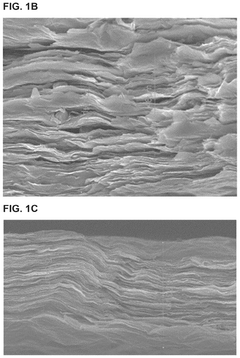
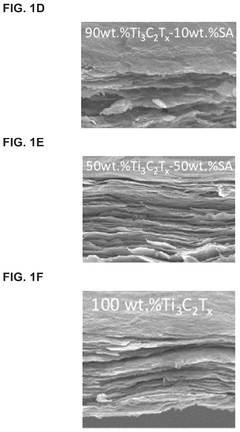
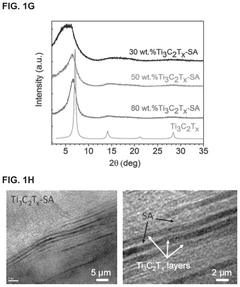
MXene-Modified Nanocarriers for Targeted Drug Delivery Systems
AUG 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MXene-Nanocarrier Background and Objectives
MXene, a class of two-dimensional transition metal carbides and nitrides, has emerged as a promising material for various applications, including targeted drug delivery systems. The development of MXene-modified nanocarriers represents a significant advancement in the field of nanomedicine, offering potential solutions to challenges in drug delivery and therapeutic efficacy.
The evolution of targeted drug delivery systems has been driven by the need to enhance therapeutic outcomes while minimizing side effects. Traditional drug administration methods often result in systemic distribution, leading to reduced efficacy and increased toxicity. The advent of nanotechnology has opened new avenues for precise drug delivery, with nanocarriers playing a crucial role in this paradigm shift.
MXene, first discovered in 2011, has rapidly gained attention due to its unique properties, including high surface area, excellent electrical conductivity, and tunable surface chemistry. These characteristics make MXene an ideal candidate for modification of nanocarriers, potentially enhancing their drug loading capacity, stability, and targeting capabilities.
The primary objective of research on MXene-modified nanocarriers is to develop a novel drug delivery platform that combines the advantages of MXene with existing nanocarrier technologies. This integration aims to address several key challenges in targeted drug delivery, such as improving drug solubility, enhancing cellular uptake, and achieving controlled release at specific sites.
Another critical goal is to explore the potential of MXene-modified nanocarriers in overcoming biological barriers, particularly the blood-brain barrier, which poses a significant challenge in treating neurological disorders. The unique properties of MXene may facilitate the design of nanocarriers capable of crossing this barrier more effectively, opening new possibilities for CNS drug delivery.
Furthermore, research in this field seeks to leverage the photothermal properties of MXene for theranostic applications, combining diagnostic imaging with therapeutic interventions. This approach could lead to the development of multifunctional nanocarriers that enable real-time monitoring of drug distribution and release.
The investigation of MXene-modified nanocarriers also aims to address concerns regarding biocompatibility and long-term safety. As a relatively new material, comprehensive studies on the in vivo behavior and potential toxicity of MXene are essential to ensure its suitability for clinical applications.
Ultimately, the research on MXene-modified nanocarriers for targeted drug delivery systems is driven by the vision of creating more effective, precise, and personalized therapeutic strategies. By harnessing the unique properties of MXene, researchers aspire to develop next-generation drug delivery platforms that can significantly improve patient outcomes across a wide range of diseases, from cancer to neurodegenerative disorders.
The evolution of targeted drug delivery systems has been driven by the need to enhance therapeutic outcomes while minimizing side effects. Traditional drug administration methods often result in systemic distribution, leading to reduced efficacy and increased toxicity. The advent of nanotechnology has opened new avenues for precise drug delivery, with nanocarriers playing a crucial role in this paradigm shift.
MXene, first discovered in 2011, has rapidly gained attention due to its unique properties, including high surface area, excellent electrical conductivity, and tunable surface chemistry. These characteristics make MXene an ideal candidate for modification of nanocarriers, potentially enhancing their drug loading capacity, stability, and targeting capabilities.
The primary objective of research on MXene-modified nanocarriers is to develop a novel drug delivery platform that combines the advantages of MXene with existing nanocarrier technologies. This integration aims to address several key challenges in targeted drug delivery, such as improving drug solubility, enhancing cellular uptake, and achieving controlled release at specific sites.
Another critical goal is to explore the potential of MXene-modified nanocarriers in overcoming biological barriers, particularly the blood-brain barrier, which poses a significant challenge in treating neurological disorders. The unique properties of MXene may facilitate the design of nanocarriers capable of crossing this barrier more effectively, opening new possibilities for CNS drug delivery.
Furthermore, research in this field seeks to leverage the photothermal properties of MXene for theranostic applications, combining diagnostic imaging with therapeutic interventions. This approach could lead to the development of multifunctional nanocarriers that enable real-time monitoring of drug distribution and release.
The investigation of MXene-modified nanocarriers also aims to address concerns regarding biocompatibility and long-term safety. As a relatively new material, comprehensive studies on the in vivo behavior and potential toxicity of MXene are essential to ensure its suitability for clinical applications.
Ultimately, the research on MXene-modified nanocarriers for targeted drug delivery systems is driven by the vision of creating more effective, precise, and personalized therapeutic strategies. By harnessing the unique properties of MXene, researchers aspire to develop next-generation drug delivery platforms that can significantly improve patient outcomes across a wide range of diseases, from cancer to neurodegenerative disorders.
Market Analysis for Targeted Drug Delivery
The targeted drug delivery market has been experiencing significant growth in recent years, driven by the increasing prevalence of chronic diseases and the need for more effective and precise treatment methods. This market segment is expected to continue its upward trajectory, with a projected compound annual growth rate (CAGR) of over 8% from 2021 to 2026. The global market value for targeted drug delivery systems is anticipated to reach several billion dollars by 2026, reflecting the growing demand for advanced therapeutic solutions.
The rise in cancer cases worldwide has been a major factor contributing to the expansion of the targeted drug delivery market. As cancer treatments require precise delivery of therapeutic agents to tumor sites while minimizing damage to healthy tissues, nanocarrier-based drug delivery systems have gained substantial attention. MXene-modified nanocarriers, in particular, have shown promising potential in this field due to their unique properties and versatility.
Geographically, North America currently holds the largest market share in targeted drug delivery systems, followed by Europe and Asia-Pacific. The United States, in particular, is at the forefront of research and development in this area, with numerous pharmaceutical companies and research institutions investing heavily in nanocarrier-based drug delivery technologies. However, emerging economies in Asia, such as China and India, are expected to witness rapid growth in this market due to increasing healthcare expenditure and growing awareness of advanced treatment options.
The pharmaceutical industry has been the primary end-user of targeted drug delivery systems, with a focus on oncology, cardiovascular diseases, and neurological disorders. However, there is a growing interest in expanding the application of these technologies to other therapeutic areas, including infectious diseases and autoimmune disorders. This diversification is expected to create new opportunities for market growth and innovation in the coming years.
Key market players in the targeted drug delivery sector include major pharmaceutical companies, biotechnology firms, and specialized nanotechnology companies. These organizations are actively engaged in research and development activities to enhance the efficacy and safety of drug delivery systems. Collaborations between academic institutions and industry partners have also become increasingly common, fostering innovation and accelerating the development of novel targeted drug delivery technologies.
The demand for personalized medicine and the increasing focus on patient-centric healthcare solutions are driving the adoption of targeted drug delivery systems. As healthcare providers and patients seek more effective and less invasive treatment options, the market for advanced drug delivery technologies, including MXene-modified nanocarriers, is expected to expand further. This trend is likely to be supported by ongoing advancements in nanotechnology, materials science, and bioengineering, which continue to push the boundaries of what is possible in targeted drug delivery.
The rise in cancer cases worldwide has been a major factor contributing to the expansion of the targeted drug delivery market. As cancer treatments require precise delivery of therapeutic agents to tumor sites while minimizing damage to healthy tissues, nanocarrier-based drug delivery systems have gained substantial attention. MXene-modified nanocarriers, in particular, have shown promising potential in this field due to their unique properties and versatility.
Geographically, North America currently holds the largest market share in targeted drug delivery systems, followed by Europe and Asia-Pacific. The United States, in particular, is at the forefront of research and development in this area, with numerous pharmaceutical companies and research institutions investing heavily in nanocarrier-based drug delivery technologies. However, emerging economies in Asia, such as China and India, are expected to witness rapid growth in this market due to increasing healthcare expenditure and growing awareness of advanced treatment options.
The pharmaceutical industry has been the primary end-user of targeted drug delivery systems, with a focus on oncology, cardiovascular diseases, and neurological disorders. However, there is a growing interest in expanding the application of these technologies to other therapeutic areas, including infectious diseases and autoimmune disorders. This diversification is expected to create new opportunities for market growth and innovation in the coming years.
Key market players in the targeted drug delivery sector include major pharmaceutical companies, biotechnology firms, and specialized nanotechnology companies. These organizations are actively engaged in research and development activities to enhance the efficacy and safety of drug delivery systems. Collaborations between academic institutions and industry partners have also become increasingly common, fostering innovation and accelerating the development of novel targeted drug delivery technologies.
The demand for personalized medicine and the increasing focus on patient-centric healthcare solutions are driving the adoption of targeted drug delivery systems. As healthcare providers and patients seek more effective and less invasive treatment options, the market for advanced drug delivery technologies, including MXene-modified nanocarriers, is expected to expand further. This trend is likely to be supported by ongoing advancements in nanotechnology, materials science, and bioengineering, which continue to push the boundaries of what is possible in targeted drug delivery.
Current Challenges in MXene-Modified Nanocarriers
Despite the promising potential of MXene-modified nanocarriers for targeted drug delivery systems, several significant challenges persist in their development and application. These obstacles span across various aspects of the technology, from synthesis and characterization to in vivo performance and safety concerns.
One of the primary challenges lies in the scalable and reproducible synthesis of MXene-modified nanocarriers. The current methods often result in inconsistent particle sizes and surface properties, which can significantly impact their drug loading capacity and release kinetics. Achieving uniform and stable MXene coatings on nanocarriers remains a complex task, requiring precise control over reaction conditions and surface chemistry.
The stability of MXene-modified nanocarriers in physiological environments presents another hurdle. MXenes are prone to oxidation and agglomeration in aqueous solutions, potentially compromising their unique properties and reducing their effectiveness as drug delivery vehicles. Developing strategies to enhance the colloidal stability and prevent degradation of MXene coatings under biological conditions is crucial for their successful application.
Controlling the drug release profile from MXene-modified nanocarriers poses a significant challenge. While MXenes offer excellent drug loading capacity, achieving sustained and controlled release of therapeutic agents remains difficult. The strong interactions between MXenes and drug molecules can sometimes hinder the timely release of drugs at target sites, necessitating the development of smart release mechanisms responsive to specific stimuli.
The potential toxicity and long-term safety of MXene-modified nanocarriers are areas of concern that require extensive investigation. The biocompatibility and biodegradability of these materials in vivo are not yet fully understood, and their potential accumulation in organs and tissues raises questions about their long-term effects on human health.
Targeting efficiency and specificity of MXene-modified nanocarriers need further improvement. While surface modifications can enhance their targeting capabilities, achieving highly selective delivery to specific cell types or tissues remains challenging. Overcoming biological barriers, such as the blood-brain barrier or tumor microenvironment, requires innovative strategies in nanocarrier design and functionalization.
Lastly, the translation of MXene-modified nanocarriers from laboratory research to clinical applications faces regulatory and manufacturing challenges. Establishing standardized production processes, ensuring batch-to-batch consistency, and meeting stringent regulatory requirements for nanomedicine products are critical steps that need to be addressed for the successful commercialization of this technology.
One of the primary challenges lies in the scalable and reproducible synthesis of MXene-modified nanocarriers. The current methods often result in inconsistent particle sizes and surface properties, which can significantly impact their drug loading capacity and release kinetics. Achieving uniform and stable MXene coatings on nanocarriers remains a complex task, requiring precise control over reaction conditions and surface chemistry.
The stability of MXene-modified nanocarriers in physiological environments presents another hurdle. MXenes are prone to oxidation and agglomeration in aqueous solutions, potentially compromising their unique properties and reducing their effectiveness as drug delivery vehicles. Developing strategies to enhance the colloidal stability and prevent degradation of MXene coatings under biological conditions is crucial for their successful application.
Controlling the drug release profile from MXene-modified nanocarriers poses a significant challenge. While MXenes offer excellent drug loading capacity, achieving sustained and controlled release of therapeutic agents remains difficult. The strong interactions between MXenes and drug molecules can sometimes hinder the timely release of drugs at target sites, necessitating the development of smart release mechanisms responsive to specific stimuli.
The potential toxicity and long-term safety of MXene-modified nanocarriers are areas of concern that require extensive investigation. The biocompatibility and biodegradability of these materials in vivo are not yet fully understood, and their potential accumulation in organs and tissues raises questions about their long-term effects on human health.
Targeting efficiency and specificity of MXene-modified nanocarriers need further improvement. While surface modifications can enhance their targeting capabilities, achieving highly selective delivery to specific cell types or tissues remains challenging. Overcoming biological barriers, such as the blood-brain barrier or tumor microenvironment, requires innovative strategies in nanocarrier design and functionalization.
Lastly, the translation of MXene-modified nanocarriers from laboratory research to clinical applications faces regulatory and manufacturing challenges. Establishing standardized production processes, ensuring batch-to-batch consistency, and meeting stringent regulatory requirements for nanomedicine products are critical steps that need to be addressed for the successful commercialization of this technology.
Existing MXene-Nanocarrier Solutions
01 MXene-based nanocarriers for drug delivery
MXene, a two-dimensional transition metal carbide/nitride, is utilized as a novel nanocarrier for targeted drug delivery. Its unique properties, including large surface area and excellent biocompatibility, make it an ideal platform for loading and transporting therapeutic agents. These MXene-modified nanocarriers can enhance drug efficacy and reduce side effects through improved targeting and controlled release mechanisms.- MXene-based nanocarriers for drug delivery: MXene, a two-dimensional transition metal carbide/nitride, is utilized as a novel nanocarrier for targeted drug delivery. Its unique properties, such as large surface area and excellent biocompatibility, make it an ideal platform for loading and transporting therapeutic agents. These MXene-modified nanocarriers can enhance drug efficacy and reduce side effects through improved targeting and controlled release mechanisms.
- Surface modification of MXene nanocarriers: Various surface modification techniques are employed to enhance the functionality of MXene nanocarriers. These modifications can improve drug loading capacity, stability, and targeting efficiency. Strategies may include polymer coating, functionalization with targeting ligands, or incorporation of stimuli-responsive elements to achieve controlled drug release at specific sites.
- Targeted delivery mechanisms: MXene-modified nanocarriers are designed with specific targeting mechanisms to improve drug delivery to desired tissues or cells. This can involve the use of targeting moieties such as antibodies, peptides, or small molecules that recognize specific receptors or biomarkers on target cells. The targeted approach enhances therapeutic efficacy while minimizing off-target effects.
- Stimuli-responsive drug release: MXene-based nanocarriers can be engineered to respond to various stimuli such as pH, temperature, or light. This allows for controlled and site-specific drug release, improving the therapeutic index of the delivered drugs. The stimuli-responsive behavior can be achieved through the incorporation of smart polymers or other responsive materials into the nanocarrier structure.
- Combination therapy and theranostics: MXene-modified nanocarriers can be designed for combination therapy, allowing the co-delivery of multiple therapeutic agents. Additionally, these nanocarriers can integrate diagnostic and therapeutic functions, enabling theranostic applications. This approach combines imaging capabilities with drug delivery, facilitating real-time monitoring of treatment efficacy and personalized medicine strategies.
02 Surface modification of MXene nanocarriers
Various surface modification techniques are employed to enhance the functionality of MXene nanocarriers. These modifications can improve drug loading capacity, stability, and targeting efficiency. Strategies may include polymer coating, functionalization with targeting ligands, or incorporation of stimuli-responsive elements to achieve controlled drug release in specific physiological environments.Expand Specific Solutions03 Integration of imaging and therapeutic capabilities
MXene-modified nanocarriers are developed to combine both imaging and therapeutic functions, enabling theranostic applications. These multifunctional nanoplatforms can simultaneously deliver drugs and provide real-time imaging for diagnosis and treatment monitoring. This approach allows for personalized medicine and improved treatment outcomes in various diseases, particularly cancer.Expand Specific Solutions04 Targeted delivery to specific organs or tissues
MXene-based nanocarriers are engineered for targeted delivery to specific organs or tissues. This is achieved through the incorporation of targeting moieties or by exploiting the unique properties of MXene materials. The targeted approach enhances therapeutic efficacy while minimizing off-target effects, making it particularly beneficial for treating diseases such as cancer, neurological disorders, or cardiovascular conditions.Expand Specific Solutions05 Stimuli-responsive drug release mechanisms
MXene-modified nanocarriers are designed with stimuli-responsive mechanisms for controlled drug release. These systems can respond to various stimuli such as pH, temperature, light, or magnetic fields, allowing for precise control over the timing and location of drug release. This approach enhances therapeutic efficacy and reduces systemic toxicity by releasing the drug payload specifically at the target site.Expand Specific Solutions
Key Players in MXene and Nanocarrier Research
The research on MXene-modified nanocarriers for targeted drug delivery systems is in an emerging stage, with significant potential for growth. The market is expanding rapidly due to increasing demand for precision medicine and nanotechnology applications in healthcare. While the technology is still evolving, several key players are making substantial progress. Companies like Beijing Inno Medicine Co., Ltd. and Suzhou Beike Nano Technology Co., Ltd. are at the forefront, developing innovative nanoliposome and nanocarrier technologies. Academic institutions such as Yale University, Shanghai Jiao Tong University, and Fudan University are contributing significantly to fundamental research. The involvement of both industry and academia indicates a collaborative ecosystem driving technological advancements in this field.
Shanghai Jiao Tong University
Technical Solution: Shanghai Jiao Tong University has made significant contributions to MXene-modified nanocarriers for targeted drug delivery systems. Their research focuses on developing stimuli-responsive MXene nanocarriers that can release drugs in response to specific tumor microenvironment cues[1]. The team has engineered MXene nanosheets with redox-sensitive linkages, allowing for glutathione-triggered drug release in cancer cells[2]. They have also explored the integration of MXene with other nanomaterials, such as gold nanoparticles, to create hybrid nanocarriers with enhanced photothermal effects and drug loading capacity[3]. Recent studies from the university have demonstrated the potential of MXene-based nanocarriers in overcoming multidrug resistance in cancer therapy, showing improved intracellular drug accumulation and efficacy against resistant tumor cells[4]. Additionally, their work on MXene-based theranostic platforms has shown promise in combining targeted drug delivery with real-time imaging capabilities[5].
Strengths: Innovative stimuli-responsive designs, effective against drug-resistant cancers, theranostic capabilities. Weaknesses: Potential for off-target effects, need for further optimization of drug loading efficiency.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering (IPE) at the Chinese Academy of Sciences has made significant strides in MXene-modified nanocarriers for targeted drug delivery systems. Their approach involves synthesizing MXene nanosheets with controlled size and surface chemistry, which are then functionalized with targeting ligands. These MXene-based nanocarriers demonstrate excellent drug loading capacity, particularly for hydrophobic drugs[1]. The institute has developed a pH-responsive drug release mechanism, allowing for controlled release in tumor microenvironments[2]. Additionally, IPE researchers have incorporated magnetic nanoparticles into the MXene structure, enabling magnetically guided drug delivery and potential for combined chemo-photothermal therapy[3]. Their recent studies have shown promising results in in vitro and in vivo models, with enhanced tumor accumulation and reduced systemic toxicity compared to conventional drug delivery systems[4].
Strengths: Advanced synthesis techniques, multifunctional nanocarriers, proven efficacy in preclinical studies. Weaknesses: Potential scalability issues, need for further long-term safety evaluations.
Core Innovations in MXene Modification
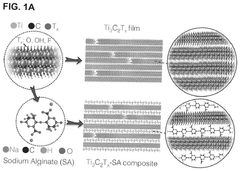
Antennas comprising MX-ENE films and composites
PatentActiveUS11862847B2
Innovation
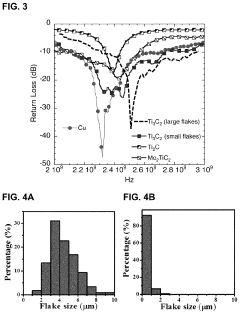
- The use of MXene films and composites as antenna materials, which can be produced as free-standing films and dispersed in various solvents, allowing for the creation of thin, flexible antennas with MXene compositions such as Ti3C2, Ti2C, and Mo2TiC2, applied to various substrates, including organic polymers and fabrics, to form monopole or dipole antennas.
Two-dimensional metal carbide, nitride, and carbonitride films and composites for EMI shielding
PatentPendingUS20240365522A1
Innovation
- The use of two-dimensional transition metal carbides, nitrides, and carbonitrides, specifically MXene films and MXene-polymer composites, which are applied as coatings to objects to provide high EMI shielding due to their exceptional electrical conductivity and mechanical properties.
Regulatory Landscape for Nanomedicine
The regulatory landscape for nanomedicine, particularly in the context of MXene-modified nanocarriers for targeted drug delivery systems, is complex and evolving. Regulatory bodies worldwide are grappling with the unique challenges posed by nanomaterials in medical applications, striving to balance innovation with safety and efficacy concerns.
In the United States, the Food and Drug Administration (FDA) has taken a leading role in developing regulatory frameworks for nanomedicine. The FDA's approach involves a product-specific, science-based assessment, considering the unique properties of nanomaterials. For MXene-modified nanocarriers, this would likely involve scrutiny of their physicochemical characteristics, biodistribution, and potential toxicity profiles.
The European Medicines Agency (EMA) has also been proactive in addressing nanomedicine regulation. The EMA's guidelines emphasize the importance of thorough characterization of nanomaterials and their interactions with biological systems. For MXene-based drug delivery systems, this would necessitate comprehensive studies on their behavior in vivo and potential long-term effects.
In Asia, regulatory bodies such as Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) are developing their own approaches to nanomedicine regulation. These agencies often look to international standards while adapting them to local contexts.
A key challenge in regulating MXene-modified nanocarriers lies in their multifunctional nature. These systems combine aspects of both drugs and devices, potentially falling under multiple regulatory categories. This complexity necessitates a collaborative approach among different regulatory departments and may require the development of new, hybrid regulatory pathways.
Safety assessment is a critical aspect of the regulatory landscape for MXene-based nanocarriers. Regulatory bodies are particularly concerned with the potential for unexpected biodistribution, accumulation in non-target tissues, and long-term toxicity. As a result, developers of these systems may need to conduct extensive preclinical studies to address these concerns before progressing to clinical trials.
The regulatory landscape also emphasizes the importance of manufacturing consistency and quality control. Given the sensitive nature of nanomaterials, regulatory bodies are likely to scrutinize the manufacturing processes for MXene-modified nanocarriers, ensuring batch-to-batch consistency and stability.
As the field of nanomedicine continues to advance, regulatory frameworks are expected to evolve. International collaboration and harmonization efforts, such as those led by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), are likely to play a crucial role in shaping global standards for nanomedicine regulation, including MXene-based drug delivery systems.
In the United States, the Food and Drug Administration (FDA) has taken a leading role in developing regulatory frameworks for nanomedicine. The FDA's approach involves a product-specific, science-based assessment, considering the unique properties of nanomaterials. For MXene-modified nanocarriers, this would likely involve scrutiny of their physicochemical characteristics, biodistribution, and potential toxicity profiles.
The European Medicines Agency (EMA) has also been proactive in addressing nanomedicine regulation. The EMA's guidelines emphasize the importance of thorough characterization of nanomaterials and their interactions with biological systems. For MXene-based drug delivery systems, this would necessitate comprehensive studies on their behavior in vivo and potential long-term effects.
In Asia, regulatory bodies such as Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) are developing their own approaches to nanomedicine regulation. These agencies often look to international standards while adapting them to local contexts.
A key challenge in regulating MXene-modified nanocarriers lies in their multifunctional nature. These systems combine aspects of both drugs and devices, potentially falling under multiple regulatory categories. This complexity necessitates a collaborative approach among different regulatory departments and may require the development of new, hybrid regulatory pathways.
Safety assessment is a critical aspect of the regulatory landscape for MXene-based nanocarriers. Regulatory bodies are particularly concerned with the potential for unexpected biodistribution, accumulation in non-target tissues, and long-term toxicity. As a result, developers of these systems may need to conduct extensive preclinical studies to address these concerns before progressing to clinical trials.
The regulatory landscape also emphasizes the importance of manufacturing consistency and quality control. Given the sensitive nature of nanomaterials, regulatory bodies are likely to scrutinize the manufacturing processes for MXene-modified nanocarriers, ensuring batch-to-batch consistency and stability.
As the field of nanomedicine continues to advance, regulatory frameworks are expected to evolve. International collaboration and harmonization efforts, such as those led by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), are likely to play a crucial role in shaping global standards for nanomedicine regulation, including MXene-based drug delivery systems.
Biocompatibility and Safety Considerations
The biocompatibility and safety considerations of MXene-modified nanocarriers for targeted drug delivery systems are crucial aspects that require thorough investigation and evaluation. MXenes, as a relatively new class of two-dimensional materials, have shown promising potential in biomedical applications, including drug delivery. However, their interaction with biological systems and potential toxicity must be carefully assessed before widespread clinical use.
One of the primary concerns is the potential cytotoxicity of MXene-modified nanocarriers. Studies have shown that the toxicity of MXenes can vary depending on their composition, size, and surface functionalization. It is essential to conduct comprehensive in vitro and in vivo studies to evaluate the cytotoxicity of these nanocarriers on various cell types and tissues. This includes assessing cell viability, proliferation, and potential genotoxic effects.
The biodegradability of MXene-modified nanocarriers is another critical factor to consider. Ideally, these nanocarriers should be designed to degrade into non-toxic byproducts that can be easily eliminated from the body. Research has shown that some MXenes can undergo gradual degradation in physiological conditions, but the long-term fate and potential accumulation of these materials in organs need further investigation.
Immunogenicity is a significant concern for any drug delivery system. MXene-modified nanocarriers must be engineered to minimize immune system recognition and activation. This involves careful surface modification and coating strategies to reduce protein adsorption and complement activation. Additionally, the potential for MXene-based nanocarriers to induce inflammatory responses or allergic reactions should be thoroughly evaluated.
The hemocompatibility of MXene-modified nanocarriers is crucial for their safe application in systemic drug delivery. Interactions with blood components, including red blood cells, platelets, and plasma proteins, must be studied to ensure that these nanocarriers do not cause hemolysis, thrombosis, or other adverse effects on blood function.
Long-term safety and potential chronic toxicity are areas that require extensive research. While acute toxicity studies are important, the effects of repeated exposure to MXene-modified nanocarriers over extended periods must be investigated. This includes assessing potential organ-specific toxicity, particularly in the liver and kidneys, which are primary sites for nanoparticle accumulation and clearance.
In conclusion, while MXene-modified nanocarriers show great promise for targeted drug delivery, their biocompatibility and safety profiles must be rigorously evaluated. This requires a multidisciplinary approach, combining expertise in materials science, pharmacology, toxicology, and clinical medicine. As research progresses, it is crucial to establish standardized protocols for safety assessment and develop regulatory guidelines specific to MXene-based nanomaterials in biomedical applications.
One of the primary concerns is the potential cytotoxicity of MXene-modified nanocarriers. Studies have shown that the toxicity of MXenes can vary depending on their composition, size, and surface functionalization. It is essential to conduct comprehensive in vitro and in vivo studies to evaluate the cytotoxicity of these nanocarriers on various cell types and tissues. This includes assessing cell viability, proliferation, and potential genotoxic effects.
The biodegradability of MXene-modified nanocarriers is another critical factor to consider. Ideally, these nanocarriers should be designed to degrade into non-toxic byproducts that can be easily eliminated from the body. Research has shown that some MXenes can undergo gradual degradation in physiological conditions, but the long-term fate and potential accumulation of these materials in organs need further investigation.
Immunogenicity is a significant concern for any drug delivery system. MXene-modified nanocarriers must be engineered to minimize immune system recognition and activation. This involves careful surface modification and coating strategies to reduce protein adsorption and complement activation. Additionally, the potential for MXene-based nanocarriers to induce inflammatory responses or allergic reactions should be thoroughly evaluated.
The hemocompatibility of MXene-modified nanocarriers is crucial for their safe application in systemic drug delivery. Interactions with blood components, including red blood cells, platelets, and plasma proteins, must be studied to ensure that these nanocarriers do not cause hemolysis, thrombosis, or other adverse effects on blood function.
Long-term safety and potential chronic toxicity are areas that require extensive research. While acute toxicity studies are important, the effects of repeated exposure to MXene-modified nanocarriers over extended periods must be investigated. This includes assessing potential organ-specific toxicity, particularly in the liver and kidneys, which are primary sites for nanoparticle accumulation and clearance.
In conclusion, while MXene-modified nanocarriers show great promise for targeted drug delivery, their biocompatibility and safety profiles must be rigorously evaluated. This requires a multidisciplinary approach, combining expertise in materials science, pharmacology, toxicology, and clinical medicine. As research progresses, it is crucial to establish standardized protocols for safety assessment and develop regulatory guidelines specific to MXene-based nanomaterials in biomedical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!