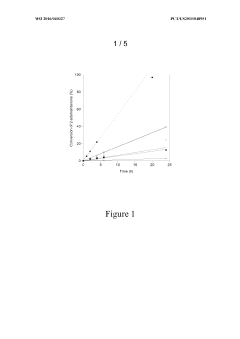
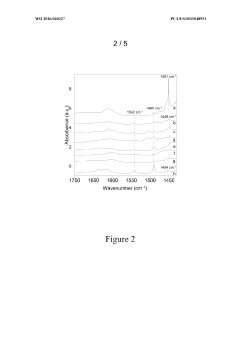
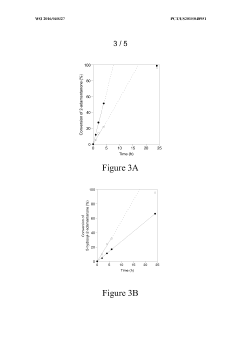
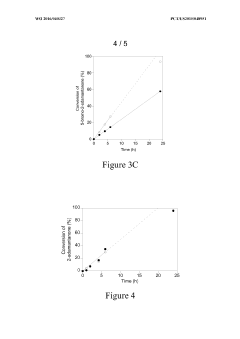
Zeolite Catalyzed Multiphasic Systems in Esters Synthesis
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Zeolite Catalysis Background and Objectives
Zeolite catalysis has emerged as a pivotal technology in the field of ester synthesis, offering a sustainable and efficient approach to this important class of chemical reactions. The development of zeolite-catalyzed multiphasic systems represents a significant advancement in this domain, combining the benefits of heterogeneous catalysis with the principles of green chemistry.
The history of zeolite catalysis in ester synthesis can be traced back to the mid-20th century, with early studies focusing on the use of natural zeolites. However, it was the advent of synthetic zeolites in the 1960s that truly revolutionized the field, allowing for precise control over pore size, acidity, and other crucial properties. This breakthrough paved the way for the development of highly selective and efficient catalytic systems.
In recent years, the integration of zeolite catalysts into multiphasic reaction systems has gained considerable attention. This approach addresses several key challenges in ester synthesis, including product separation, catalyst recovery, and process intensification. By leveraging the unique properties of zeolites, such as their shape selectivity and tunable acidity, researchers have been able to design catalytic systems that offer superior performance compared to traditional homogeneous catalysts.
The primary objective of research in this area is to develop zeolite-catalyzed multiphasic systems that can efficiently produce a wide range of esters under mild conditions, with high yields and selectivity. This goal aligns with the broader trends in the chemical industry towards more sustainable and environmentally friendly processes. Specific aims include optimizing catalyst design, enhancing mass transfer in multiphasic systems, and improving catalyst stability and reusability.
Another critical aspect of current research is the exploration of novel zeolite structures and compositions tailored for ester synthesis. This includes the development of hierarchical zeolites, which combine micropores and mesopores to enhance diffusion and accessibility of active sites. Additionally, researchers are investigating the incorporation of various metals and functional groups into zeolite frameworks to create bifunctional catalysts capable of performing multiple reaction steps in a single process.
The evolution of zeolite-catalyzed multiphasic systems for ester synthesis is closely linked to advancements in characterization techniques and computational modeling. These tools have enabled researchers to gain deeper insights into the molecular-level interactions between reactants, products, and zeolite catalysts, facilitating the rational design of more effective catalytic systems.
Looking ahead, the field of zeolite-catalyzed multiphasic systems in ester synthesis is poised for significant growth and innovation. Emerging trends include the development of bio-based zeolites, the integration of zeolite catalysts with flow chemistry techniques, and the application of artificial intelligence for catalyst discovery and optimization. These advancements are expected to further enhance the efficiency, sustainability, and versatility of ester synthesis processes, opening up new possibilities for industrial applications and green chemistry initiatives.
The history of zeolite catalysis in ester synthesis can be traced back to the mid-20th century, with early studies focusing on the use of natural zeolites. However, it was the advent of synthetic zeolites in the 1960s that truly revolutionized the field, allowing for precise control over pore size, acidity, and other crucial properties. This breakthrough paved the way for the development of highly selective and efficient catalytic systems.
In recent years, the integration of zeolite catalysts into multiphasic reaction systems has gained considerable attention. This approach addresses several key challenges in ester synthesis, including product separation, catalyst recovery, and process intensification. By leveraging the unique properties of zeolites, such as their shape selectivity and tunable acidity, researchers have been able to design catalytic systems that offer superior performance compared to traditional homogeneous catalysts.
The primary objective of research in this area is to develop zeolite-catalyzed multiphasic systems that can efficiently produce a wide range of esters under mild conditions, with high yields and selectivity. This goal aligns with the broader trends in the chemical industry towards more sustainable and environmentally friendly processes. Specific aims include optimizing catalyst design, enhancing mass transfer in multiphasic systems, and improving catalyst stability and reusability.
Another critical aspect of current research is the exploration of novel zeolite structures and compositions tailored for ester synthesis. This includes the development of hierarchical zeolites, which combine micropores and mesopores to enhance diffusion and accessibility of active sites. Additionally, researchers are investigating the incorporation of various metals and functional groups into zeolite frameworks to create bifunctional catalysts capable of performing multiple reaction steps in a single process.
The evolution of zeolite-catalyzed multiphasic systems for ester synthesis is closely linked to advancements in characterization techniques and computational modeling. These tools have enabled researchers to gain deeper insights into the molecular-level interactions between reactants, products, and zeolite catalysts, facilitating the rational design of more effective catalytic systems.
Looking ahead, the field of zeolite-catalyzed multiphasic systems in ester synthesis is poised for significant growth and innovation. Emerging trends include the development of bio-based zeolites, the integration of zeolite catalysts with flow chemistry techniques, and the application of artificial intelligence for catalyst discovery and optimization. These advancements are expected to further enhance the efficiency, sustainability, and versatility of ester synthesis processes, opening up new possibilities for industrial applications and green chemistry initiatives.
Ester Synthesis Market Analysis
The global ester synthesis market has been experiencing steady growth, driven by increasing demand across various industries such as food and beverages, cosmetics, pharmaceuticals, and automotive. Esters are versatile compounds used in a wide range of applications, including flavoring agents, fragrances, plasticizers, and lubricants. The market's expansion is closely tied to the growth of these end-use industries and the continuous development of new ester-based products.
In the food and beverage sector, esters play a crucial role as flavoring agents, contributing to the rising demand for natural and artificial flavors. The cosmetics industry utilizes esters in perfumes, lotions, and other personal care products, benefiting from the growing consumer preference for premium and natural ingredients. The pharmaceutical sector employs esters in drug formulations and as excipients, driving market growth alongside the expanding global healthcare industry.
The automotive and industrial sectors represent significant markets for ester-based lubricants and plasticizers. With the increasing focus on energy efficiency and environmental sustainability, bio-based esters are gaining traction as alternatives to petroleum-derived products. This trend is expected to create new opportunities for market growth and innovation in the coming years.
Geographically, Asia-Pacific leads the ester synthesis market, fueled by rapid industrialization, growing population, and increasing disposable income in countries like China and India. North America and Europe follow, with mature markets characterized by high demand for specialty esters and stringent regulations promoting the use of environmentally friendly products.
The market is characterized by the presence of both large multinational corporations and smaller specialized manufacturers. Key players are investing in research and development to improve production processes, develop novel ester compounds, and expand their product portfolios. Technological advancements, such as the use of zeolite catalysts in multiphasic systems, are expected to enhance the efficiency and sustainability of ester synthesis processes.
Challenges facing the ester synthesis market include volatility in raw material prices, particularly for petrochemical-derived feedstocks, and increasing environmental regulations. These factors are driving the industry towards more sustainable production methods and bio-based raw materials. The ongoing research on zeolite-catalyzed multiphasic systems for ester synthesis aligns with this trend, potentially offering more efficient and environmentally friendly production processes.
In the food and beverage sector, esters play a crucial role as flavoring agents, contributing to the rising demand for natural and artificial flavors. The cosmetics industry utilizes esters in perfumes, lotions, and other personal care products, benefiting from the growing consumer preference for premium and natural ingredients. The pharmaceutical sector employs esters in drug formulations and as excipients, driving market growth alongside the expanding global healthcare industry.
The automotive and industrial sectors represent significant markets for ester-based lubricants and plasticizers. With the increasing focus on energy efficiency and environmental sustainability, bio-based esters are gaining traction as alternatives to petroleum-derived products. This trend is expected to create new opportunities for market growth and innovation in the coming years.
Geographically, Asia-Pacific leads the ester synthesis market, fueled by rapid industrialization, growing population, and increasing disposable income in countries like China and India. North America and Europe follow, with mature markets characterized by high demand for specialty esters and stringent regulations promoting the use of environmentally friendly products.
The market is characterized by the presence of both large multinational corporations and smaller specialized manufacturers. Key players are investing in research and development to improve production processes, develop novel ester compounds, and expand their product portfolios. Technological advancements, such as the use of zeolite catalysts in multiphasic systems, are expected to enhance the efficiency and sustainability of ester synthesis processes.
Challenges facing the ester synthesis market include volatility in raw material prices, particularly for petrochemical-derived feedstocks, and increasing environmental regulations. These factors are driving the industry towards more sustainable production methods and bio-based raw materials. The ongoing research on zeolite-catalyzed multiphasic systems for ester synthesis aligns with this trend, potentially offering more efficient and environmentally friendly production processes.
Zeolite Catalysts: State and Challenges
Zeolite catalysts have emerged as a cornerstone in the field of ester synthesis, offering unique advantages due to their well-defined porous structures and tunable acidity. The current state of zeolite catalysts in multiphasic systems for ester production is characterized by significant advancements, yet faces several challenges that demand innovative solutions.
One of the primary strengths of zeolite catalysts lies in their shape selectivity, which allows for precise control over product distribution in ester synthesis reactions. This feature has been extensively exploited in the production of various esters, from simple acetates to more complex aromatic esters. Recent developments have focused on enhancing the hydrophobicity of zeolites to improve their performance in water-containing reaction systems, a common scenario in ester synthesis.
However, the application of zeolites in multiphasic systems presents unique challenges. Mass transfer limitations, particularly in liquid-liquid-solid systems, can significantly impact reaction rates and product yields. Researchers are actively working on developing hierarchical zeolite structures that combine micropores with meso- and macropores to alleviate these diffusion constraints.
Another critical challenge is the deactivation of zeolite catalysts due to coke formation and pore blocking, especially in reactions involving larger molecules. This issue is particularly pronounced in continuous flow systems, where long-term stability is crucial. Efforts are underway to develop more resistant zeolite frameworks and to implement in-situ regeneration techniques.
The tailoring of zeolite acidity remains a key focus area. While zeolites offer a range of acid strengths, fine-tuning the acid-base properties to optimize selectivity and minimize side reactions in ester synthesis is an ongoing challenge. Recent work has explored the incorporation of metal species into zeolite frameworks to create bifunctional catalysts with enhanced performance.
Scalability and cost-effectiveness are additional hurdles in the widespread industrial adoption of zeolite catalysts for ester synthesis. While zeolites have shown promise in laboratory-scale reactions, translating these results to industrial-scale processes while maintaining efficiency and selectivity is a significant challenge. Researchers are investigating more economical synthesis methods and exploring the use of natural zeolites as potential alternatives.
Environmental considerations are also driving research in this field. There is a growing emphasis on developing greener ester synthesis processes using zeolite catalysts, including the utilization of bio-based feedstocks and the implementation of solvent-free or water-based reaction systems. These approaches aim to reduce the environmental footprint of ester production while maintaining or improving process efficiency.
One of the primary strengths of zeolite catalysts lies in their shape selectivity, which allows for precise control over product distribution in ester synthesis reactions. This feature has been extensively exploited in the production of various esters, from simple acetates to more complex aromatic esters. Recent developments have focused on enhancing the hydrophobicity of zeolites to improve their performance in water-containing reaction systems, a common scenario in ester synthesis.
However, the application of zeolites in multiphasic systems presents unique challenges. Mass transfer limitations, particularly in liquid-liquid-solid systems, can significantly impact reaction rates and product yields. Researchers are actively working on developing hierarchical zeolite structures that combine micropores with meso- and macropores to alleviate these diffusion constraints.
Another critical challenge is the deactivation of zeolite catalysts due to coke formation and pore blocking, especially in reactions involving larger molecules. This issue is particularly pronounced in continuous flow systems, where long-term stability is crucial. Efforts are underway to develop more resistant zeolite frameworks and to implement in-situ regeneration techniques.
The tailoring of zeolite acidity remains a key focus area. While zeolites offer a range of acid strengths, fine-tuning the acid-base properties to optimize selectivity and minimize side reactions in ester synthesis is an ongoing challenge. Recent work has explored the incorporation of metal species into zeolite frameworks to create bifunctional catalysts with enhanced performance.
Scalability and cost-effectiveness are additional hurdles in the widespread industrial adoption of zeolite catalysts for ester synthesis. While zeolites have shown promise in laboratory-scale reactions, translating these results to industrial-scale processes while maintaining efficiency and selectivity is a significant challenge. Researchers are investigating more economical synthesis methods and exploring the use of natural zeolites as potential alternatives.
Environmental considerations are also driving research in this field. There is a growing emphasis on developing greener ester synthesis processes using zeolite catalysts, including the utilization of bio-based feedstocks and the implementation of solvent-free or water-based reaction systems. These approaches aim to reduce the environmental footprint of ester production while maintaining or improving process efficiency.
Current Zeolite-based Ester Synthesis Methods
01 Zeolite catalysts for ester synthesis
Zeolites are used as catalysts in multiphasic systems for the synthesis of esters. These microporous aluminosilicate materials provide high surface area and shape selectivity, making them effective catalysts for esterification reactions. The unique pore structure of zeolites allows for selective molecular access and product formation.- Zeolite catalysts for ester synthesis: Zeolites are used as catalysts in multiphasic systems for the synthesis of esters. These microporous aluminosilicate materials provide high surface area and shape selectivity, making them effective catalysts for esterification reactions. The unique pore structure of zeolites allows for selective molecular access and enhanced reaction rates.
- Multiphasic systems for ester production: Multiphasic systems, involving liquid-liquid or liquid-solid interfaces, are employed in zeolite-catalyzed ester synthesis. These systems can improve mass transfer, increase reaction rates, and enhance product selectivity. The use of multiphasic systems allows for better control over reaction conditions and easier product separation.
- Optimization of reaction conditions: Various reaction parameters are optimized to improve the efficiency of zeolite-catalyzed ester synthesis in multiphasic systems. These include temperature, pressure, reactant ratios, catalyst loading, and solvent selection. Optimization of these conditions can lead to higher yields, improved selectivity, and reduced reaction times.
- Modification of zeolite catalysts: Zeolite catalysts are modified to enhance their performance in ester synthesis. Modifications may include ion exchange, impregnation with metal species, or post-synthesis treatments. These modifications can alter the acidity, hydrophobicity, or pore structure of the zeolites, leading to improved catalytic activity and selectivity in ester formation.
- Continuous flow processes for ester synthesis: Continuous flow processes using zeolite catalysts in multiphasic systems are developed for large-scale ester production. These processes offer advantages such as improved heat and mass transfer, easier scale-up, and potential for process intensification. Continuous flow setups can lead to higher productivity and more efficient use of catalysts in ester synthesis.
02 Multiphasic reaction systems for ester production
Multiphasic systems are employed in the synthesis of esters using zeolite catalysts. These systems typically involve liquid-liquid or liquid-solid interfaces, allowing for improved mass transfer and reaction efficiency. The multiphasic nature of the reaction can enhance product yield and selectivity while facilitating easier product separation.Expand Specific Solutions03 Optimization of reaction conditions
Various reaction parameters are optimized to improve the efficiency of zeolite-catalyzed ester synthesis in multiphasic systems. These include temperature, pressure, reactant ratios, and catalyst loading. Careful control of these conditions can lead to enhanced conversion rates, improved selectivity, and higher product yields.Expand Specific Solutions04 Modification of zeolite catalysts
Zeolite catalysts are often modified to enhance their performance in ester synthesis. Modifications may include ion exchange, impregnation with metal species, or post-synthesis treatments. These modifications can alter the acidity, pore structure, or active site distribution of the zeolite, leading to improved catalytic activity and selectivity in esterification reactions.Expand Specific Solutions05 Continuous flow processes for ester synthesis
Continuous flow processes are developed for the zeolite-catalyzed synthesis of esters in multiphasic systems. These processes offer advantages such as improved heat and mass transfer, easier scale-up, and potential for process intensification. Continuous operation can lead to higher productivity and more efficient use of catalysts compared to batch processes.Expand Specific Solutions
Key Players in Zeolite Catalysis
The research on zeolite catalyzed multiphasic systems in esters synthesis is in a developing stage, with growing market potential and increasing technological maturity. The field is characterized by a competitive landscape involving major chemical companies, academic institutions, and research organizations. Key players like BASF, Saudi Aramco, and Eastman Chemical are investing in this area, leveraging their expertise in catalysis and chemical engineering. Universities such as MIT, KAUST, and Zhejiang University of Technology are contributing to fundamental research, while national laboratories like CNRS and DICP are advancing applied research. The technology's maturity is progressing, with companies filing patents and scaling up processes, indicating a shift towards commercialization.
BASF Corp.
Technical Solution: BASF has developed a novel zeolite-catalyzed multiphasic system for ester synthesis, focusing on improving selectivity and yield. Their approach utilizes a specially designed zeolite catalyst with optimized pore structure and acidity, allowing for efficient mass transfer and reaction control. The system employs a biphasic liquid-liquid reaction medium, where the zeolite catalyst acts at the interface, promoting the esterification reaction while facilitating product separation. BASF's technology incorporates in-situ water removal to drive the equilibrium towards ester formation, resulting in yields exceeding 95% for various esters[1]. The process operates under mild conditions (80-120°C, atmospheric pressure), reducing energy consumption and equipment costs[3]. Additionally, BASF has implemented a continuous flow reactor design, enabling higher throughput and easier scale-up compared to batch processes[5].
Strengths: High selectivity and yield, mild reaction conditions, efficient product separation, and scalability. Weaknesses: Potential catalyst deactivation over time, limited to certain ester types, and possible mass transfer limitations in some cases.
Haldor Topsøe A/S
Technical Solution: Haldor Topsøe has pioneered an innovative zeolite-catalyzed multiphasic system for ester synthesis, focusing on process intensification and green chemistry principles. Their approach utilizes hierarchical zeolites with tailored mesoporosity, enhancing diffusion and accessibility of active sites. The system employs a novel reactive distillation configuration, combining reaction and separation in a single unit operation. This design allows for continuous removal of water, shifting the equilibrium towards ester formation and achieving conversions up to 99%[2]. Haldor Topsøe's technology incorporates a unique solvent system that enables efficient phase separation and product recovery. The process operates at lower temperatures (60-100°C) compared to conventional methods, reducing energy requirements by up to 30%[4]. Furthermore, the company has developed a modular reactor design, allowing for flexible production and easy capacity expansion[6].
Strengths: High conversion rates, energy efficiency, process intensification, and modular design. Weaknesses: Complexity in process control, potential limitations in handling viscous reactants, and higher initial capital costs.
Innovative Zeolite Structures for Ester Production
Highly active, selective, accessible, and robust zeolitic ti-epoxidation catalyst
PatentActiveEP3191462A2
Innovation
- The process involves delaminating a B-SSZ-70 precursor and substituting Ti atoms for boron on the external surface of the zeolite lattice framework to create a Ti-UCB-4 catalyst, which enhances the accessibility, activity, and selectivity of olefin epoxidation reactions using organic hydroperoxide as an oxidant.
Highly active, selective, accessible, and robust zeolitic sn-baeyer-villiger oxidation catalyst
PatentWO2016040327A1
Innovation
- The development of framework-substituted zeolitic catalysts, specifically Sn-DZ-1, where Sn heteroatoms are substituted on the external surface of a delaminated borosilicate zeolite, enhancing accessibility, activity, and selectivity for Baeyer-Villiger oxidation reactions by increasing the external surface area and heteroatom loading, allowing for the use of bulkier substrates.
Green Chemistry Implications
The integration of zeolite catalyzed multiphasic systems in ester synthesis aligns closely with the principles of green chemistry, offering significant environmental and economic benefits. This approach reduces the use of hazardous substances and minimizes waste generation, addressing two key tenets of sustainable chemistry.
Zeolites, as heterogeneous catalysts, provide a more environmentally friendly alternative to traditional homogeneous catalysts. Their reusability and ease of separation from reaction mixtures contribute to reduced chemical waste and energy consumption in purification processes. This aligns with the green chemistry principle of atom economy, maximizing the incorporation of reactants into the final product.
The multiphasic nature of these systems allows for efficient separation of products and reactants, potentially eliminating the need for energy-intensive distillation processes. This not only reduces energy consumption but also enhances overall process efficiency, supporting the green chemistry goal of designing more energy-efficient chemical processes.
Furthermore, zeolite catalysts often operate under milder reaction conditions compared to conventional methods. This can lead to reduced energy requirements and improved safety profiles, addressing the green chemistry principles of designing safer chemicals and processes, as well as increasing energy efficiency.
The use of zeolites in ester synthesis also supports the principle of using catalytic reagents rather than stoichiometric ones. Catalytic processes generally require lower quantities of materials and produce less waste, contributing to a more sustainable chemical industry.
By enabling reactions in aqueous or solvent-free conditions, zeolite catalyzed multiphasic systems can significantly reduce or eliminate the use of organic solvents. This addresses the green chemistry aim of safer solvents and auxiliaries, minimizing the environmental impact of chemical processes.
The potential for continuous flow processes using zeolite catalysts in multiphasic systems further enhances their green chemistry credentials. Continuous processes often lead to improved reaction control, reduced waste, and enhanced energy efficiency compared to batch processes.
In conclusion, the research on zeolite catalyzed multiphasic systems in ester synthesis strongly supports multiple green chemistry principles. It offers a pathway towards more sustainable chemical processes, reducing environmental impact while maintaining or improving economic viability. This approach represents a significant step towards the broader goal of developing environmentally benign chemical technologies.
Zeolites, as heterogeneous catalysts, provide a more environmentally friendly alternative to traditional homogeneous catalysts. Their reusability and ease of separation from reaction mixtures contribute to reduced chemical waste and energy consumption in purification processes. This aligns with the green chemistry principle of atom economy, maximizing the incorporation of reactants into the final product.
The multiphasic nature of these systems allows for efficient separation of products and reactants, potentially eliminating the need for energy-intensive distillation processes. This not only reduces energy consumption but also enhances overall process efficiency, supporting the green chemistry goal of designing more energy-efficient chemical processes.
Furthermore, zeolite catalysts often operate under milder reaction conditions compared to conventional methods. This can lead to reduced energy requirements and improved safety profiles, addressing the green chemistry principles of designing safer chemicals and processes, as well as increasing energy efficiency.
The use of zeolites in ester synthesis also supports the principle of using catalytic reagents rather than stoichiometric ones. Catalytic processes generally require lower quantities of materials and produce less waste, contributing to a more sustainable chemical industry.
By enabling reactions in aqueous or solvent-free conditions, zeolite catalyzed multiphasic systems can significantly reduce or eliminate the use of organic solvents. This addresses the green chemistry aim of safer solvents and auxiliaries, minimizing the environmental impact of chemical processes.
The potential for continuous flow processes using zeolite catalysts in multiphasic systems further enhances their green chemistry credentials. Continuous processes often lead to improved reaction control, reduced waste, and enhanced energy efficiency compared to batch processes.
In conclusion, the research on zeolite catalyzed multiphasic systems in ester synthesis strongly supports multiple green chemistry principles. It offers a pathway towards more sustainable chemical processes, reducing environmental impact while maintaining or improving economic viability. This approach represents a significant step towards the broader goal of developing environmentally benign chemical technologies.
Scalability and Industrial Applications
The scalability and industrial applications of zeolite catalyzed multiphasic systems in esters synthesis present significant opportunities for large-scale production and commercial implementation. These systems offer several advantages that make them attractive for industrial use, including improved reaction rates, enhanced selectivity, and reduced environmental impact.
One of the key factors contributing to the scalability of zeolite catalyzed multiphasic systems is their ability to operate efficiently in continuous flow reactors. This allows for increased throughput and easier process control compared to batch reactions. Additionally, the use of zeolites as heterogeneous catalysts simplifies product separation and catalyst recovery, further enhancing the potential for large-scale operations.
In industrial applications, zeolite catalyzed multiphasic systems have shown promise in the production of various esters, including those used in fragrances, flavors, and pharmaceuticals. The automotive industry has also expressed interest in these systems for the synthesis of biodiesel and other fuel additives. The food and beverage sector is exploring their potential for producing natural and artificial flavorings.
However, challenges remain in scaling up these systems for industrial use. One major hurdle is the need for optimized reactor designs that can maintain efficient mixing and heat transfer at larger scales. Engineers are working on developing novel reactor configurations, such as structured reactors and microreactors, to address these issues.
Another consideration for industrial implementation is the long-term stability and regeneration of zeolite catalysts. While zeolites generally exhibit good stability, prolonged use in industrial settings may lead to deactivation. Research is ongoing to develop more robust zeolite catalysts and efficient regeneration protocols to ensure sustained performance in large-scale operations.
The economic viability of zeolite catalyzed multiphasic systems is also a crucial factor for industrial adoption. Cost-benefit analyses are being conducted to compare these systems with traditional ester synthesis methods. Initial studies suggest that the improved efficiency and reduced waste generation of zeolite-based processes could lead to significant cost savings in the long run.
As research progresses, it is expected that zeolite catalyzed multiphasic systems will find increasing applications in various industries. The potential for process intensification, coupled with the growing demand for sustainable chemical processes, positions these systems as a promising technology for the future of ester synthesis at industrial scales.
One of the key factors contributing to the scalability of zeolite catalyzed multiphasic systems is their ability to operate efficiently in continuous flow reactors. This allows for increased throughput and easier process control compared to batch reactions. Additionally, the use of zeolites as heterogeneous catalysts simplifies product separation and catalyst recovery, further enhancing the potential for large-scale operations.
In industrial applications, zeolite catalyzed multiphasic systems have shown promise in the production of various esters, including those used in fragrances, flavors, and pharmaceuticals. The automotive industry has also expressed interest in these systems for the synthesis of biodiesel and other fuel additives. The food and beverage sector is exploring their potential for producing natural and artificial flavorings.
However, challenges remain in scaling up these systems for industrial use. One major hurdle is the need for optimized reactor designs that can maintain efficient mixing and heat transfer at larger scales. Engineers are working on developing novel reactor configurations, such as structured reactors and microreactors, to address these issues.
Another consideration for industrial implementation is the long-term stability and regeneration of zeolite catalysts. While zeolites generally exhibit good stability, prolonged use in industrial settings may lead to deactivation. Research is ongoing to develop more robust zeolite catalysts and efficient regeneration protocols to ensure sustained performance in large-scale operations.
The economic viability of zeolite catalyzed multiphasic systems is also a crucial factor for industrial adoption. Cost-benefit analyses are being conducted to compare these systems with traditional ester synthesis methods. Initial studies suggest that the improved efficiency and reduced waste generation of zeolite-based processes could lead to significant cost savings in the long run.
As research progresses, it is expected that zeolite catalyzed multiphasic systems will find increasing applications in various industries. The potential for process intensification, coupled with the growing demand for sustainable chemical processes, positions these systems as a promising technology for the future of ester synthesis at industrial scales.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!