol, necessitating careful formulation strategies and extensive stability studies.
The development of targeted delivery mechanisms using glycerol-based systems is also an area of ongoing research. While glycerol itself is biocompatible, achieving specific targeting to diseased tissues or cells requires the incorporation of additional targeting moieties. The integration of these targeting elements without compromising the overall stability and efficacy of the delivery system remains a complex task.
Scalability and manufacturing considerations present additional hurdles. The production of consistent, high-quality glycerol-based drug delivery systems on an industrial scale requires sophisticated processing techniques and quality control measures. Ensuring batch-to-batch reproducibility and long-term stability during storage and transportation are critical aspects that need to be addressed.
Regulatory challenges also play a significant role in the development of glycerol-based drug delivery systems. As a novel carrier, extensive safety and efficacy data are required to gain regulatory approval. The lack of established regulatory pathways for such innovative delivery systems can lead to prolonged development timelines and increased costs.
Existing Glycerol Carrier Technologies
01 Glycerol as a carrier for active ingredients
Glycerol is used as an effective carrier for various active ingredients in pharmaceutical and cosmetic formulations. Its hygroscopic properties help to improve the solubility and stability of the active compounds, enhancing their bioavailability and effectiveness. This carrier system can be particularly useful for delivering hydrophilic and lipophilic substances.- Glycerol as a carrier for active ingredients: Glycerol is used as an effective carrier for various active ingredients in pharmaceutical and cosmetic formulations. Its hygroscopic properties help to improve the solubility and stability of active compounds, enhancing their delivery and effectiveness. This carrier system can be particularly useful for hydrophilic substances.
- Glycerol in transdermal drug delivery systems: Glycerol is utilized in transdermal drug delivery systems to enhance skin penetration of active ingredients. It acts as a penetration enhancer and moisturizer, improving the overall effectiveness of topical formulations. This approach can lead to increased bioavailability of drugs administered through the skin.
- Glycerol as a stabilizer in formulations: Glycerol serves as a stabilizer in various formulations, including suspensions and emulsions. It helps maintain the physical and chemical stability of active ingredients, preventing degradation and ensuring consistent effectiveness throughout the product's shelf life. This property is particularly valuable in pharmaceutical and cosmetic preparations.
- Glycerol in oral drug delivery systems: Glycerol is employed in oral drug delivery systems to improve the solubility and bioavailability of poorly water-soluble drugs. It can act as a solubilizing agent and help in the formation of self-emulsifying drug delivery systems, enhancing the overall effectiveness of oral medications.
- Glycerol in nanocarrier systems: Glycerol is utilized in the development of nanocarrier systems for drug delivery. It can be incorporated into nanoparticles, liposomes, or other nanostructures to improve their stability, drug loading capacity, and release characteristics. This approach can lead to enhanced therapeutic efficacy and reduced side effects of various drugs.
02 Glycerol in transdermal drug delivery systems
Glycerol is utilized in transdermal drug delivery systems to enhance skin penetration of active ingredients. It acts as a penetration enhancer and moisturizer, improving the absorption of drugs through the skin. This application is particularly beneficial for topical medications and cosmetic products.Expand Specific Solutions03 Glycerol as a stabilizer in formulations
Glycerol serves as a stabilizer in various formulations, including pharmaceuticals and cosmetics. It helps maintain the stability of active ingredients, prevents crystallization, and extends the shelf life of products. This property makes glycerol an valuable component in many liquid and semi-solid preparations.Expand Specific Solutions04 Glycerol in microencapsulation technology
Glycerol is employed in microencapsulation processes to improve the encapsulation efficiency and release characteristics of active ingredients. It can act as a core material or be part of the shell formation, enhancing the protection and controlled release of encapsulated substances.Expand Specific Solutions05 Glycerol as a cryoprotectant in biological applications
Glycerol is used as an effective cryoprotectant in various biological applications, including the preservation of cells, tissues, and organisms at low temperatures. Its ability to prevent ice crystal formation and maintain cellular integrity during freezing and thawing processes makes it valuable in cryopreservation techniques.Expand Specific Solutions
Key Players in Glycerol-based Drug Delivery
The research on glycerol as a carrier in targeted drug delivery systems is in a developing stage, with significant potential for growth. The market size is expanding as pharmaceutical companies and research institutions increasingly focus on improving drug delivery efficiency. The technology's maturity is progressing, with various players contributing to advancements. Companies like Nippon Shinyaku Co., Ltd. and NOF Corp. are leveraging their expertise in pharmaceuticals and functional chemicals to explore glycerol-based delivery systems. Academic institutions such as Zhejiang University, Fudan University, and the University of California are conducting fundamental research to enhance the technology's efficacy. Collaborations between industry and academia, exemplified by partnerships involving Advanced Industrial Science & Technology and EnGeneIC Molecular Delivery Pty Ltd., are accelerating innovation in this field.
Hangzhou DAC Biotechnology Co., Ltd.
Technical Solution: Hangzhou DAC Biotechnology has developed a glycerol-based linker technology for antibody-drug conjugates (ADCs) in targeted cancer therapy. Their approach utilizes glycerol as a core component in designing cleavable linkers that connect potent cytotoxic drugs to antibodies. The glycerol-based linkers are engineered to be stable in circulation but readily cleaved in the tumor microenvironment or within cancer cells[13]. This technology has enabled the development of ADCs with improved therapeutic index by allowing higher drug-to-antibody ratios while maintaining stability. DAC's glycerol linkers have shown compatibility with various classes of cytotoxic payloads, including auristatins and maytansinoids[15]. In preclinical studies, ADCs utilizing these glycerol-based linkers demonstrated enhanced tumor penetration and sustained drug release compared to conventional ADC technologies[17]. The company has also explored the use of their glycerol linker platform in developing bispecific antibodies and immune-stimulating antibody conjugates.
Strengths: Improved stability and efficacy of ADCs, versatile application across different antibody and payload types. Weaknesses: Potential manufacturing challenges and regulatory hurdles for novel linker chemistry.
EnGeneIC Molecular Delivery Pty Ltd.
Technical Solution: EnGeneIC has developed a proprietary glycerol-based nanocell technology for targeted cancer drug delivery. Their EDV™ (EnGeneIC Delivery Vehicle) nanocells incorporate glycerol in their formulation to enhance stability and drug loading capacity. The nanocells are engineered to be 400nm in size, allowing them to passively target tumors through the enhanced permeability and retention (EPR) effect[10]. EnGeneIC's technology includes a surface modification strategy using glycerol-derived linkers to attach tumor-targeting antibodies, enabling active targeting to cancer cells[12]. The company has demonstrated the ability to package various payloads, including chemotherapeutics, siRNAs, and miRNAs, into their glycerol-enhanced nanocells. Clinical trials have shown promising results in treating various solid tumors, with reduced systemic toxicity compared to conventional chemotherapy[14]. The glycerol component of the nanocells contributes to their biodegradability and favorable pharmacokinetic profile.
Strengths: Versatile payload capacity, proven clinical efficacy, and reduced side effects. Weaknesses: Complex manufacturing process and potential immunogenicity concerns.
Innovative Glycerol Formulations for Drug Delivery
Compound modified with glycerol derivative
PatentWO2005023844A1
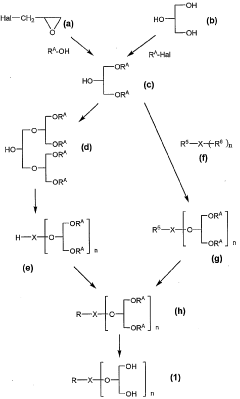
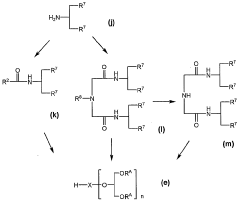
Innovation
- Development of compounds modified with glycerol derivatives as surface modifiers for drug carriers, which enhance blood retention and improve drug delivery by modifying amphipathic or hydrophobic substances with glycerol derivatives, creating microparticles that can effectively target and retain drugs in the bloodstream.
Drug delivery
PatentInactiveUS20210228725A1
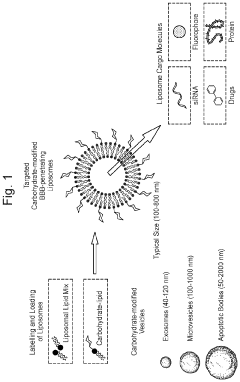
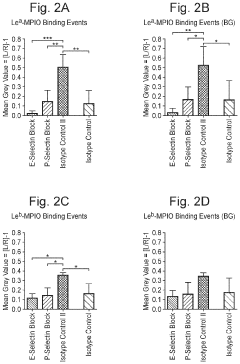
Innovation
- Conjugating vesicles containing therapeutic agents with specific targeting oligosaccharides, such as Lewis A or Lewis B, which bind to cell adhesion molecules like E-selectin, allowing targeted transport across the blood-brain barrier and accumulation in microglial cells, thereby selectively delivering drugs to brain pathology sites.
Regulatory Considerations for Glycerol Carriers
The regulatory landscape for glycerol as a carrier in targeted drug delivery systems is complex and multifaceted. Regulatory bodies, such as the FDA in the United States and the EMA in Europe, have established guidelines for the use of excipients in pharmaceutical formulations. Glycerol, being a common excipient, is generally recognized as safe (GRAS) by the FDA. However, its use in targeted drug delivery systems requires additional considerations.
One of the primary regulatory concerns is the safety profile of glycerol when used as a carrier. While glycerol is well-tolerated in most applications, its use in targeted drug delivery may involve novel formulations or routes of administration. Regulatory agencies require comprehensive toxicology studies to assess the safety of these new applications, including potential interactions with the active pharmaceutical ingredient (API) and other excipients.
The quality and purity of glycerol used in drug delivery systems are subject to stringent regulatory standards. Manufacturers must adhere to Good Manufacturing Practices (GMP) and provide detailed documentation on the sourcing, production, and quality control of glycerol. Impurities in glycerol can significantly impact drug stability and efficacy, making quality assurance a critical regulatory focus.
Regulatory bodies also scrutinize the functionality of glycerol as a carrier in targeted drug delivery systems. Manufacturers must demonstrate that glycerol enhances drug delivery without compromising the safety or efficacy of the API. This involves providing data on the pharmacokinetics and biodistribution of the drug when delivered using glycerol-based carriers.
The regulatory pathway for glycerol-based drug delivery systems may vary depending on whether the formulation is considered a new drug application (NDA) or a generic drug application (ANDA). For NDAs, extensive clinical trials may be required to establish safety and efficacy. ANDAs may face challenges in demonstrating bioequivalence, particularly if the glycerol carrier significantly alters the drug's pharmacokinetics.
Regulatory agencies also consider the environmental impact of glycerol-based drug delivery systems. Manufacturers may need to provide data on the biodegradability and environmental fate of glycerol and any associated compounds used in the formulation.
As targeted drug delivery technologies advance, regulatory frameworks continue to evolve. Manufacturers developing glycerol-based carriers must stay abreast of changing regulations and engage in early and frequent communication with regulatory agencies to ensure compliance throughout the development process.
One of the primary regulatory concerns is the safety profile of glycerol when used as a carrier. While glycerol is well-tolerated in most applications, its use in targeted drug delivery may involve novel formulations or routes of administration. Regulatory agencies require comprehensive toxicology studies to assess the safety of these new applications, including potential interactions with the active pharmaceutical ingredient (API) and other excipients.
The quality and purity of glycerol used in drug delivery systems are subject to stringent regulatory standards. Manufacturers must adhere to Good Manufacturing Practices (GMP) and provide detailed documentation on the sourcing, production, and quality control of glycerol. Impurities in glycerol can significantly impact drug stability and efficacy, making quality assurance a critical regulatory focus.
Regulatory bodies also scrutinize the functionality of glycerol as a carrier in targeted drug delivery systems. Manufacturers must demonstrate that glycerol enhances drug delivery without compromising the safety or efficacy of the API. This involves providing data on the pharmacokinetics and biodistribution of the drug when delivered using glycerol-based carriers.
The regulatory pathway for glycerol-based drug delivery systems may vary depending on whether the formulation is considered a new drug application (NDA) or a generic drug application (ANDA). For NDAs, extensive clinical trials may be required to establish safety and efficacy. ANDAs may face challenges in demonstrating bioequivalence, particularly if the glycerol carrier significantly alters the drug's pharmacokinetics.
Regulatory agencies also consider the environmental impact of glycerol-based drug delivery systems. Manufacturers may need to provide data on the biodegradability and environmental fate of glycerol and any associated compounds used in the formulation.
As targeted drug delivery technologies advance, regulatory frameworks continue to evolve. Manufacturers developing glycerol-based carriers must stay abreast of changing regulations and engage in early and frequent communication with regulatory agencies to ensure compliance throughout the development process.
Biocompatibility and Safety of Glycerol Carriers
Glycerol has emerged as a promising carrier in targeted drug delivery systems due to its unique properties and potential for biocompatibility. The safety profile of glycerol carriers is a critical aspect that requires thorough investigation to ensure their suitability for pharmaceutical applications.
Glycerol, also known as glycerin, is a naturally occurring compound found in various biological systems. Its inherent biocompatibility stems from its presence in cellular membranes and its role in metabolic processes. This natural occurrence contributes to the generally low toxicity profile of glycerol when used as a carrier in drug delivery systems.
Extensive research has been conducted to evaluate the biocompatibility of glycerol-based carriers in various biological environments. In vitro studies have demonstrated minimal cytotoxicity when glycerol carriers are exposed to different cell lines, including human fibroblasts and epithelial cells. These findings suggest that glycerol-based systems are well-tolerated at the cellular level.
In vivo studies have further supported the safety of glycerol carriers. Animal models have shown that glycerol-based drug delivery systems exhibit low systemic toxicity when administered through various routes, including oral, intravenous, and topical applications. The biodegradable nature of glycerol contributes to its favorable safety profile, as it can be metabolized and eliminated from the body through normal physiological processes.
One of the key advantages of glycerol as a carrier is its ability to enhance the solubility and stability of various drug molecules. This property not only improves the efficacy of drug delivery but also potentially reduces the required dosage, thereby minimizing the risk of adverse effects associated with high drug concentrations.
However, it is important to note that the safety and biocompatibility of glycerol carriers can be influenced by factors such as concentration, formulation, and the specific drug being delivered. Higher concentrations of glycerol may lead to osmotic effects, which could potentially cause local irritation or tissue damage. Therefore, careful optimization of glycerol-based formulations is essential to maintain an appropriate balance between efficacy and safety.
The long-term effects of repeated exposure to glycerol carriers in targeted drug delivery systems are an area that requires further investigation. While acute toxicity studies have shown promising results, more research is needed to assess the potential cumulative effects of prolonged use, particularly in chronic disease management scenarios.
Regulatory bodies, including the FDA and EMA, have recognized glycerol as a generally recognized as safe (GRAS) substance for various applications. This designation provides a foundation for its use in pharmaceutical formulations, but specific glycerol-based drug delivery systems still require rigorous safety evaluations on a case-by-case basis to ensure their suitability for clinical use.
Glycerol, also known as glycerin, is a naturally occurring compound found in various biological systems. Its inherent biocompatibility stems from its presence in cellular membranes and its role in metabolic processes. This natural occurrence contributes to the generally low toxicity profile of glycerol when used as a carrier in drug delivery systems.
Extensive research has been conducted to evaluate the biocompatibility of glycerol-based carriers in various biological environments. In vitro studies have demonstrated minimal cytotoxicity when glycerol carriers are exposed to different cell lines, including human fibroblasts and epithelial cells. These findings suggest that glycerol-based systems are well-tolerated at the cellular level.
In vivo studies have further supported the safety of glycerol carriers. Animal models have shown that glycerol-based drug delivery systems exhibit low systemic toxicity when administered through various routes, including oral, intravenous, and topical applications. The biodegradable nature of glycerol contributes to its favorable safety profile, as it can be metabolized and eliminated from the body through normal physiological processes.
One of the key advantages of glycerol as a carrier is its ability to enhance the solubility and stability of various drug molecules. This property not only improves the efficacy of drug delivery but also potentially reduces the required dosage, thereby minimizing the risk of adverse effects associated with high drug concentrations.
However, it is important to note that the safety and biocompatibility of glycerol carriers can be influenced by factors such as concentration, formulation, and the specific drug being delivered. Higher concentrations of glycerol may lead to osmotic effects, which could potentially cause local irritation or tissue damage. Therefore, careful optimization of glycerol-based formulations is essential to maintain an appropriate balance between efficacy and safety.
The long-term effects of repeated exposure to glycerol carriers in targeted drug delivery systems are an area that requires further investigation. While acute toxicity studies have shown promising results, more research is needed to assess the potential cumulative effects of prolonged use, particularly in chronic disease management scenarios.
Regulatory bodies, including the FDA and EMA, have recognized glycerol as a generally recognized as safe (GRAS) substance for various applications. This designation provides a foundation for its use in pharmaceutical formulations, but specific glycerol-based drug delivery systems still require rigorous safety evaluations on a case-by-case basis to ensure their suitability for clinical use.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!